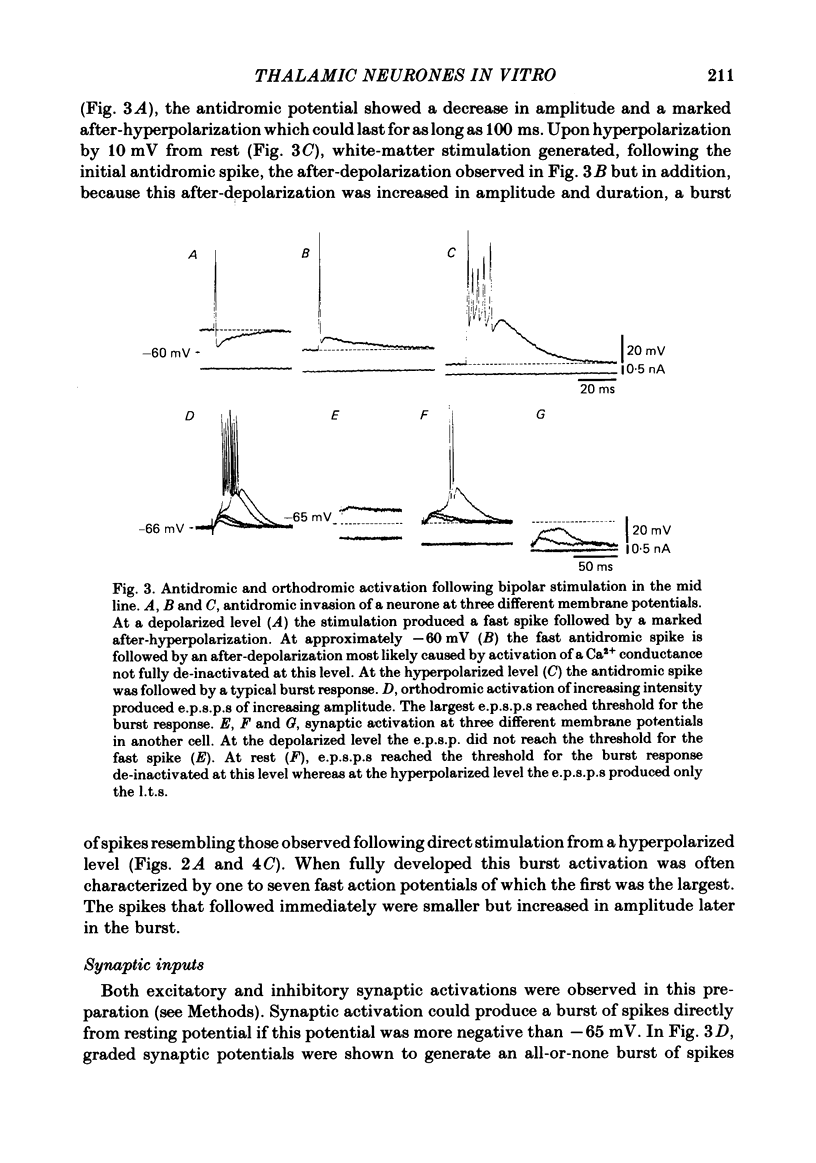
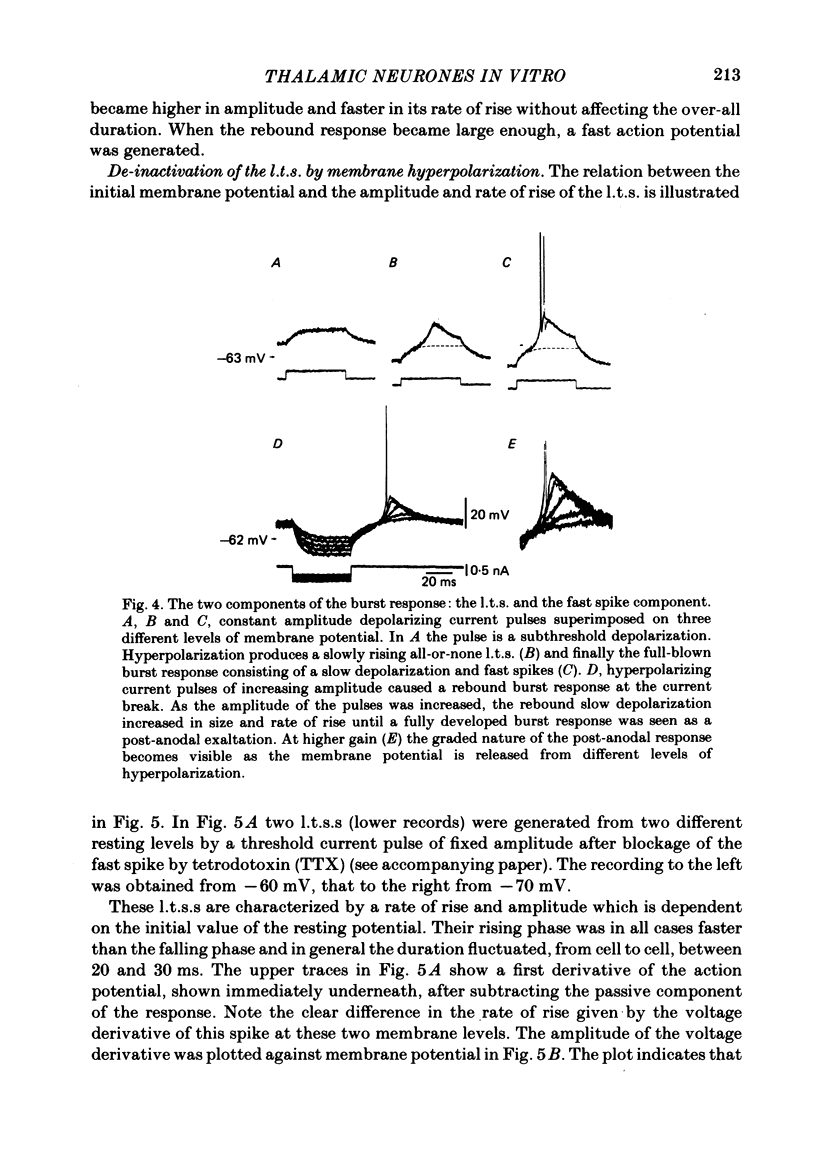
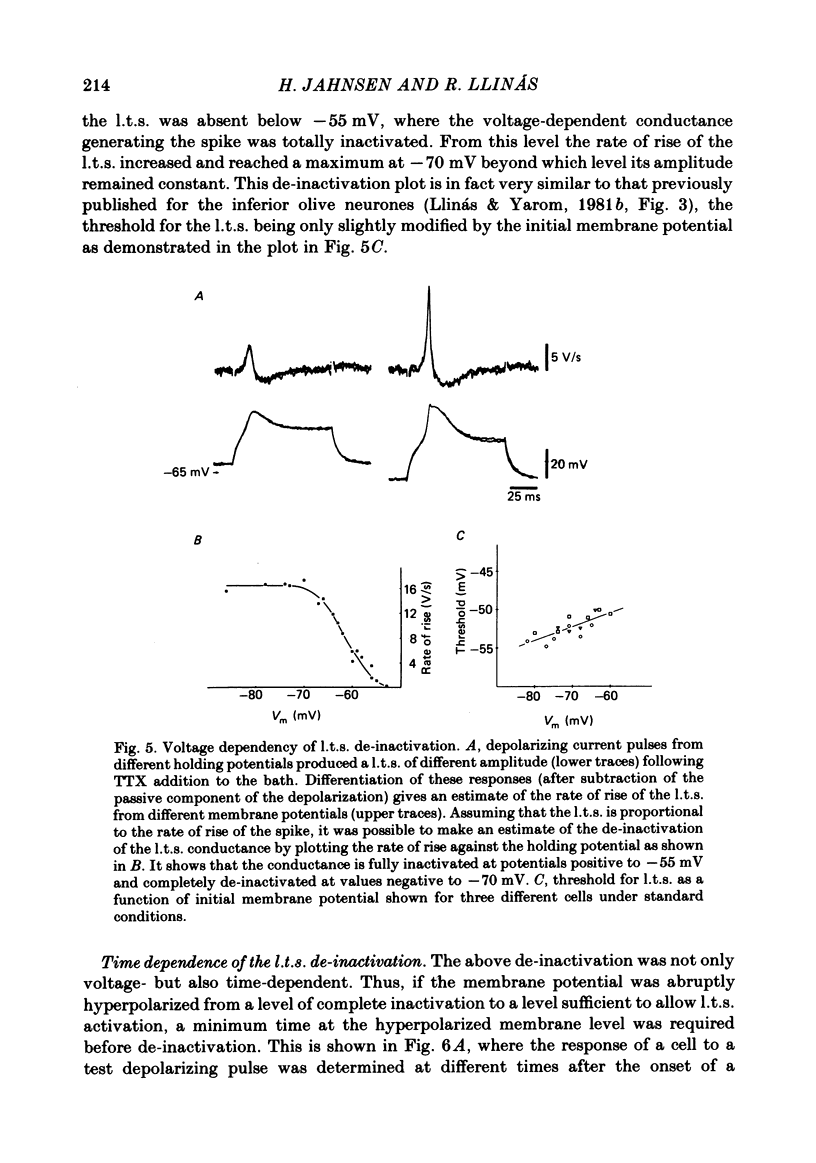
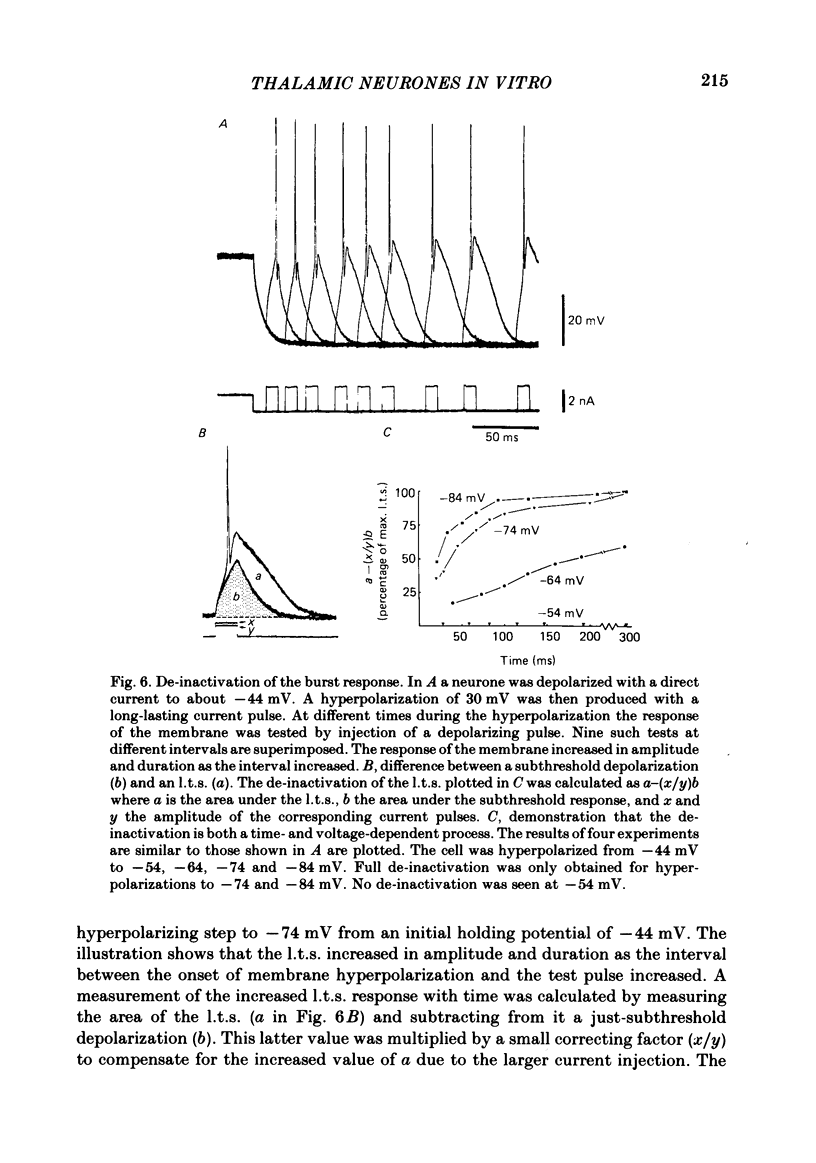
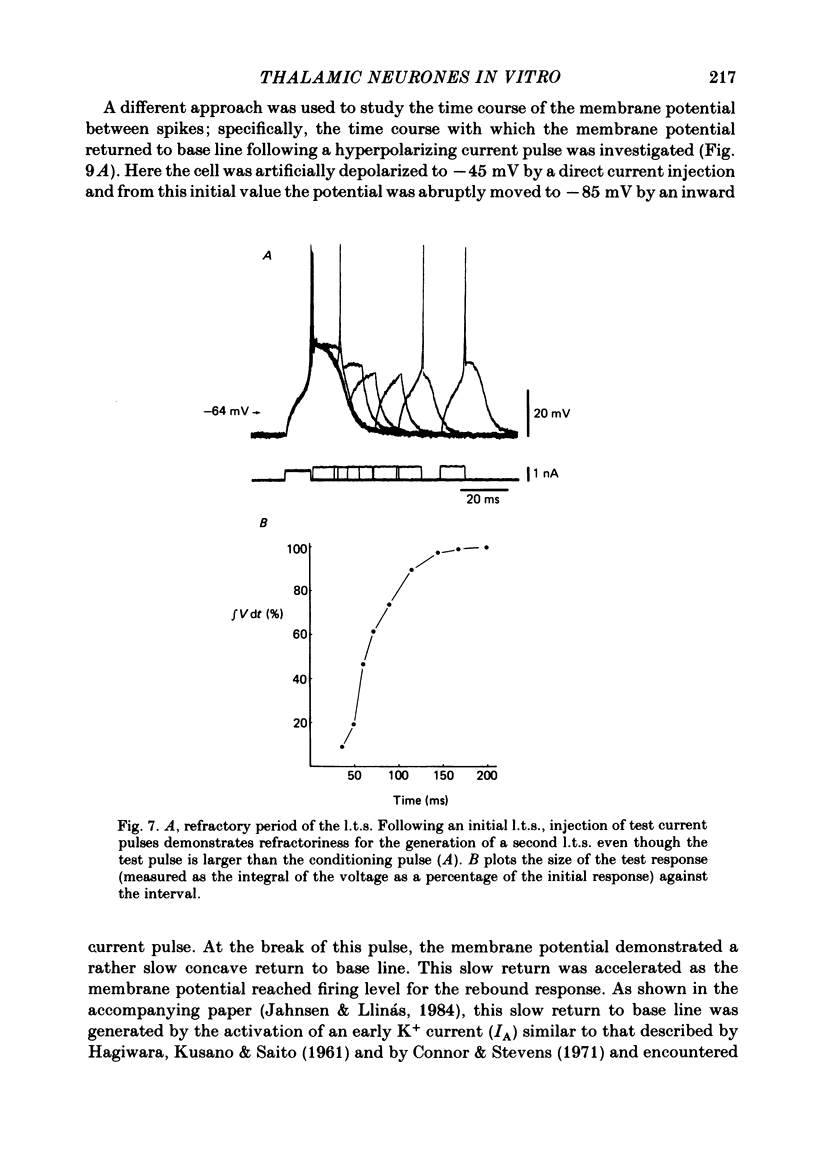
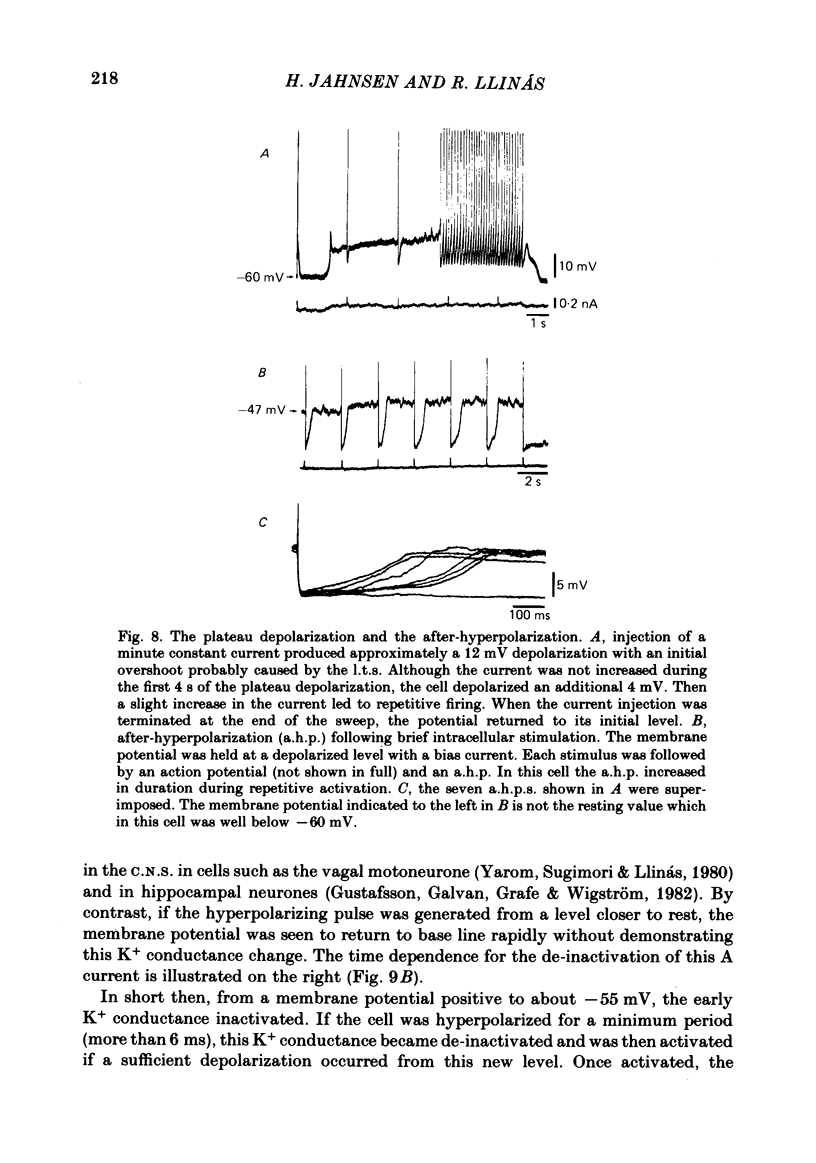
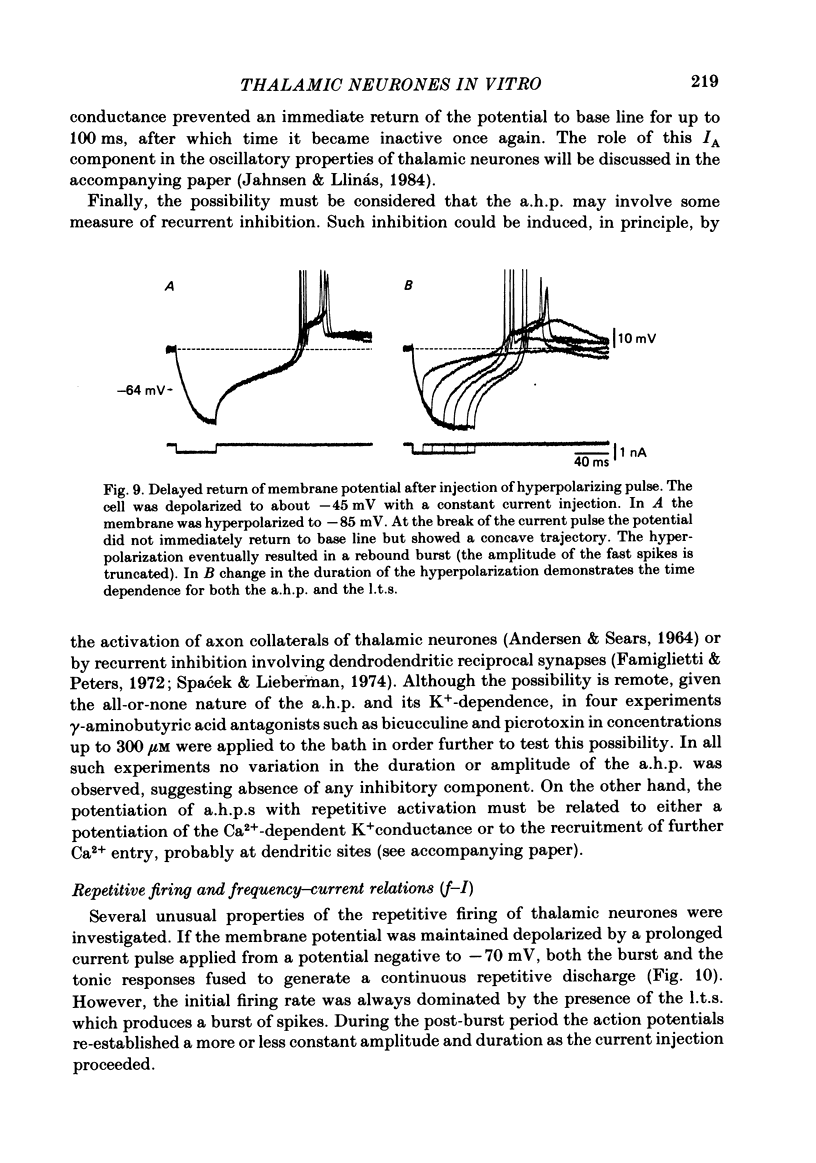
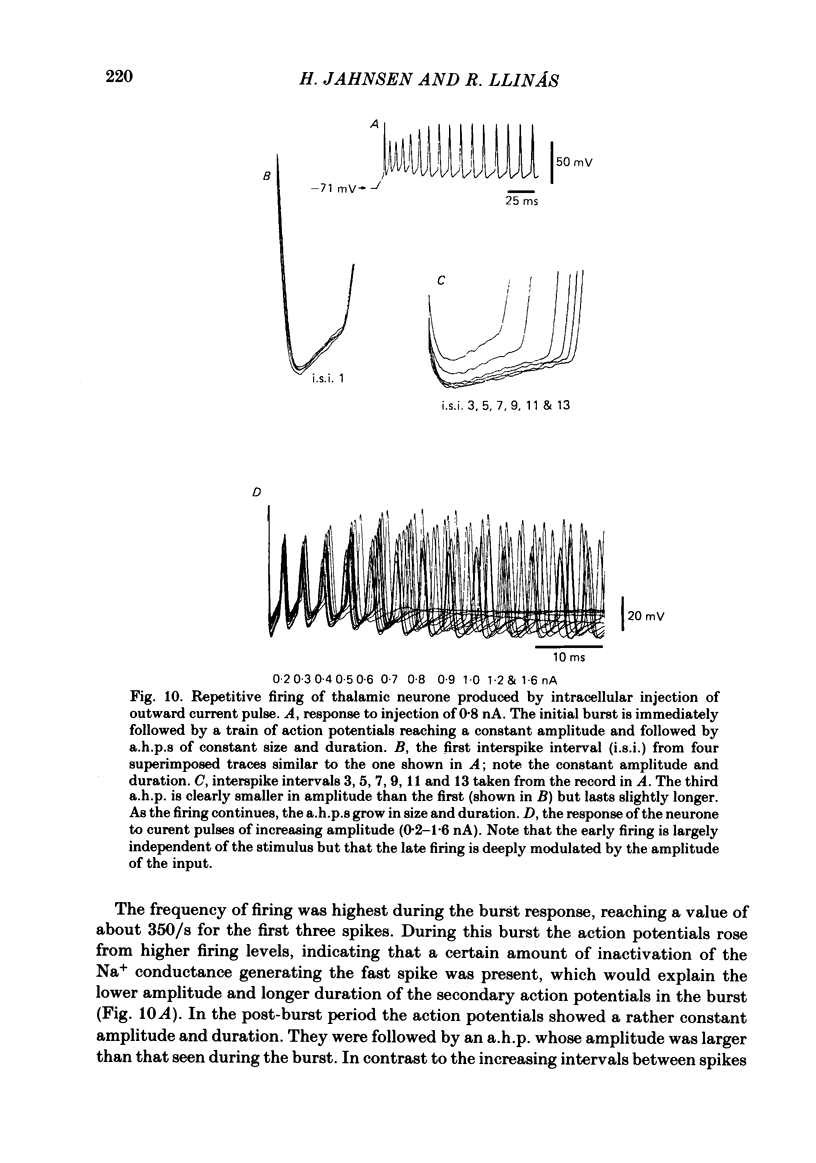
Abstract
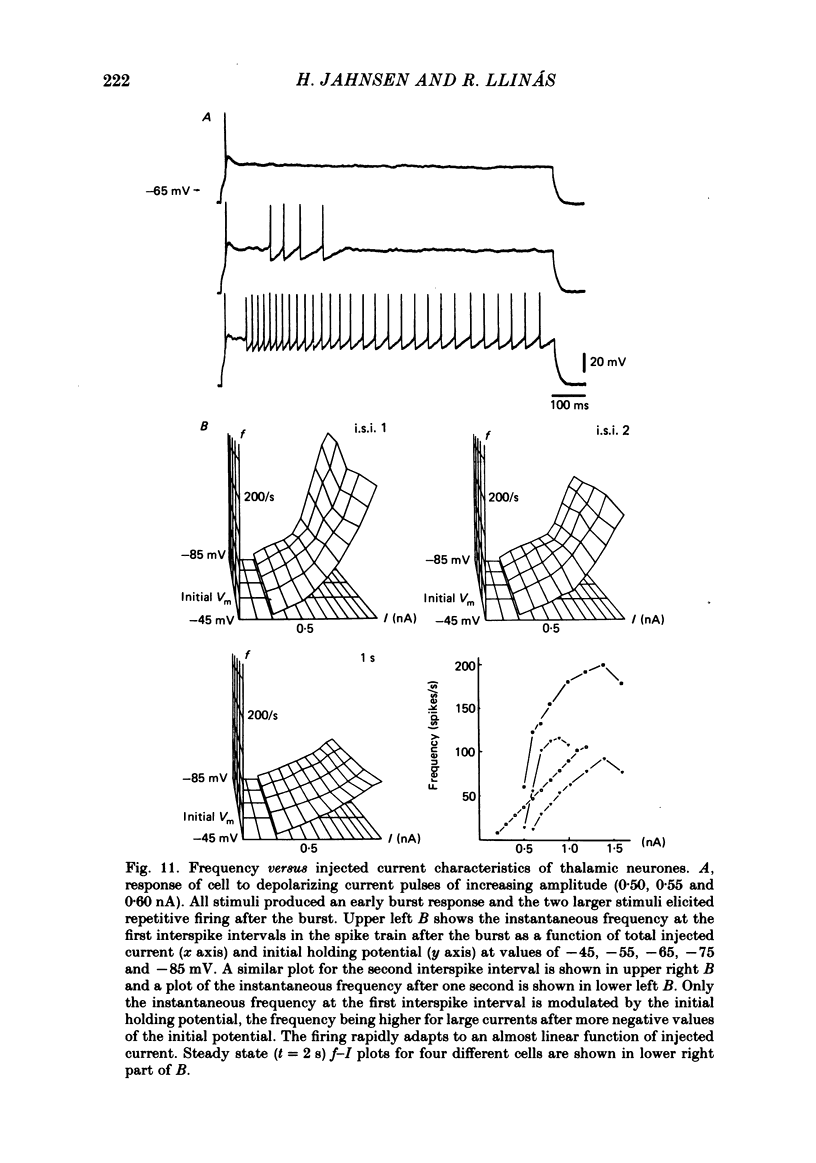
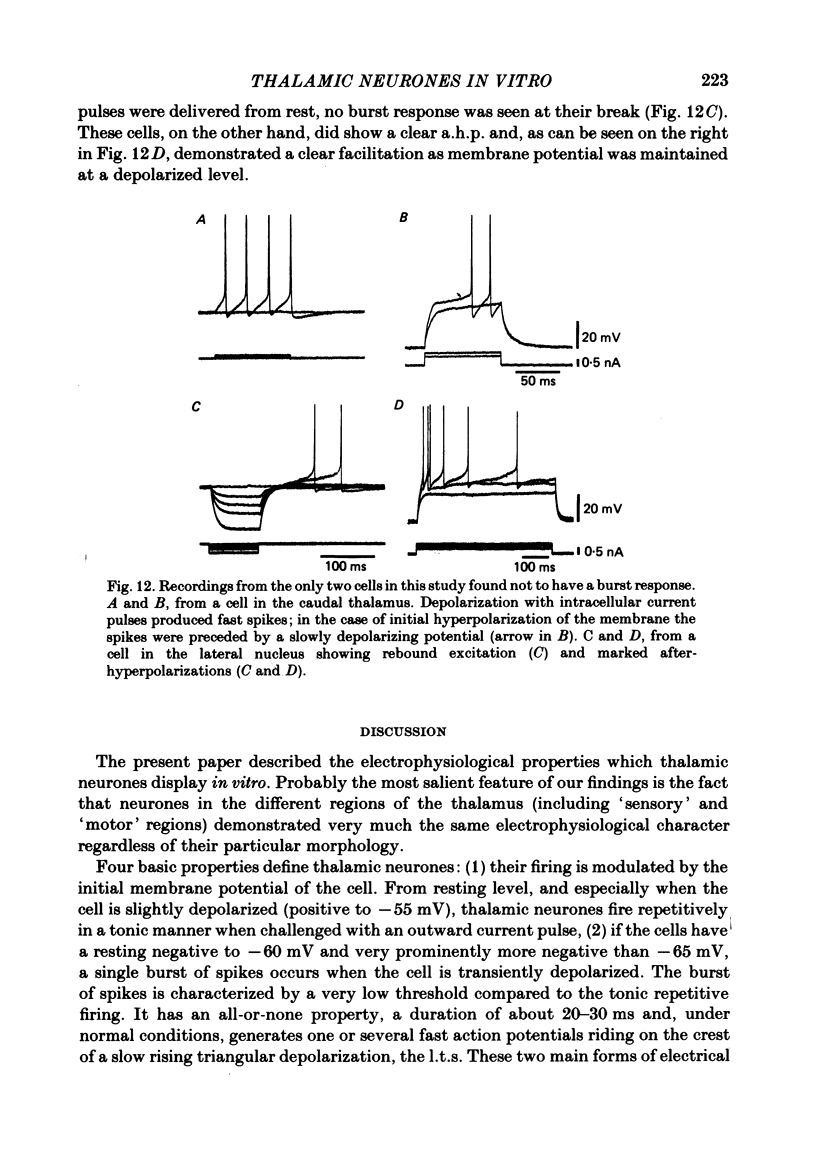
The electroresponsive properties of guinea-pig thalamic neurones were studied using an in vitro slice preparation. A total of 650 cells were recorded intracellularly comprising all regions of the thalamus; of these 229 fulfilled our criterion for recording stability and were used as the data base for this report. The resting membrane potential for thirty-four representative neurones which were analysed in detail was -64 +/- 5 mV (mean +/- S.D.), input resistance 42 +/- 18 M omega, and action