Abstract
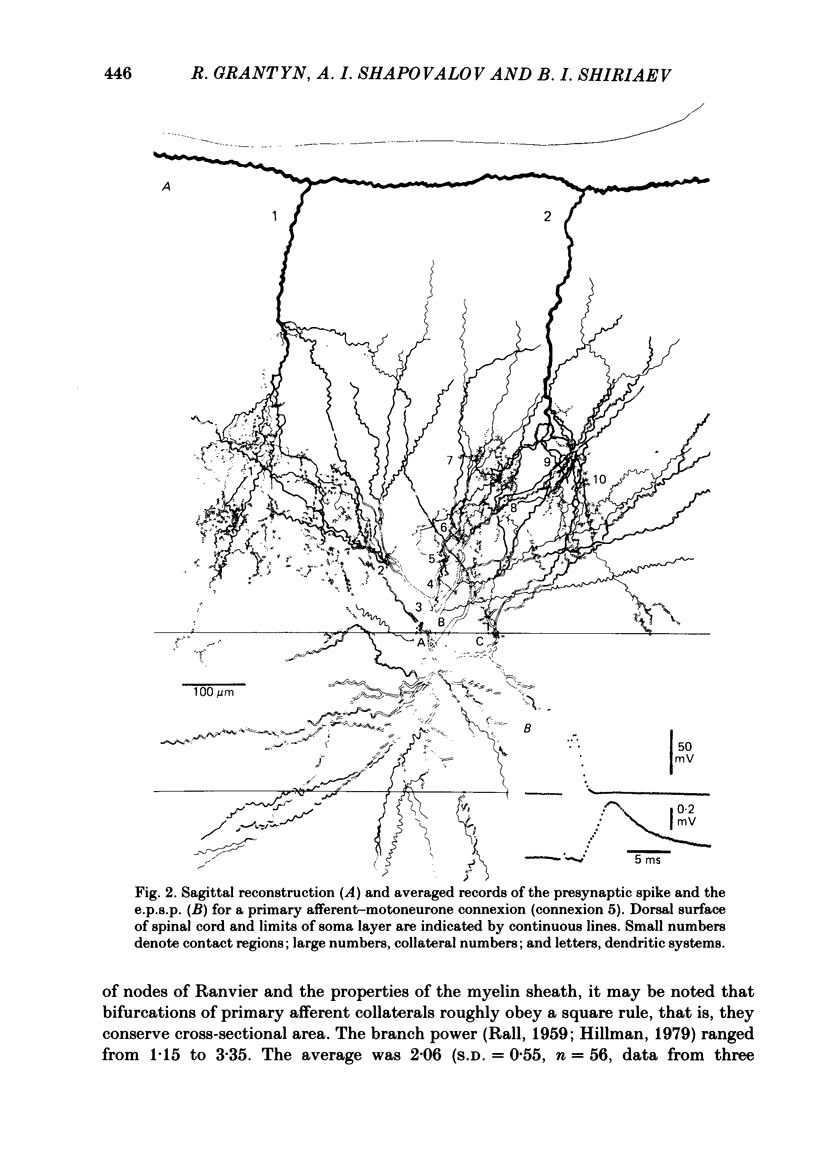
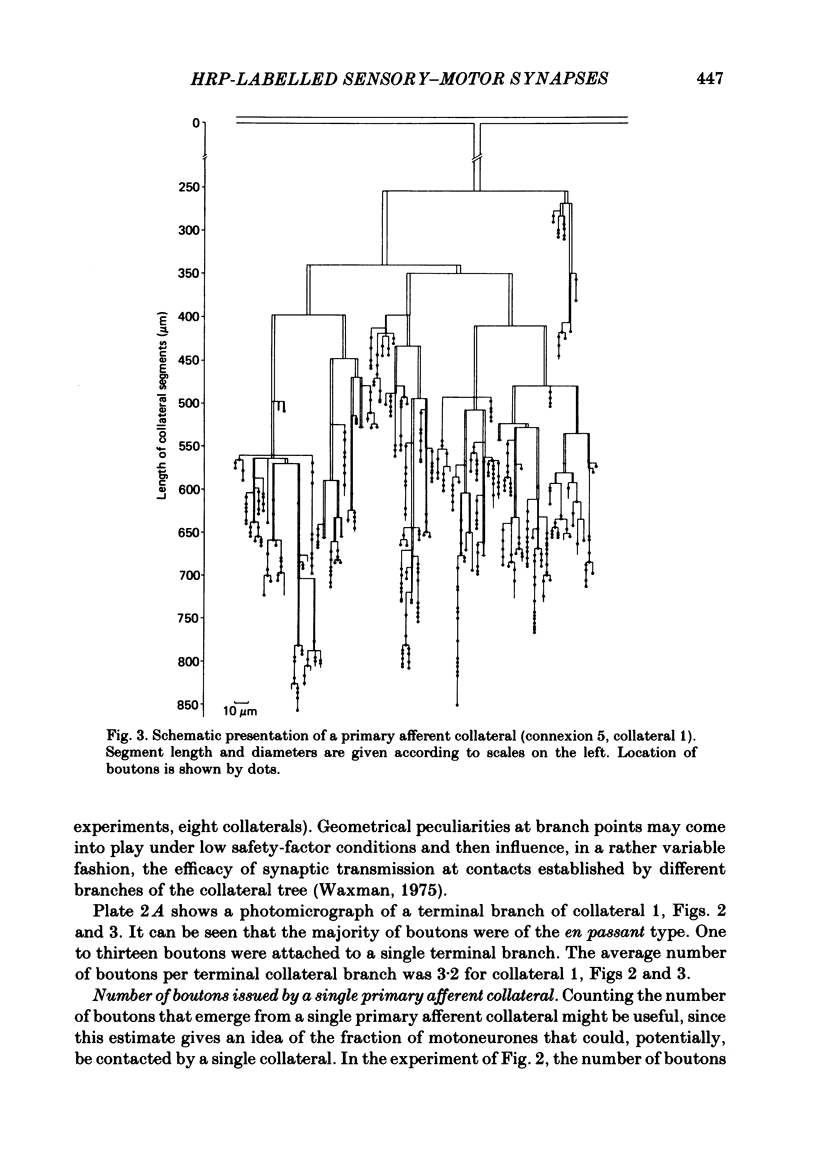
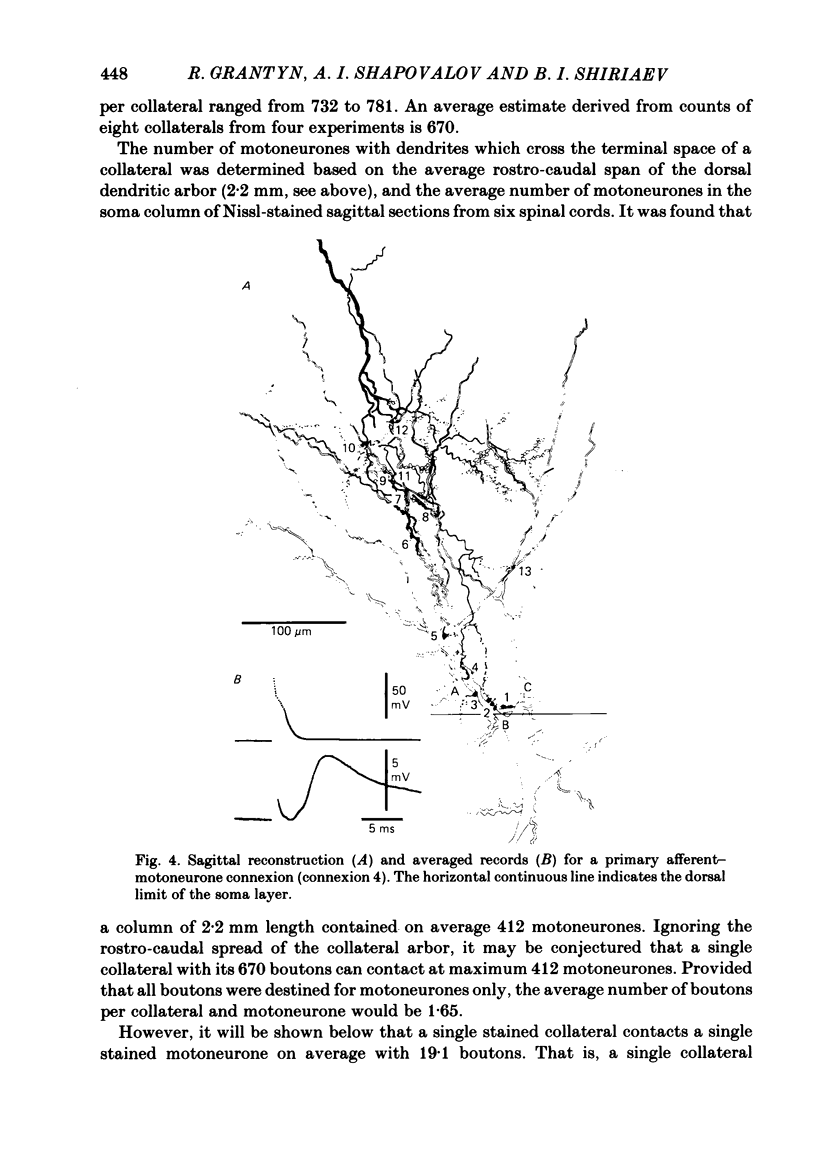
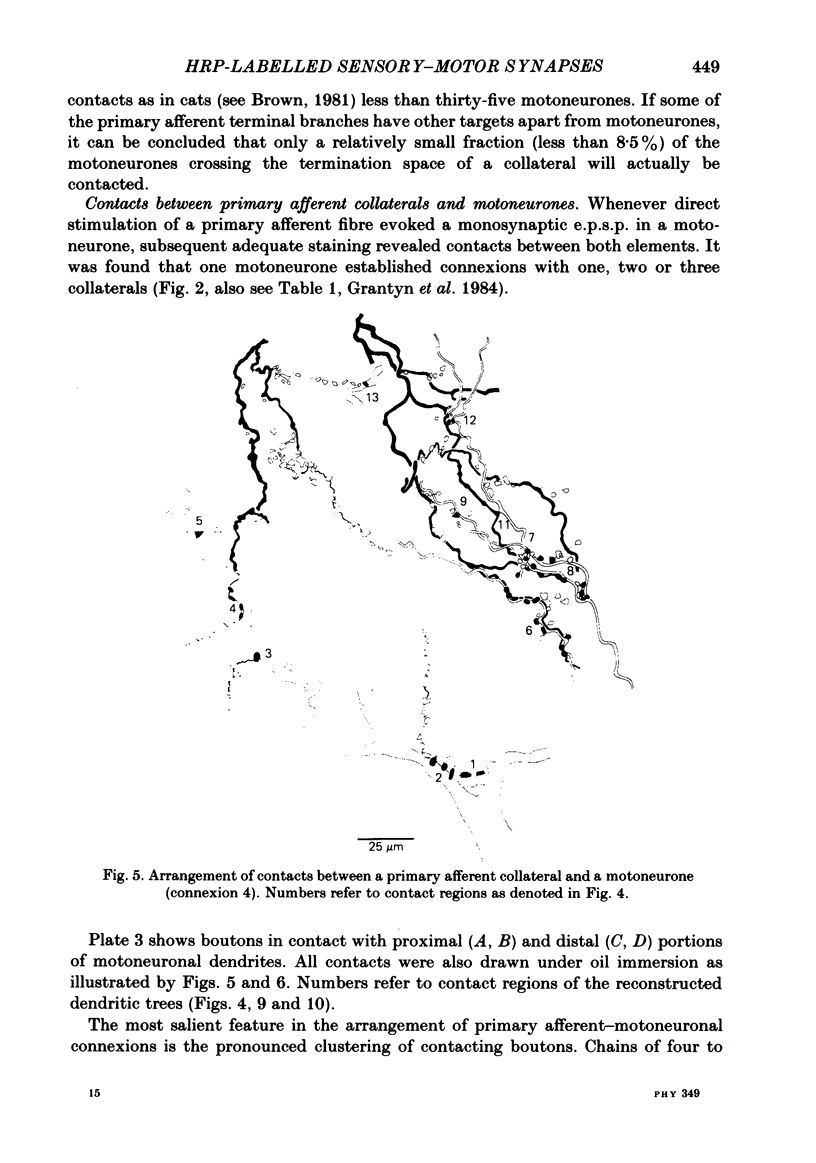
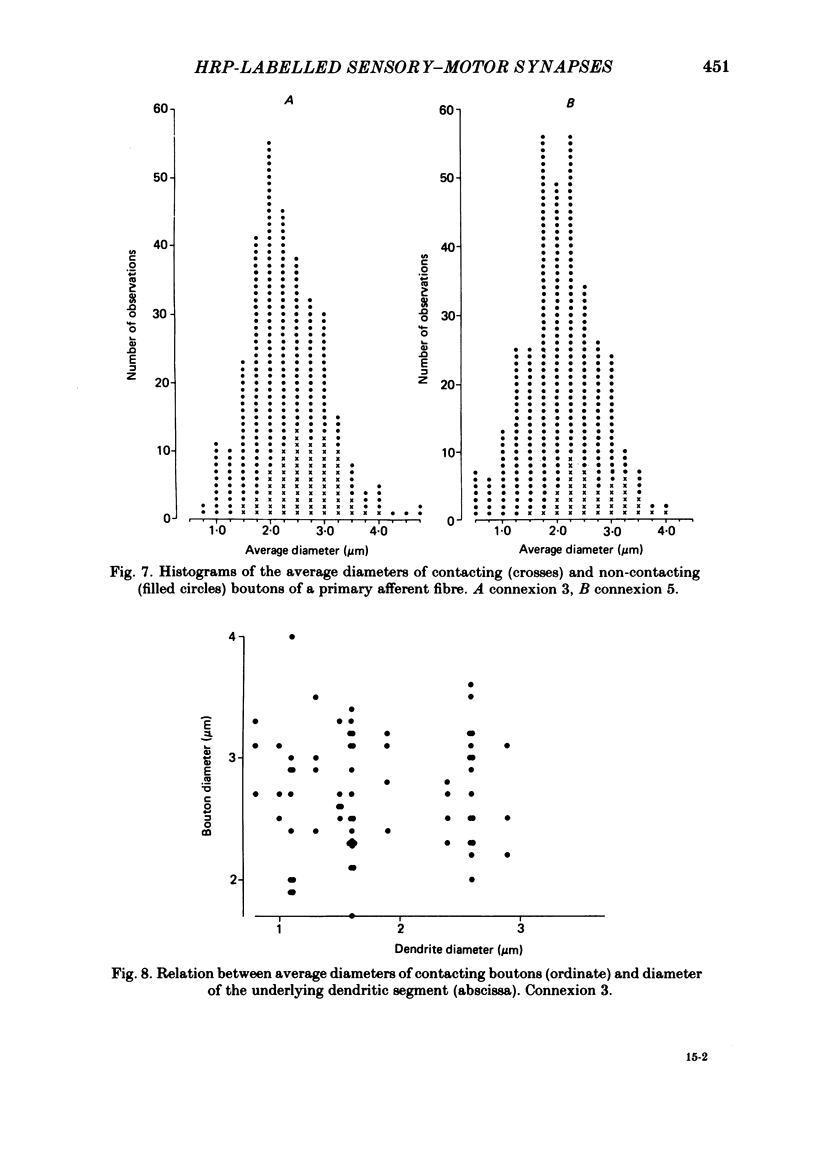
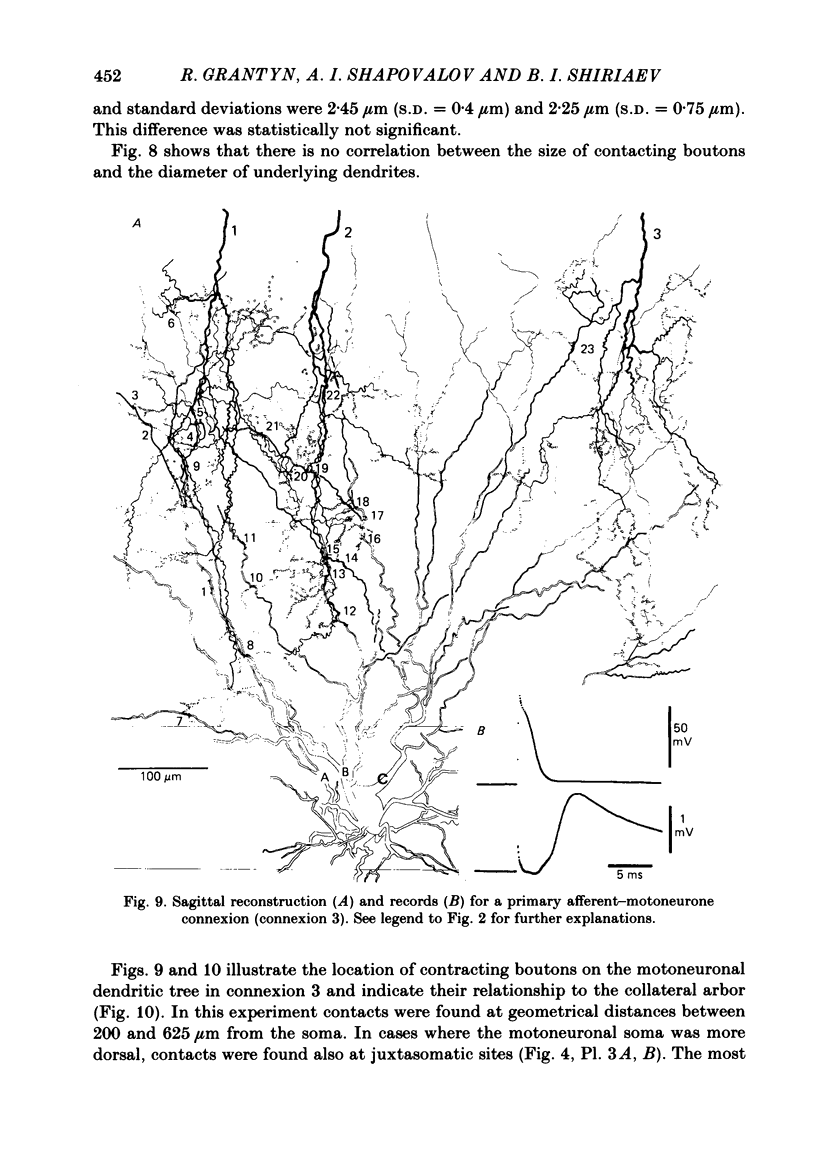
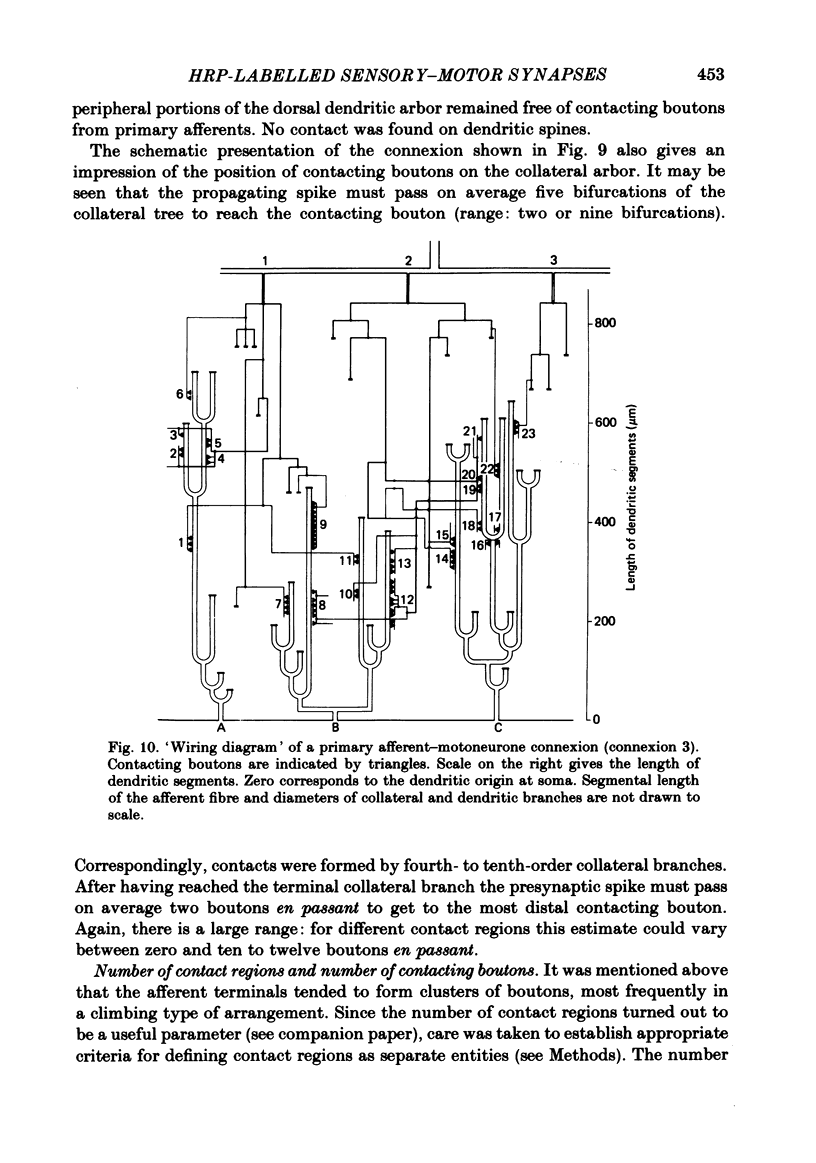
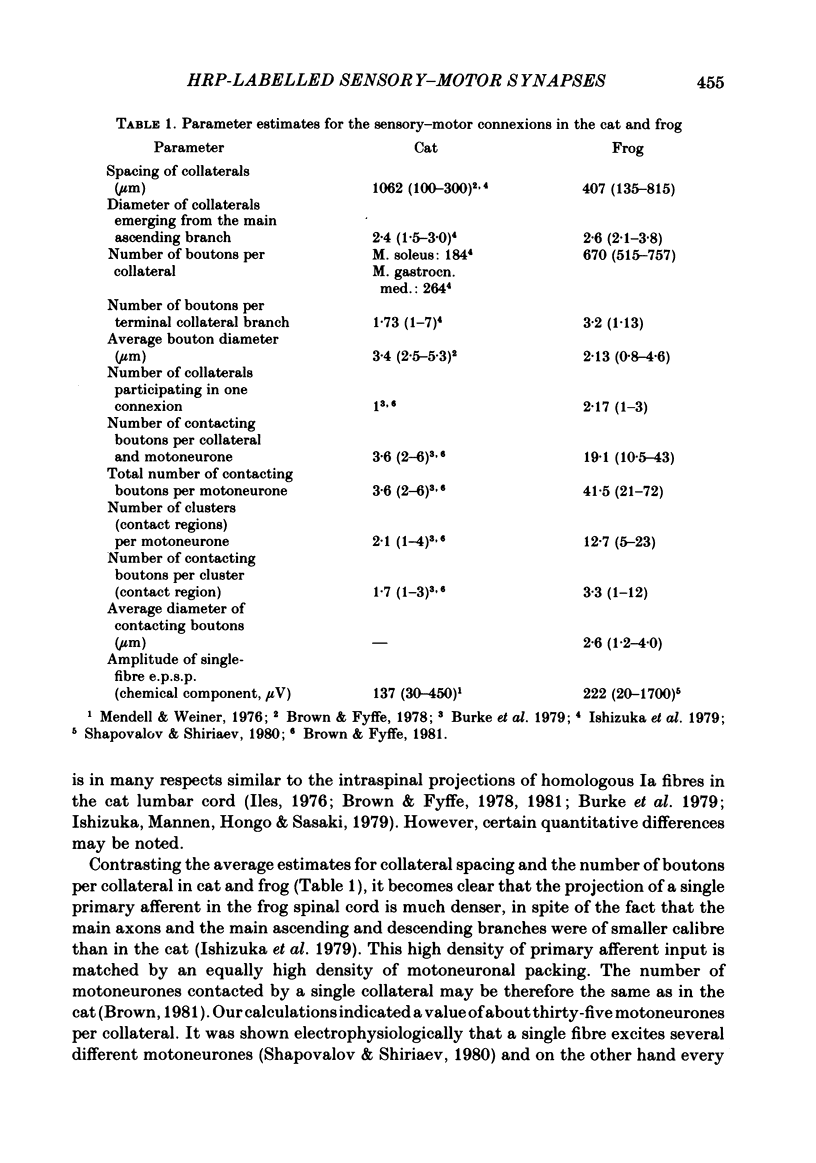
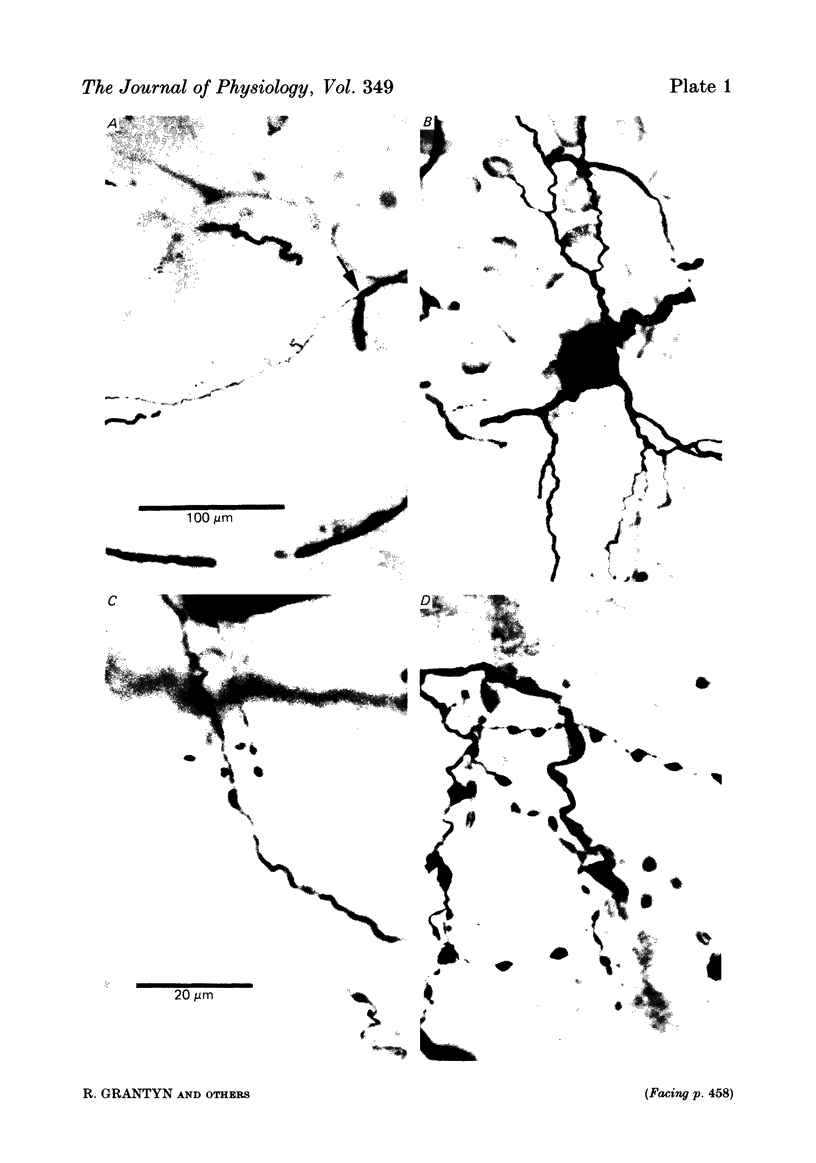
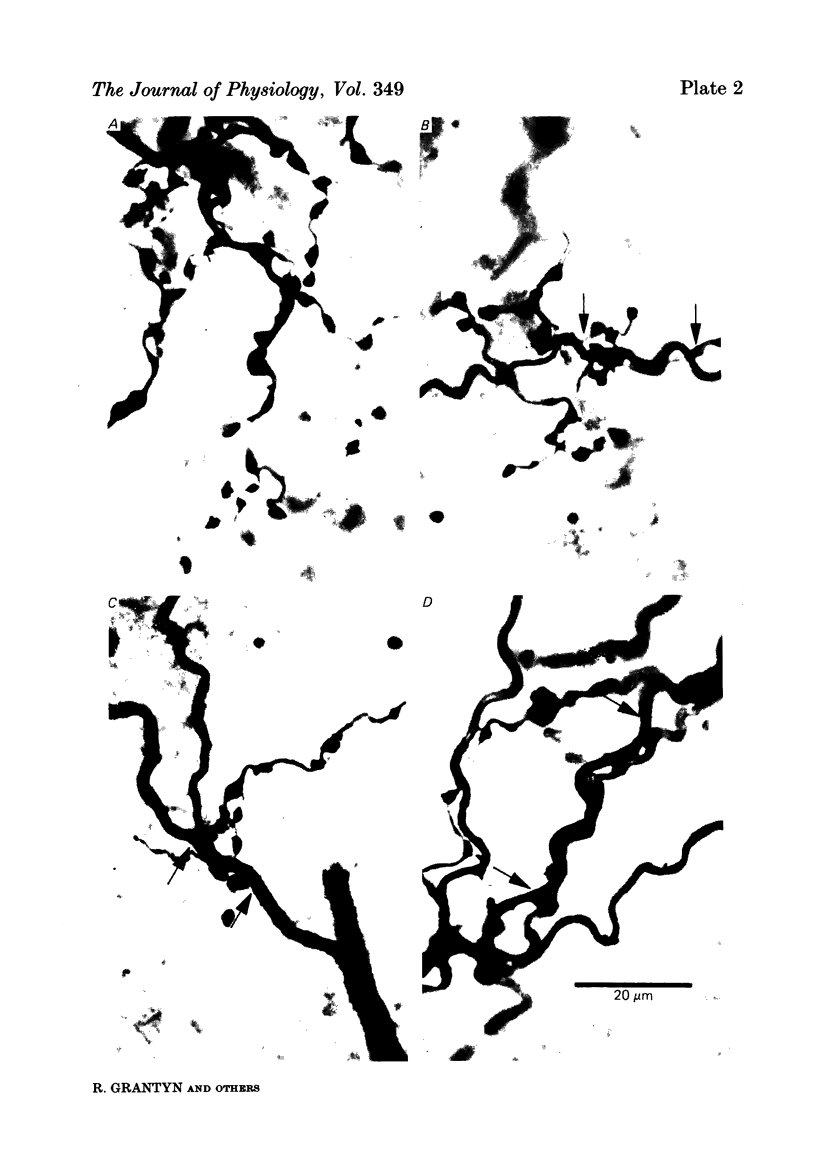
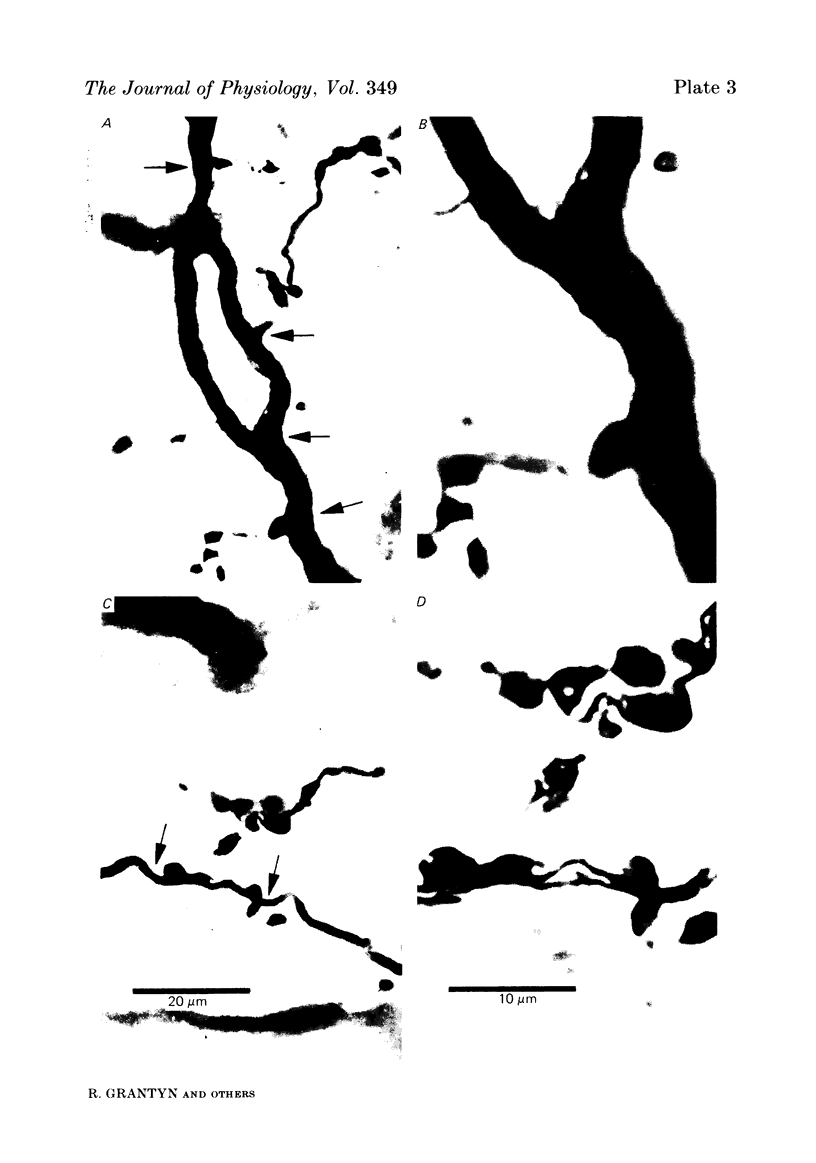
Monosynaptically connected primary afferent fibres and motoneurones of the isolated spinal cord of the frog were injected with horseradish peroxidase (HRP). Six labelled afferent fibre-motoneurone pairs were reconstructed and subjected to detailed analysis. Frog motoneurones possess eight to twelve dendritic arrays displaying some dorso-ventral asymmetry. Dorsal dendrites exhibit a rostro-caudal extent of 1.7-2.6 mm (average 2.2 mm). Primary afferent fibres bifurcate in the dorsal funiculus. First-order collaterals emanate from the main ascending and descending branches, at an average distance of 407 micron. The average number of boutons per collateral is 670. To reach a contacting bouton the presynaptic spike must pass on average five bifurcations and then