Abstract
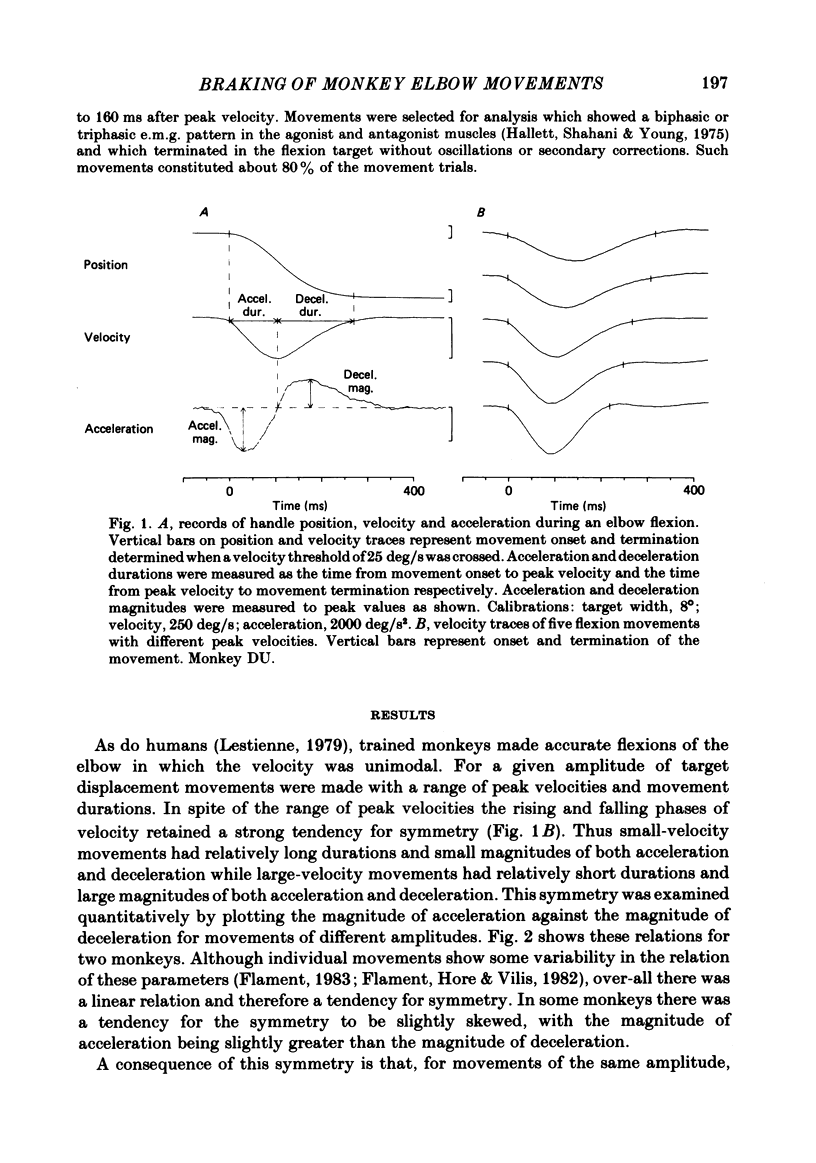
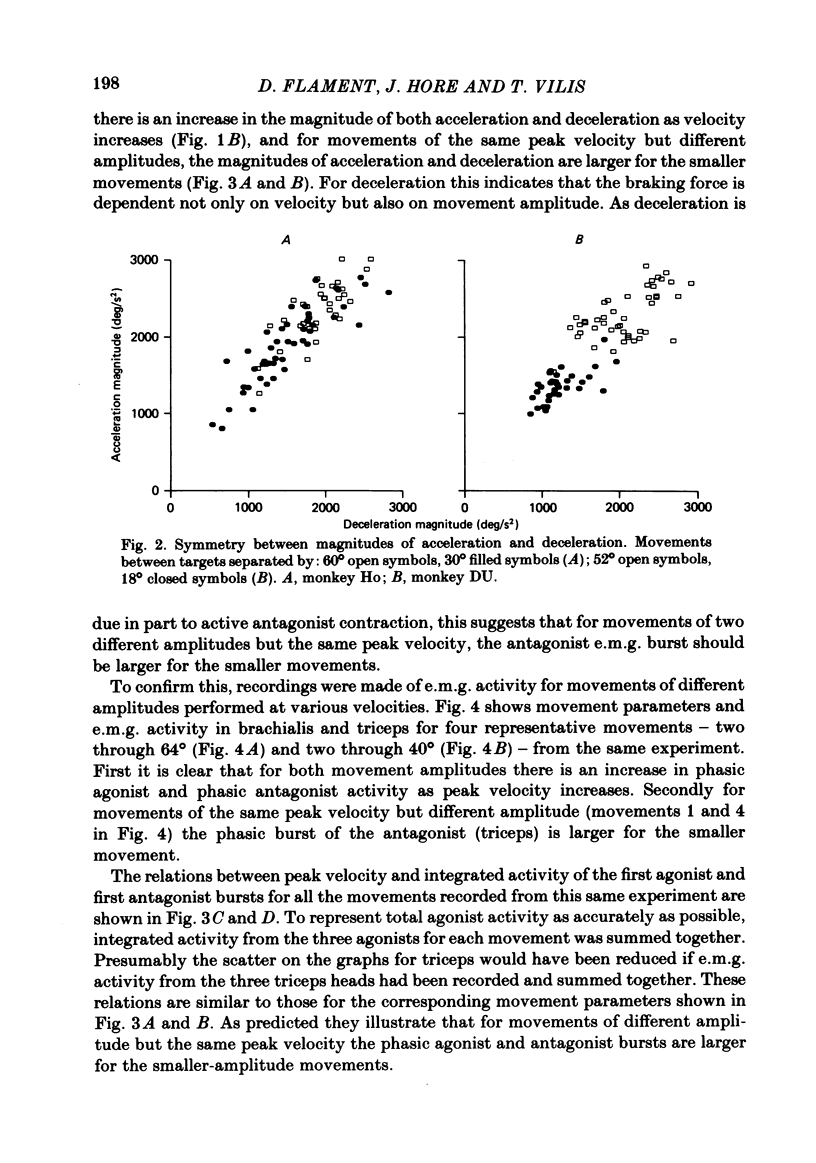
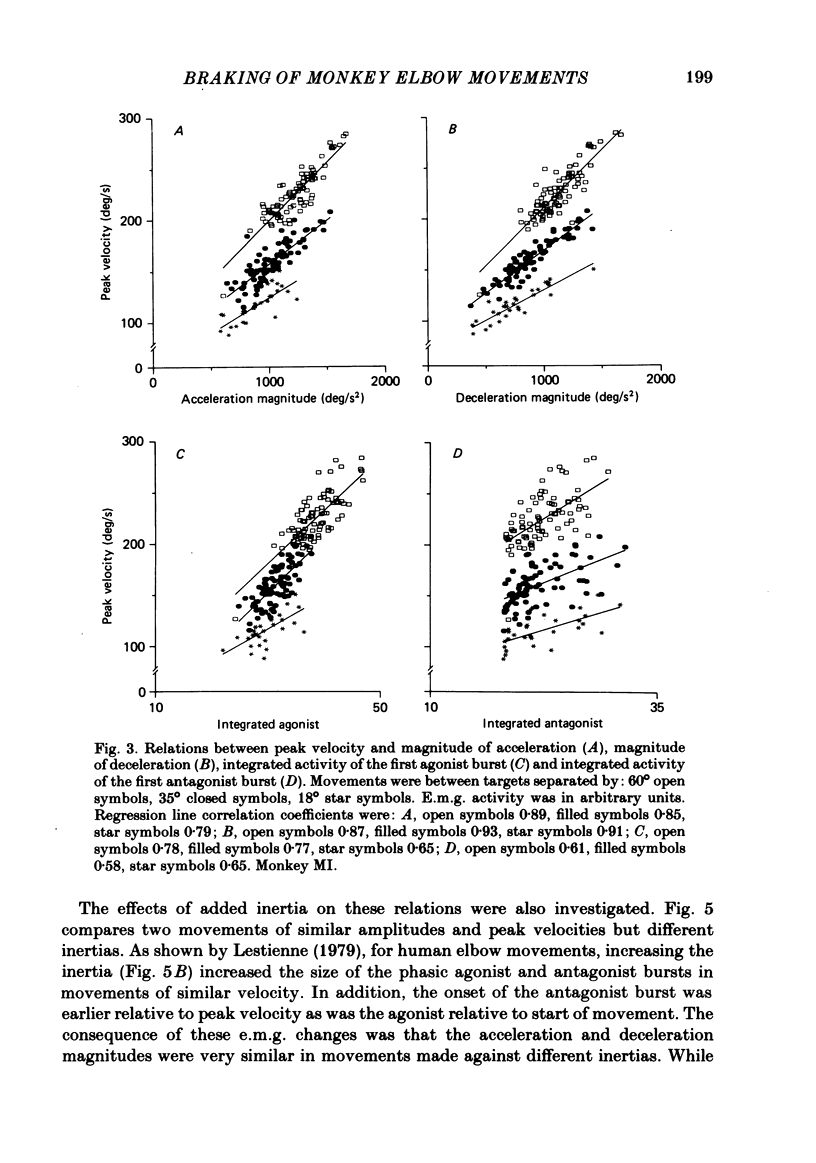
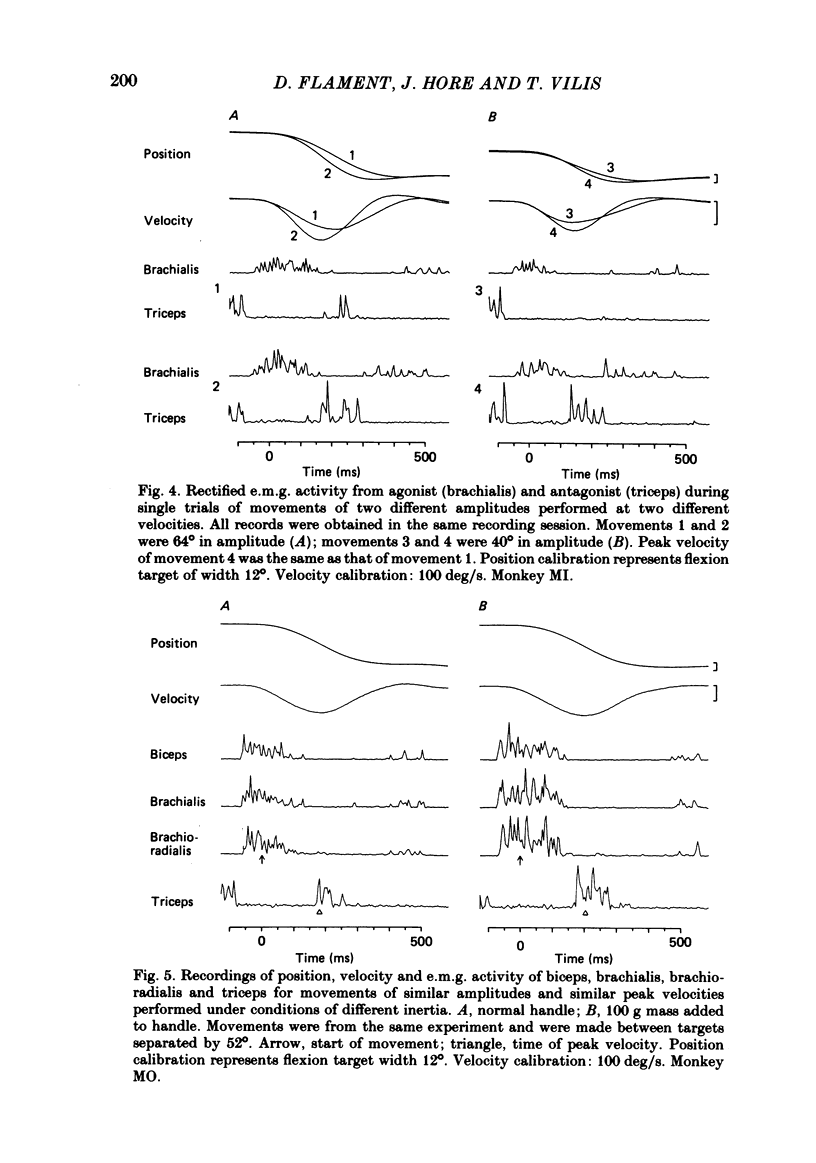
The processes responsible for braking fast and accurate elbow movements were studied in the monkey. The movements studied were made over different amplitudes and against different inertias . All were made to the same end position. Only fast movements that showed the typical biphasic or triphasic pattern of activity in agonists and antagonists were analysed in detail. For movements made over different amplitudes and at different velocities there was symmetry between the acceleration and deceleration phases of the movements. For movements of the same amplitude performed at different velocities there was a direct linear relation between peak velocity and both the peak acceleration (and integrated agonist