potential amplitude 80 +/- 7 mV. Intracellular staining with horseradish peroxidase and Lucifer Yellow revealed that the recorded cells had different morphology. In some their axonal trajectory characterized them as thalamo-cortical relay cells. Two main types of neuronal firing were observed. From a membrane potential negative to -60 mV, anti- or orthodromic and direct activation generated a single burst of spikes, consisting of a low-threshold spike (l.t.s.) of low amplitude and a set of fast superimposed spikes. Tonic repetitive firing was observed if the neurones were activated from a more positive membrane potential; this was a constant finding in all but two of the cells which fulfilled the stability criteria. The l.t.s. response was totally inactivated at membrane potentials positive to -55 mV. As the membrane was hyperpolarized from this level the amplitude of the l.t.s. increased and became fully developed at potentials negative to -70 mV. This increase is due to a de-inactivation of the ionic conductance generating this response. After activation the l.t.s. showed refractoriness for approximately 170 ms. Deinactivation of l.t.s. is a voltage- and time-dependent process; full de-inactivation after a step hyperpolarization to maximal l.t.s. amplitude (-75 to -80 mV) requires 150-180 ms. Membrane depolarization positive to -55 mV generated sudden sustained depolarizing 'plateau potentials', capable of supporting repetitive firing (each action potential being followed by a marked after-hyperpolarization, a.h.p.). The a.h.p. and the plateau potential controlled the voltage trajectory during the interspike interval and, with the fast spike, constitute a functional state where the thalamic neurone displayed oscillatory properties. Frequency-current (f-I) plots from different initial levels of membrane potential