zero to twelve boutons en passant, attached to a single terminal collateral branch. The structural equivalent of the axon cylinder of the collateral tree roughly preserves cross-sectional area. The branch power ranged between 1.15 and 3.35 (average 2.06). Primary afferent fibres usually form clusters of contacting boutons (contact regions). Connexions between an afferent fibre and a motoneurone comprise from five to twenty-three contact regions (average 12.5). Each contact region contains one to twelve contacting boutons (average 3.3). In two of three experiments contacting boutons were found to be significantly larger than non-contacting boutons. The average diameter of the former was 2.6 micron (range 1.2-4.0). In five out of six cases more than one collateral belonging to the same fibre participated in the connexion with a given motoneurone. The average number of contacting boutons per motoneurone and collateral is 19.1. It was estimated that each collateral could supply not more than thirty-five motoneurones. This would be less than 8.5% of the motoneurones with their dendrites which cross the termination space of a single collateral. The average number of contacting boutons forming one primary motoneurone connexion was 41.5 (range 21-72).
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AITKEN J. T., BRIDGER J. E. Neuron size and neuron population density in the lumbosacral region of the cat's spinal cord. J Anat. 1961 Jan;95:38–53. [PMC free article] [PubMed] [Google Scholar]
- Adams J. C. Technical considerations on the use of horseradish peroxidase as a neuronal marker. Neuroscience. 1977;2(1):141–145. doi: 10.1016/0306-4522(77)90074-4. [DOI] [PubMed] [Google Scholar]
- Adanina V. O., Shapovalov A. I. Smeshannye sinapsy pervichnykh afferentnykh volokon na motoneironakh liagushki, identifitsirovannye s pomoshch'iu neiroplaz-maticheskogo transporta peroksidazy khrena i élektronnoi mikroskopii. Dokl Akad Nauk SSSR. 1983;269(1):231–232. [PubMed] [Google Scholar]
- Bregman B. S., Cruce W. L. Normal dendritic morphology of frog spinal motoneurons: a Golgi study. J Comp Neurol. 1980 Oct 15;193(4):1035–1045. doi: 10.1002/cne.901930415. [DOI] [PubMed] [Google Scholar]
- Brown A. G., Fyffe R. E. Direct observations on the contacts made between Ia afferent fibres and alpha-motoneurones in the cat's lumbosacral spinal cord. J Physiol. 