Abstract
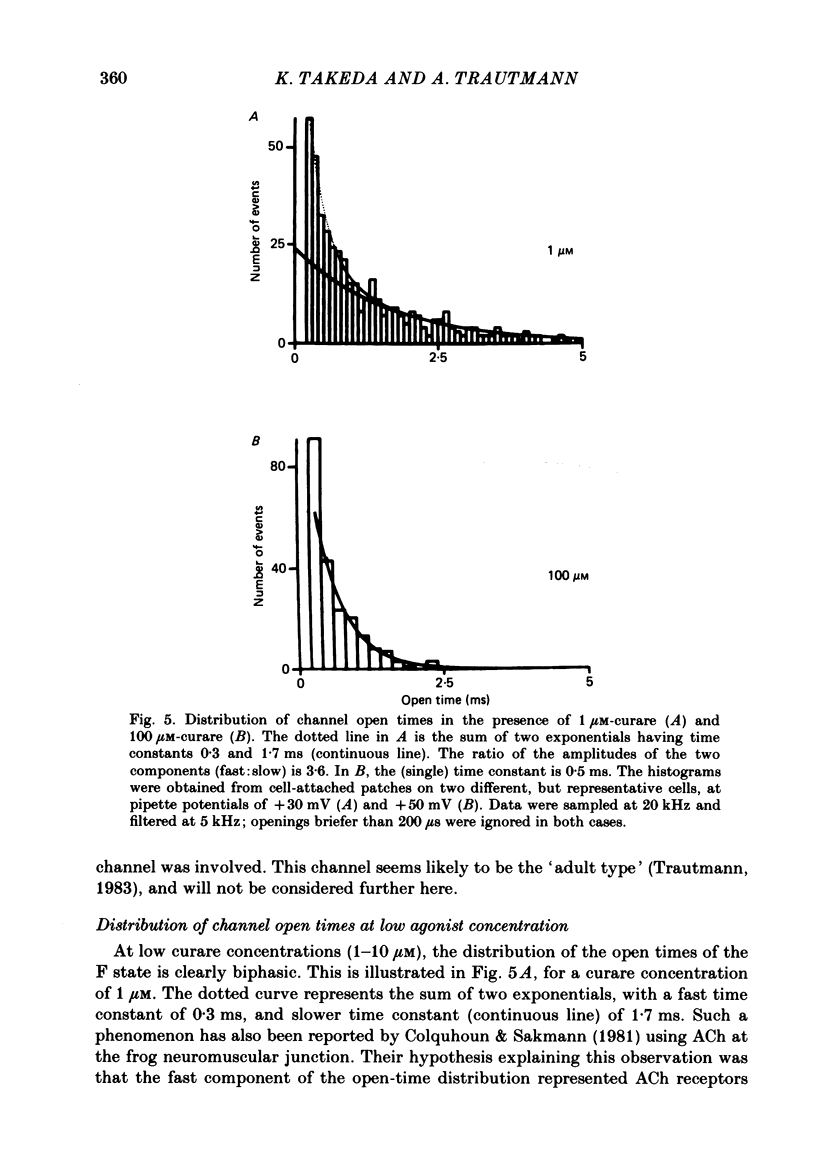
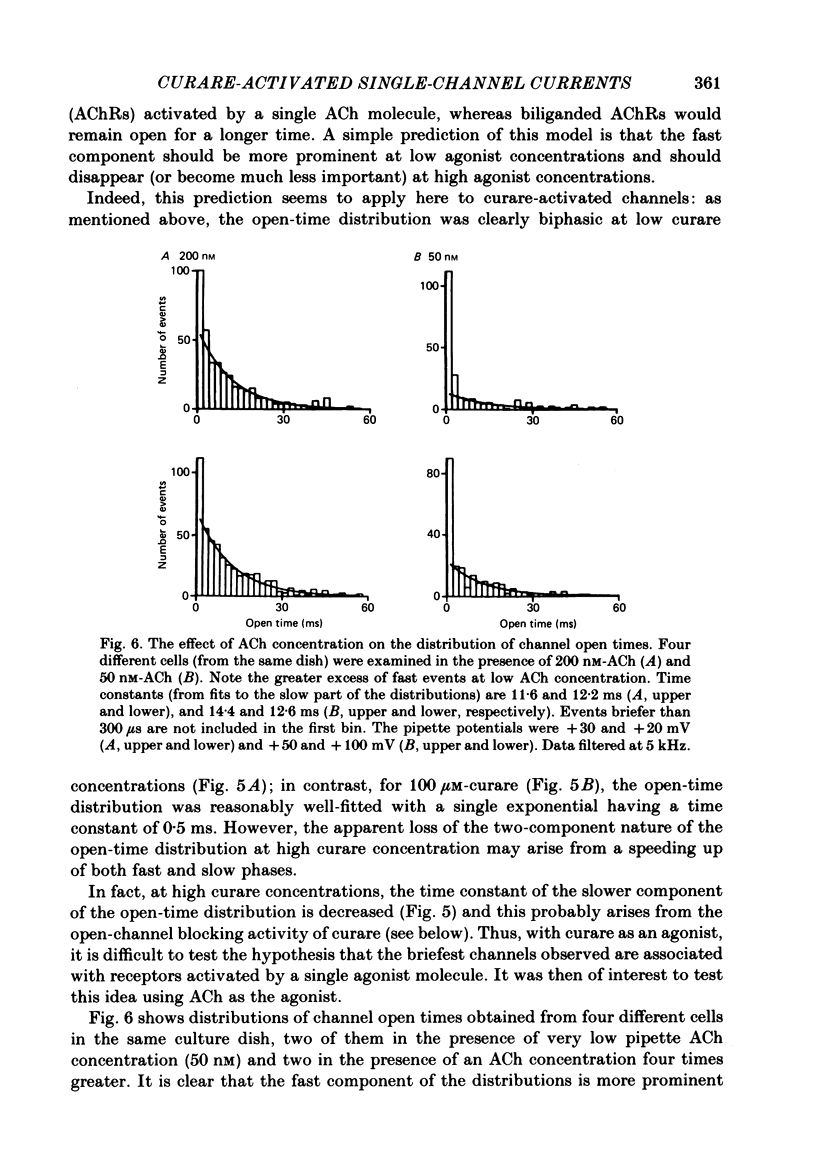
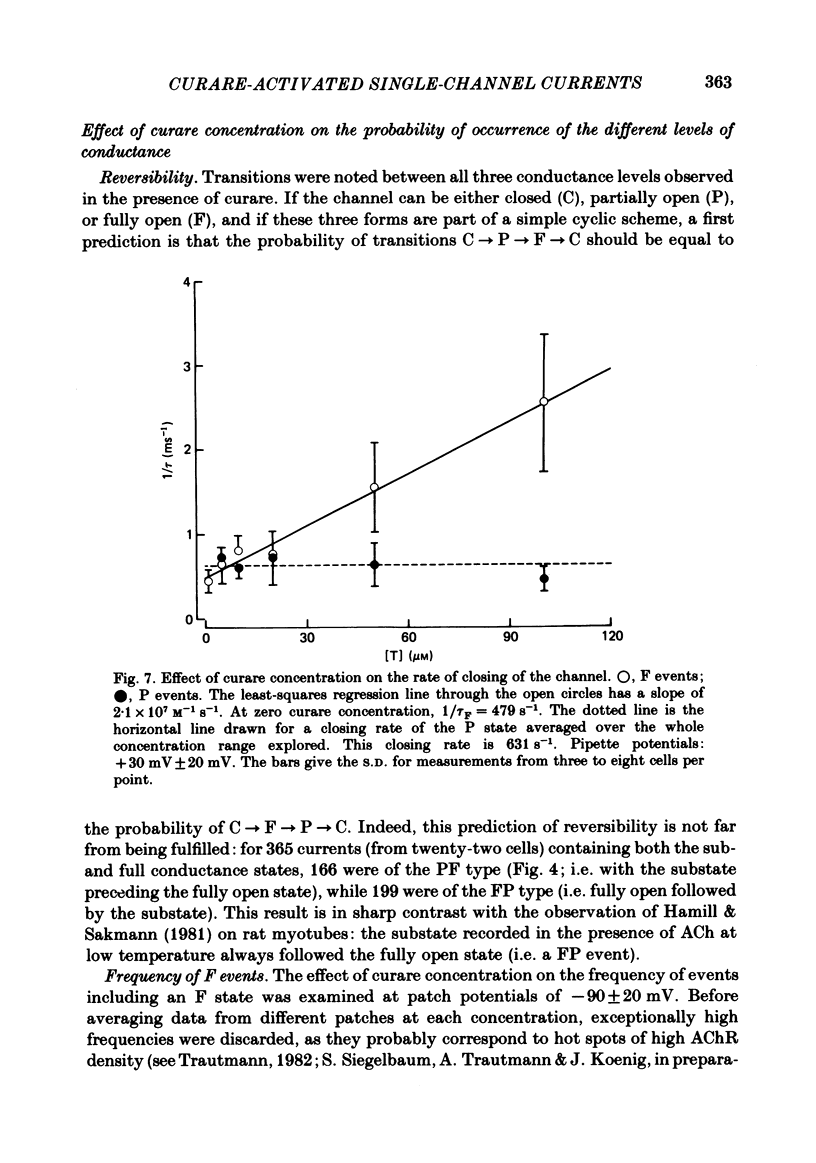
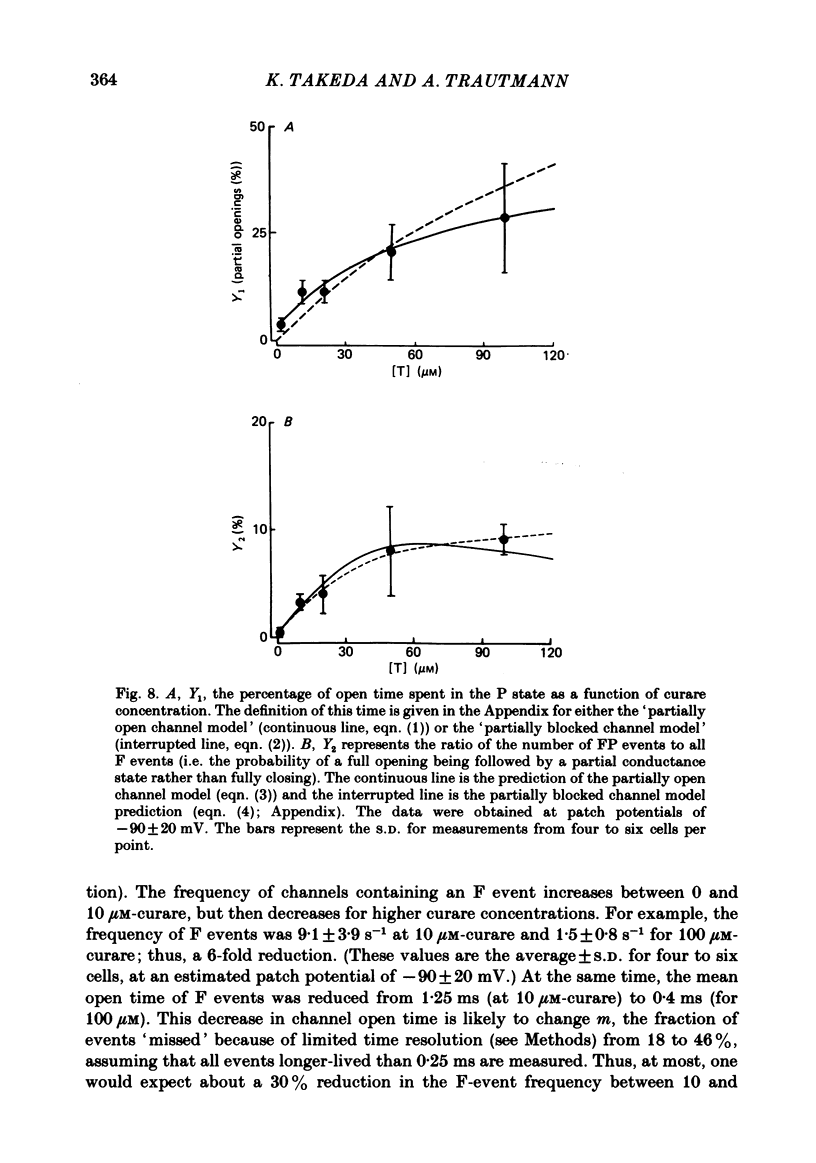
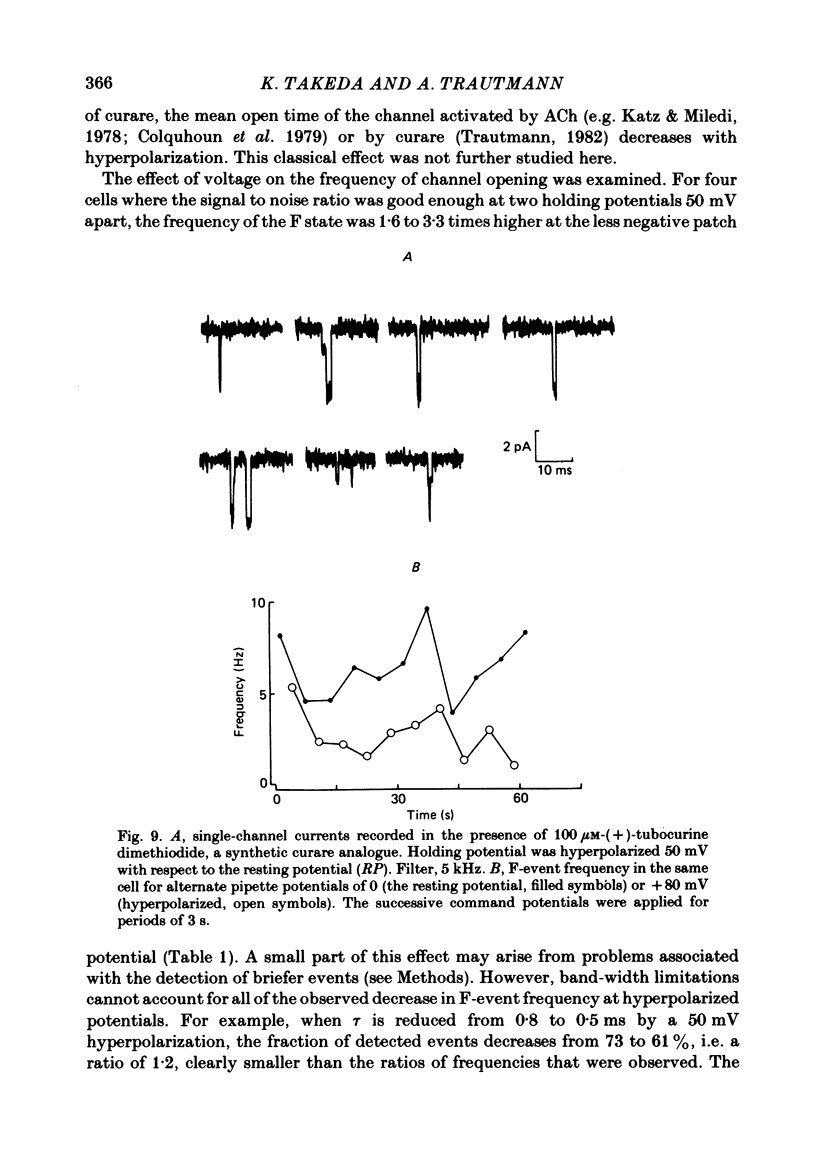
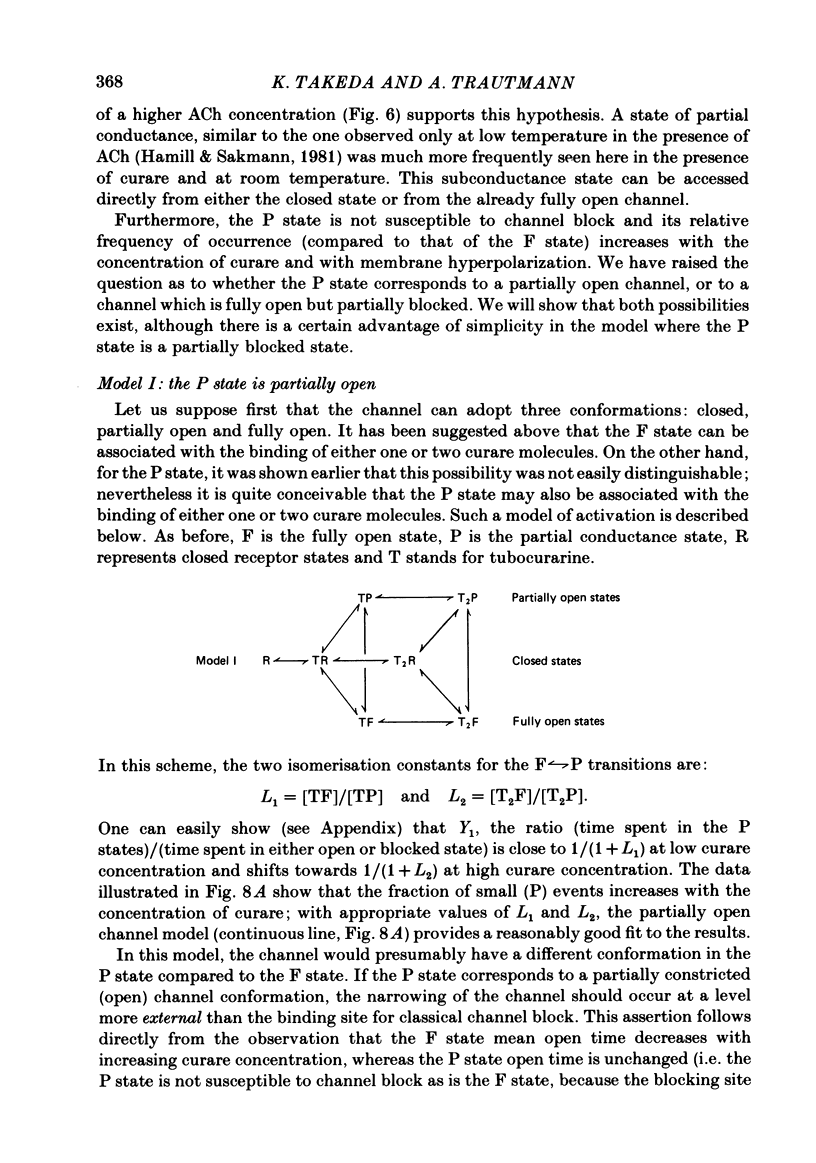
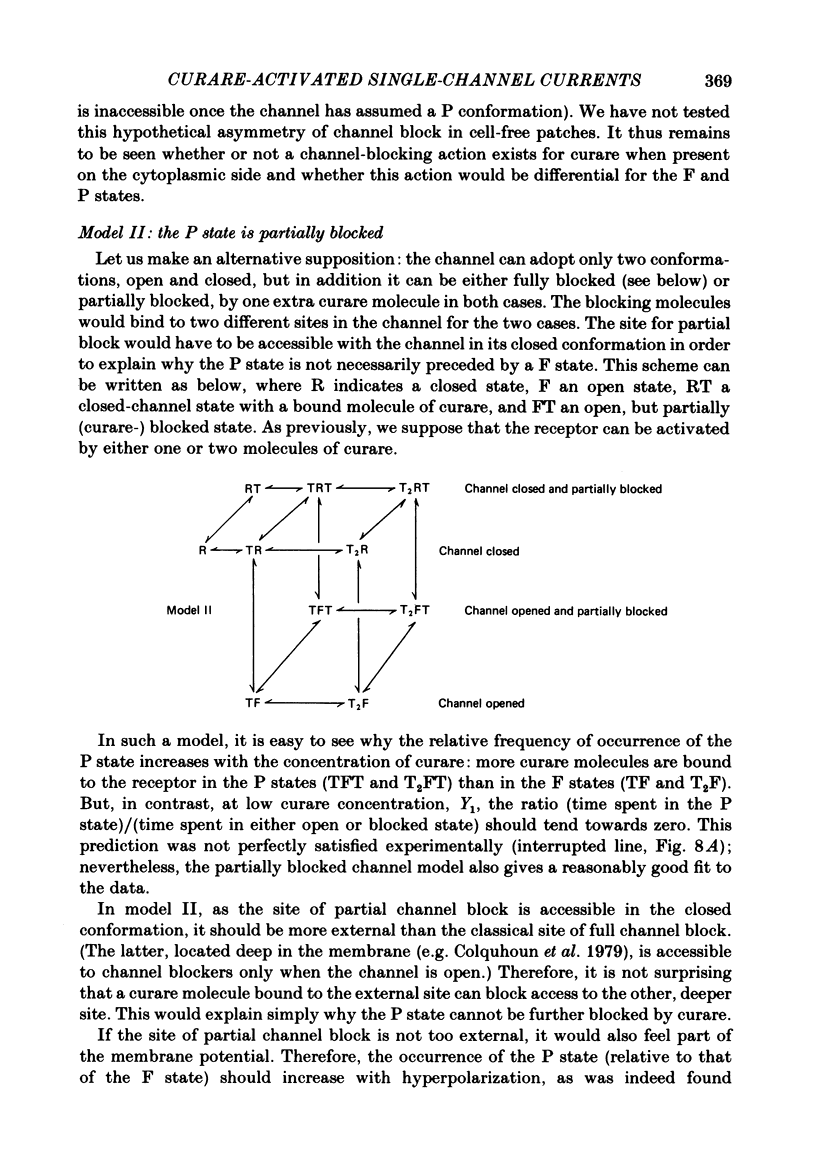
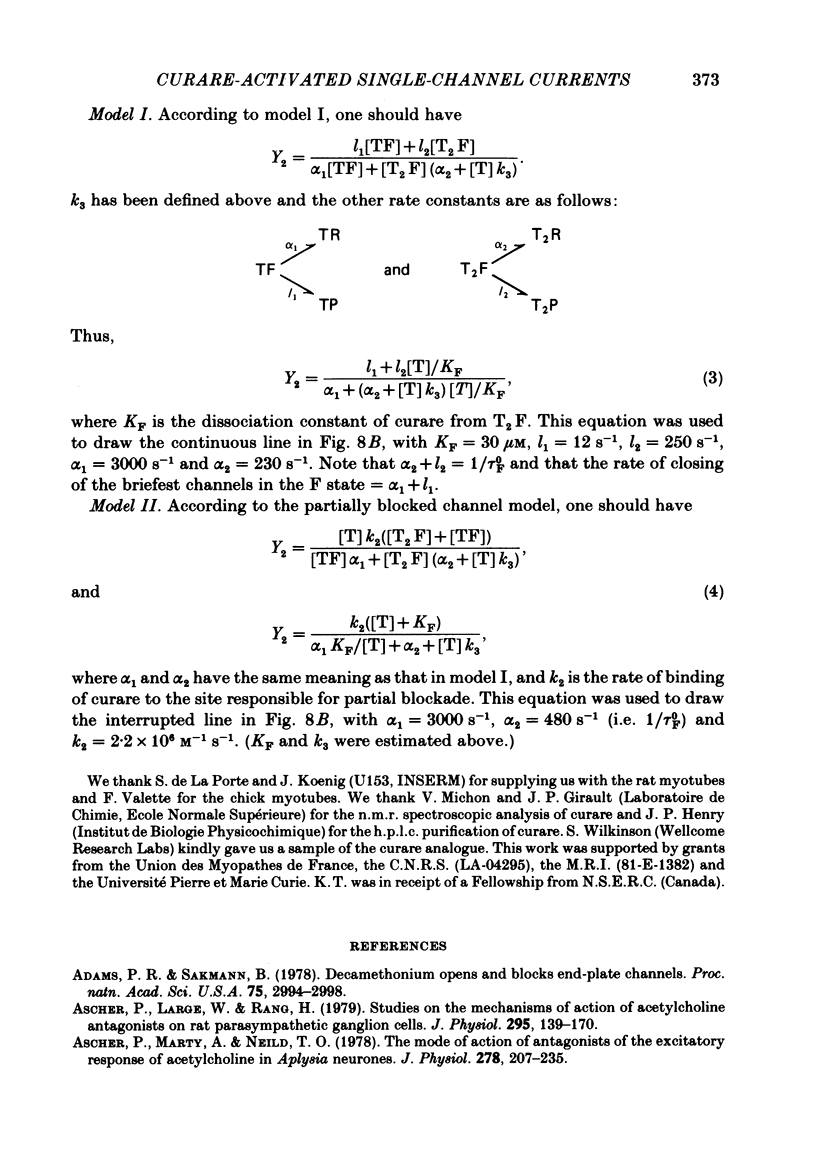
Single channels activated by (+)-tubocurarine (curare) were recorded from rat myotubes using the patch-clamp technique. The agonist-like action of curare does not result from a