were obtained by the application of square current pulses of long duration (2s). From resting membrane potential and from hyperpolarized levels a rather stereotyped onset firing rate was observed due to the presence of the l.t.s.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSEN P., SEARS T. A. THE ROLE OF INHIBITION IN THE PHASING OF SPONTANEOUS THALAMO-CORTICAL DISCHARGE. J Physiol. 1964 Oct;173:459–480. doi: 10.1113/jphysiol.1964.sp007468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971 Feb;213(1):21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschênes M., Roy J. P., Steriade M. Thalamic bursting mechanism: an inward slow current revealed by membrane hyperpolarization. Brain Res. 1982 May 6;239(1):289–293. doi: 10.1016/0006-8993(82)90854-x. [DOI] [PubMed] [Google Scholar]
- Famiglietti E. V., Jr, Peters A. The synaptic glomerulus and the intrinsic neuron in the dorsal lateral geniculate nucleus of the cat. J Comp Neurol. 1972 Mar;144(3):285–334. doi: 10.1002/cne.901440304. [DOI] [PubMed] [Google Scholar]
- Friedlander M. J., Lin C. S., Stanford L. R., Sherman S. M. Morphology of functionally identified neurons in lateral geniculate nucleus of the cat. J Neurophysiol. 1981 Jul;46(1):80–129. doi: 10.1152/jn.1981.46.1.80. [DOI] [PubMed] [Google Scholar]
- Grossman A., Lieberman A. R., Webster K. E. A Golgi study of the rat dorsal lateral geniculate nucleus. J Comp Neurol. 1973 Aug;150(4):441–466. doi: 10.1002/cne.901500404. [DOI] [PubMed] [Google Scholar]
- Guillery R. W. A study of Golgi preparations from the dorsal lateral geniculate nucleus of the adult cat. J Comp Neurol. 1966 Sep;128(1):21–50. doi: 10.1002/cne.901280104. [DOI] [PubMed] [Google Scholar]
- Gustafsson B., Galvan M., Grafe P., Wigström H. A transient outward current in a mammalian central neurone blocked by 4-aminopyridine. Nature. 1982 Sep 16;299(5880):252–254. doi: 10.1038/299252a0. [DOI] [PubMed] [Google Scholar]