1981;313:121–140. doi: 10.1113/jphysiol.1981.sp013654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Fyffe R. E. The morphology of group Ia afferent fibre collaterals in the spinal cord of the cat. J Physiol. 1978 Jan;274:111–127. doi: 10.1113/jphysiol.1978.sp012137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Walmsley B., Hodgson J. A. HRP anatomy of group Ia afferent contacts on alpha motoneurones. Brain Res. 1979 Jan 12;160(2):347–352. doi: 10.1016/0006-8993(79)90430-x. [DOI] [PubMed] [Google Scholar]
- Cruce W. L. A supraspinal monosynaptic input to hindlimb motoneurons in lumbar spinal cord of the frog, Rana catesbiana. J Neurophysiol. 1974 Jul;37(4):691–704. doi: 10.1152/jn.1974.37.4.691. [DOI] [PubMed] [Google Scholar]
- Cullheim S., Kellerth J. O. A morphological study of the axons and recurrent axon collaterals of cat sciatic alpha-motoneurons after intracellular staining with horseradish peroxidase. J Comp Neurol. 1978 Apr 1;178(3):537–557. doi: 10.1002/cne.901780309. [DOI] [PubMed] [Google Scholar]
- Edwards F. R., Redman S. J., Walmsley B. Non-quantal fluctuations and transmission failures in charge transfer at Ia synapses on spinal motoneurones. J Physiol. 