burst) and peak deceleration (and integrated antagonist burst). The slopes of these relations and their intercept with the peak velocity axis were a function of movement amplitude. This was such that for large and small movements of the same peak velocity and the same end position (i) peak acceleration and phasic agonist activity were larger for the small movements and (ii) peak deceleration and phasic antagonist activity were larger for the small movements. The slope of these relations and the symmetry between acceleration and deceleration were not affected by the addition of an inertial load to the handle held by the monkey. The results indicate that fast and accurate elbow movements in the monkey are braked by antagonist activity that is centrally programmed. As all movements were made to the same end position, the larger antagonist burst in small movements, made at the same peak velocity as large movements, cannot be due to differences in the viscoelastic contribution to braking (cf. Marsden, Obeso & Rothwell , 1983).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown S. H., Cooke J. D. Amplitude- and instruction-dependent modulation of movement-related electromyogram activity in humans. J Physiol. 1981 Jul;316:97–107. doi: 10.1113/jphysiol.1981.sp013775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghez C., Martin J. H. The control of rapid limb movement in the cat. III. Agonist - antagonist coupling. Exp Brain Res. 1982;45(1-2):115–125. doi: 10.1007/BF00235770. [DOI] [PubMed] [Google Scholar]
- Hallett M., Marsden C. D. Ballistic flexion movements of the human thumb. J Physiol. 1979 Sep;294:33–50. doi: 10.1113/jphysiol.1979.sp012913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M., Shahani B. T., Young R. R. EMG analysis of stereotyped voluntary movements in man. J Neurol Neurosurg Psychiatry. 1975 Dec;38(12):1154–1162. doi: 10.1136/jnnp.38.12.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lestienne F. Effects of inertial load and velocity on the braking process of voluntary limb movements. Exp Brain Res. 1979 May 2;35(3):407–418. doi: 10.1007/BF00236760. [DOI] [PubMed] [Google Scholar]
- Marsden C. D., Obeso J. A., Rothwell J. C. The function of the antagonist muscle during fast limb movements in man. J Physiol. 1983 Feb;335:1–13. doi: 10.1113/jphysiol.1983.sp014514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soechting J. F., Ranish N. A., Palminteri R., Terzuolo C. A. Changes in a motor pattern following cerebellar and olivary lesions in the squirrel monkey. Brain Res. 1976 Mar 19;105(1):21–44. doi: 10.1016/0006-8993(76)90920-3. [DOI] [PubMed] [Google Scholar]