- HAGIWARA S., KUSANO K., SAITO N. Membrane changes of Onchidium nerve cell in potassium-rich media. J Physiol. 1961 Mar;155:470–489. doi: 10.1113/jphysiol.1961.sp006640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnsen H., Llinás R. Ionic basis for the electro-responsiveness and oscillatory properties of guinea-pig thalamic neurones in vitro. J Physiol. 1984 Apr;349:227–247. doi: 10.1113/jphysiol.1984.sp015154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Jahnsen H. Electrophysiology of mammalian thalamic neurones in vitro. Nature. 1982 Jun 3;297(5865):406–408. doi: 10.1038/297406a0. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M. Electrophysiological properties of in vitro Purkinje cell somata in mammalian cerebellar slices. J Physiol. 1980 Aug;305:171–195. doi: 10.1113/jphysiol.1980.sp013357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Yarom Y. Electrophysiology of mammalian inferior olivary neurones in vitro. Different types of voltage-dependent ionic conductances. J Physiol. 1981 Jun;315:549–567. doi: 10.1113/jphysiol.1981.sp013763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Yarom Y. Properties and distribution of ionic conductances generating electroresponsiveness of mammalian inferior olivary neurones in vitro. J Physiol. 1981 Jun;315:569–584. doi: 10.1113/jphysiol.1981.sp013764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOUNTCASTLE V. B., POGGIO G. F., WERNER G. THE RELATION OF THALAMIC CELL RESPONSE TO PERIPHERAL STIMULI VARIED OVER AN INTENSIVE CONTINUUM. J Neurophysiol. 1963 Sep;26:807–834. doi: 10.1152/jn.1963.26.5.807. [DOI] [PubMed] [Google Scholar]