1976 Aug;259(3):689–704. doi: 10.1113/jphysiol.1976.sp011489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger M. D., Freeman N. C., Proshansky E. Morphology of spinal motoneurones mediating a cutaneous spinal reflex in the cat. J Physiol. 1980 Sep;306:349–363. doi: 10.1113/jphysiol.1980.sp013401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evinger C., Baker R., McCrea R. A. Axon collaterals of cat medial rectus motoneurons. Brain Res. 1979 Sep 28;174(1):153–160. doi: 10.1016/0006-8993(79)90810-2. [DOI] [PubMed] [Google Scholar]
- Frank E., Westerfield M. Synaptic organization of sensory and motor neurones innervating triceps brachii muscles in the bullfrog. J Physiol. 1982 Mar;324:479–494. doi: 10.1113/jphysiol.1982.sp014125. [DOI] [PMC free article] [PubMed] [Google Scholar]
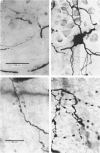
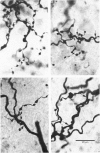
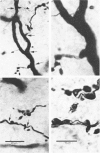
- Grantyn R., Shapovalov A. I., Shiriaev B. I. Combined morphological and electrophysiological description of connections between single primary afferent fibres and individual motoneurons in the frog spinal cord. Exp Brain Res. 1982;48(3):459–462. doi: 10.1007/BF00238624. [DOI] [PubMed] [Google Scholar]