contaminant molecule, as the same effects were observed with curare purified by high-performance liquid chromatography. A curare-activated channel can adopt two levels of conductance: full (F) or partial (P). The F state has a slope conductance of 40 pS (identical to that of the acetylcholine (ACh)-activated channel) and the P state has a conductance of 13 pS. At low concentrations of agonist (ACh or curare), the distribution of channel open times is biphasic. The briefer channels may result from the binding of a single agonist molecule whereas the longer-lived channels probably occur following the binding of two agonist molecules. The mean open time of the F state decreases with increasing curare concentration. It is shown that band-width limitations are likely to account for only a very small part of this observed reduction. In contrast, the mean open time of the P state is independent of the concentration of curare. A simple interpretation is that the F state is susceptible to channel blockade by curare, whereas the P state is not. The P state preceded the F state almost as often as it followed the F state; it can also be observed separately from the F state. The fraction of events including a P state increases from about 4% in the presence of 1 microM-curare to 30% at 100 microM-curare. This fraction is also increased by hyperpolarization. When the curare concentration is increased, the F-state frequency first increases and then decreases at higher concentration. This frequency is also decreased by hyperpolarization. The decrease in F-state frequency is probably related to channel blockade by curare; it cannot be wholly accounted for by problems associated with limited time resolution. A synthetic analogue of curare, (+)- tubocurine dimethiodide presents an agonist activity similar to that of curare but with a faster closing rate for both F and P states. The various actions of curare are summarized in two possible models where the P state is interpreted as either a partially open channel or a channel which is partially blocked.