- Murase K., Randić M. Electrophysiological properties of rat spinal dorsal horn neurones in vitro: calcium-dependent action potentials. J Physiol. 1983 Jan;334:141–153. doi: 10.1113/jphysiol.1983.sp014485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POGGIO G. F., MOUNTCASTLE V. B. THE FUNCTIONAL PROPERTIES OF VENTROBASAL THALAMIC NEURONSSTUDIED IN UNANESTHETIZED MONKEYS. J Neurophysiol. 1963 Sep;26:775–806. doi: 10.1152/jn.1963.26.5.775. [DOI] [PubMed] [Google Scholar]
- Spacek J., Lieberman A. R. Ultrastructure and three-dimensional organization of synaptic glomeruli in rat somatosensory thalamus. J Anat. 1974 Jul;117(Pt 3):487–516. [PMC free article] [PubMed] [Google Scholar]
- TINDAL J. S. THE FOREBRAIN OF THE GUINEA PIG IN STEREOTAXIC COORDINATES. J Comp Neurol. 1965 Apr;124:259–266. doi: 10.1002/cne.901240210. [DOI] [PubMed] [Google Scholar]
- Tsien R. W. Calcium channels in excitable cell membranes. Annu Rev Physiol. 1983;45:341–358. doi: 10.1146/annurev.ph.45.030183.002013. [DOI] [PubMed] [Google Scholar]