- Grantyn R., Shapovalov A. I., Shiriaev B. I. Relation between structural and release parameters at the frog sensory-motor synapse. J Physiol. 1984 Apr;349:459–474. doi: 10.1113/jphysiol.1984.sp015167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iles J. F. Central terminations of muscle afferents on motoneurones in the cat spinal cord. J Physiol. 1976 Oct;262(1):91–117. doi: 10.1113/jphysiol.1976.sp011587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka N., Mannen H., Hongo T., Sasaki S. Trajectory of group Ia afferent fibers stained with horseradish peroxidase in the lumbosacral spinal cord of the cat: three dimensional reconstructions from serial sections. J Comp Neurol. 1979 Jul 15;186(2):189–211. doi: 10.1002/cne.901860206. [DOI] [PubMed] [Google Scholar]
- Itoh K., Konishi A., Nomura S., Mizuno N., Nakamura Y., Sugimoto T. Application of coupled oxidation reaction to electron microscopic demonstration of horseradish peroxidase: cobalt-glucose oxidase method. Brain Res. 1979 Oct 19;175(2):341–346. doi: 10.1016/0006-8993(79)91013-8. [DOI] [PubMed] [Google Scholar]
- Joseph B. S., Whitlock D. G. Central projections of selected spinal dorsal roots in anuran amphibians. Anat Rec. 1968 Feb;160(2):279–288. doi: 10.1002/ar.1091600214. [DOI] [PubMed] [Google Scholar]