Full text
PDF


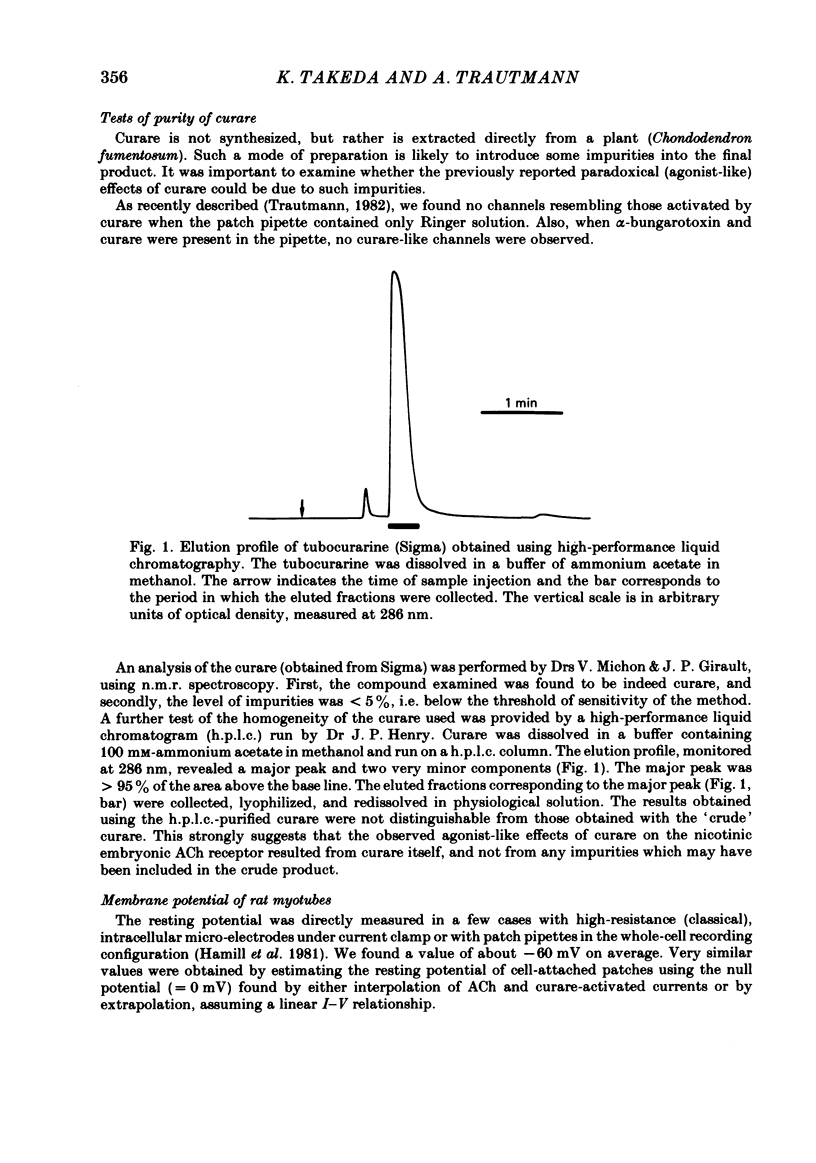
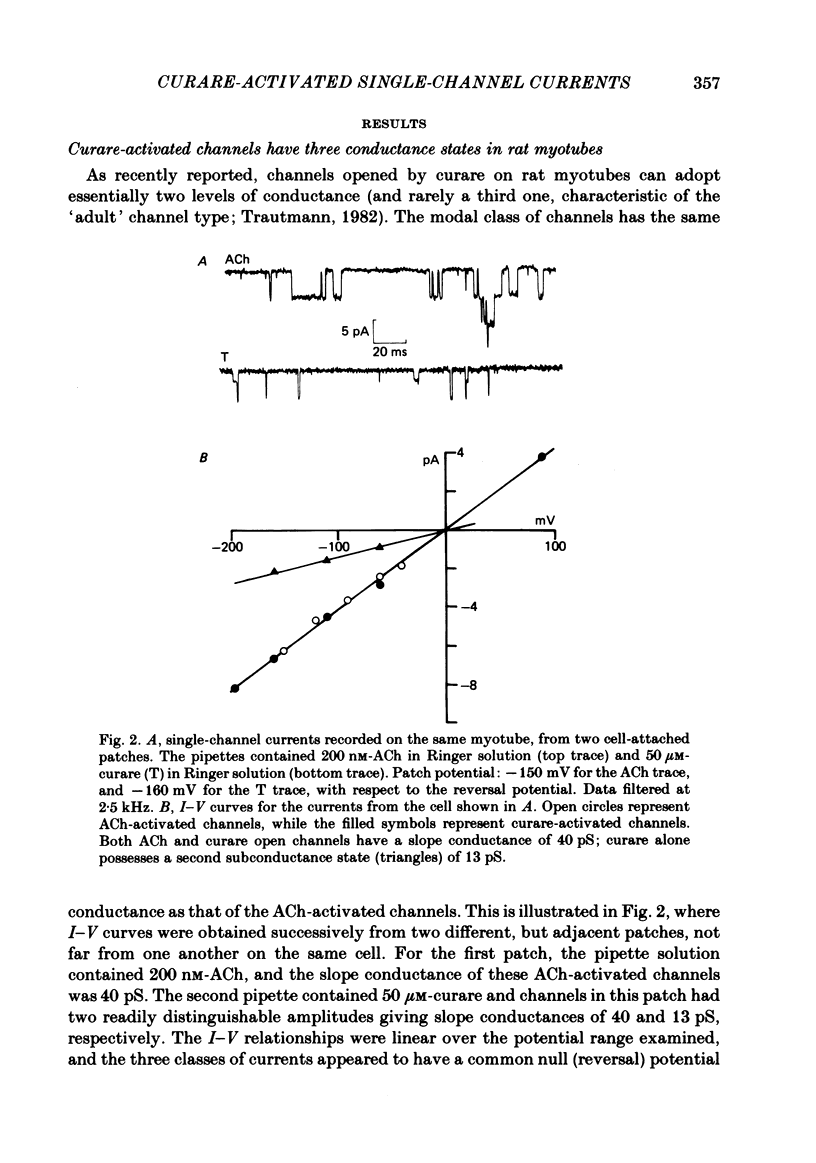
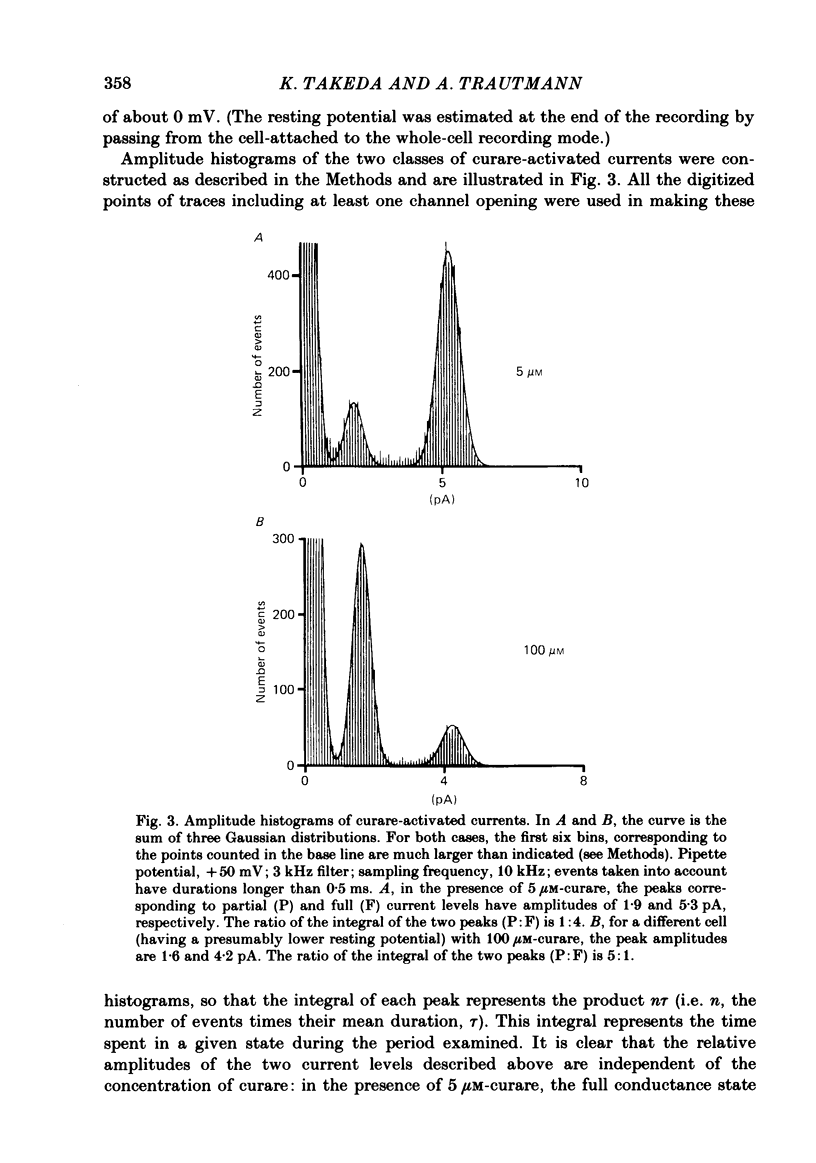
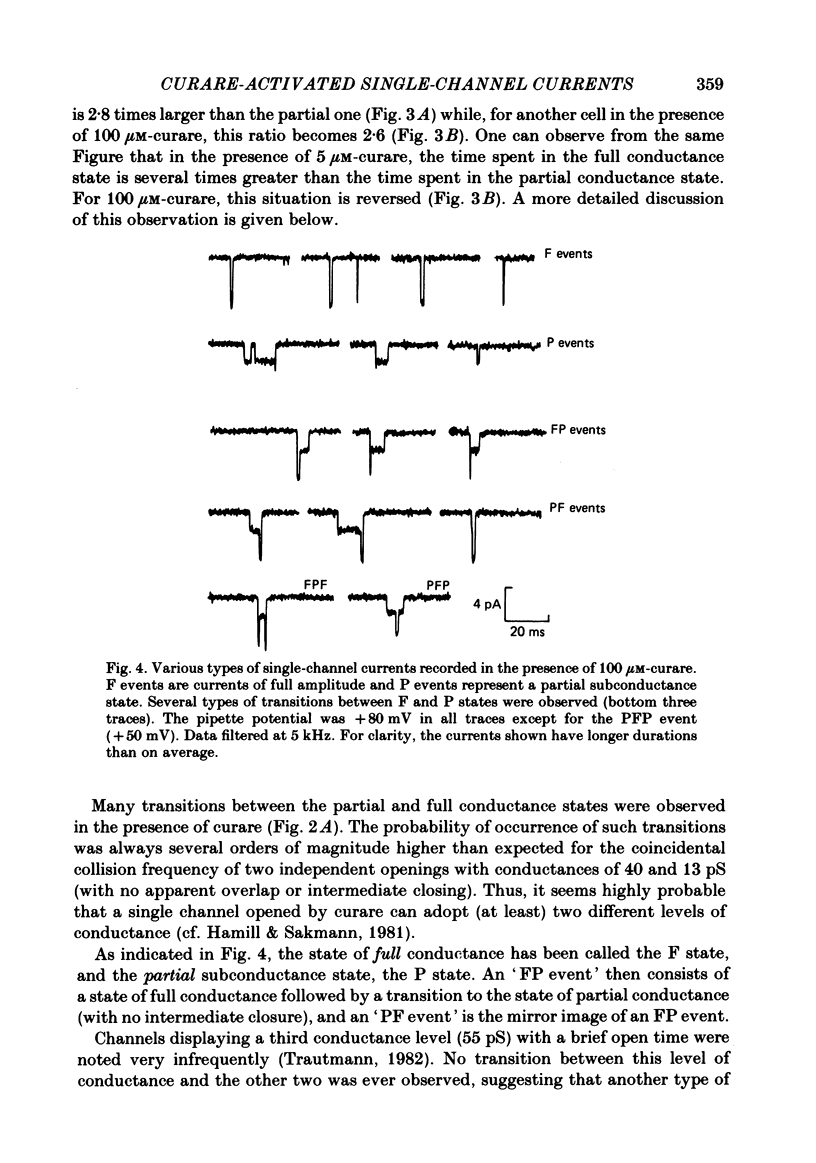







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Sakmann B. Decamethonium both opens and blocks endplate channels. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2994–2998. doi: 10.1073/pnas.75.6.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Large W. A., Rang H. P. Studies on the mechanism of action of acetylcholine antagonists on rat parasympathetic ganglion cells. J Physiol. 1979 Oct;295:139–170. doi: 10.1113/jphysiol.1979.sp012958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P., Marty A., Neild T. O. The mode of action of antagonists of the excitatory response to acetylcholine in Aplysia neurones. J Physiol. 1978 May;278:207–235. doi: 10.1113/jphysiol.1978.sp012300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J. P. The acetylcholine receptor: an "allosteric" membrane protein. Harvey Lect. 1979 1980;75:85–254. [PubMed] [Google Scholar]
- Colquhoun D., Dreyer F., Sheridan R. E. The actions of tubocurarine at the frog neuromuscular junction. J Physiol. 1979 Aug;293:247–284. doi: 10.1113/jphysiol.1979.sp012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Sakmann B. Fluctuations in the microsecond time range of the current through single acetylcholine receptor ion channels. Nature. 1981 Dec 3;294(5840):464–466. doi: 10.1038/294464a0. [DOI] [PubMed] [Google Scholar]
- Conti-Tronconi B. M., Gotti C. M., Hunkapiller M. W., Raftery M. A. Mammalian muscle acetylcholine receptor: a supramolecular structure formed by four related proteins. Science. 1982 Dec 17;218(4578):1227–1229. doi: 10.1126/science.7146904. [DOI] [PubMed] [Google Scholar]