- Lüscher H. R., Ruenzel P., Henneman E. How the size of motoneurones determines their susceptibility to discharge. Nature. 1979 Dec 20;282(5741):859–861. doi: 10.1038/282859a0. [DOI] [PubMed] [Google Scholar]
- Mendell L. M., Weiner R. Analysis of pairs of individual Ia-E.P.S.P.S in single motoneurones. J Physiol. 1976 Feb;255(1):81–104. doi: 10.1113/jphysiol.1976.sp011271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motorina M. V., Tamarova Z. A., Shapovalov A. I., Shiriaev B. I. Issledovanie sinapticheskikh sviazei mezhdu pervichnymi afferentami i motoneironami spinnogo mozga liagushki s pomoshch'iu vnutrikletochnogo vvedeniia peroksidazy khrena. Neirofiziologiia. 1982;14(1):60–68. [PubMed] [Google Scholar]
- Motorina M. V., Tamarova Z. A., Shapovalov A. I., Shiriaev B. I. Raspredelenie terminalei pervichnykh afferentnykh volokon, polisinapticheski sviazannykh s motoneironami, v spinnom mozge liagushki. Neirofiziologiia. 1982;14(6):615–621. [PubMed] [Google Scholar]
- RALL W. Branching dendritic trees and motoneuron membrane resistivity. Exp Neurol. 1959 Nov;1:491–527. doi: 10.1016/0014-4886(59)90046-9. [DOI] [PubMed] [Google Scholar]
- Redman S. Junctional mechanisms at group Ia synapses. Prog Neurobiol. 1979;12(1):33–83. doi: 10.1016/0301-0082(79)90010-8. [DOI] [PubMed] [Google Scholar]