- Feltz A., Trautmann A. Desensitization at the frog neuromuscular junction: a biphasic process. J Physiol. 1982 Jan;322:257–272. doi: 10.1113/jphysiol.1982.sp014036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbach G. D., Schuetze S. M. A post-natal decrease in acetylcholine channel open time at rat end-plates. J Physiol. 1980 Jun;303:125–137. doi: 10.1113/jphysiol.1980.sp013275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Sakmann B. Multiple conductance states of single acetylcholine receptor channels in embryonic muscle cells. Nature. 1981 Dec 3;294(5840):462–464. doi: 10.1038/294462a0. [DOI] [PubMed] [Google Scholar]
- Isreal J. M., Meunier J. M. Procaine as an acetylcholine agonist in snail neuron. J Pharmacol Exp Ther. 1979 Oct;211(1):93–98. [PubMed] [Google Scholar]
- JENKINSON D. H. The antagonism between tubocurarine and substances which depolarize the motor end-plate. J Physiol. 1960 Jul;152:309–324. doi: 10.1113/jphysiol.1960.sp006489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. B., Lecar H., Askanas V., Engel W. K. Single cholinergic receptor channel currents in cultured human muscle. J Neurosci. 1982 Oct;2(10):1465–1473. doi: 10.1523/JNEUROSCI.02-10-01465.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. A re-examination of curare action at the motor endplate. Proc R Soc Lond B Biol Sci. 1978 Dec 4;203(1151):119–133. doi: 10.1098/rspb.1978.0096. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The characteristics of 'end-plate noise' produced by different depolarizing drugs. J Physiol. 1973 May;230(3):707–717. doi: 10.1113/jphysiol.1973.sp010213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manalis R. S. Voltage-dependent effect of curare at the frog neuromuscular junction. Nature. 1977 May 26;267(5609):366–368. doi: 10.1038/267366a0. [DOI] [PubMed] [Google Scholar]
- Marty A., Neild T., Ascher P. Voltage sensitivity of acetylcholine currents in Aplysia neurones in the presence of curare. Nature. 1976 Jun 10;261(5560):501–503. doi: 10.1038/261501a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Takahashi H., Tanabe T., Toyosato M., Kikyotani S., Furutani Y., Hirose T., Takashima H., Inayama S., Miyata T. Structural homology of Torpedo californica acetylcholine receptor subunits. Nature. 1983 Apr 7;302(5908):528–532. doi: 10.1038/302528a0. [DOI] [PubMed] [Google Scholar]
- Sakmann B., Brenner H. R. Change in synaptic channel gating during neuromuscular development. Nature. 1978 Nov 23;276(5686):401–402. doi: 10.1038/276401a0. [DOI] [PubMed] [Google Scholar]
- Schuetze S. M. The acetylcholine channel open time in chick muscle is not decreased following innervation. J Physiol. 1980 Jun;303:111–124. doi: 10.1113/jphysiol.1980.sp013274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaker N., Eldefrawi A. T., Aguayo L. G., Warnick J. E., Albuquerque E. X., Eldefrawi M. E. Interactions of d-tubocurarine with the nicotinic acetylcholine receptor/channel molecule. J Pharmacol Exp Ther. 1982 Jan;220(1):172–177. [PubMed] [Google Scholar]
- Trautmann A. Tubocurarine, a partial agonist for cholinergic receptors. J Neural Transm Suppl. 1983;18:353–361. [PubMed] [Google Scholar]
- Ziskind L., Dennis M. J. Depolarising effect of curare on embryonic rat muscles. Nature. 1978 Dec 7;276(5688):622–623. doi: 10.1038/276622a0. [DOI] [PubMed] [Google Scholar]