- Rose P. K. Distribution of dendrites from biventer cervicis and complexus motoneurons stained intracellularly with horseradish peroxidase in the adult cat. J Comp Neurol. 1981 Apr 10;197(3):395–409. doi: 10.1002/cne.901970304. [DOI] [PubMed] [Google Scholar]
- Shapovalov A. I. Interneuronal synapses with electrical, dual and chemical mode of transmission in vertebrates. Neuroscience. 1980;5(7):1113–1124. doi: 10.1016/0306-4522(80)90190-6. [DOI] [PubMed] [Google Scholar]
- Shapovalov A. I., Shiriaev B. I. Dual mode of junctional transmission at synapses between single primary afferent fibres and motoneurones in the amphibian. J Physiol. 1980 Sep;306:1–15. doi: 10.1113/jphysiol.1980.sp013381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapovalov A. I., Shiriaev B. I. Single-fiber EPSPs in amphibian motoneurons. Brain Res. 1979 Jan 19;160(3):519–523. doi: 10.1016/0006-8993(79)91079-5. [DOI] [PubMed] [Google Scholar]
- Shiriaev B. I. Sinapticheskie éffekty, vyzyvaemye v motoneironakh spinnogo mozga liagushki adekvatnym razdrazheniem myshechnykh vereten. Fiziol Zh SSSR Im I M Sechenova. 1983 Sep;69(9):1157–1163. [PubMed] [Google Scholar]
- Spencer R. F., Evinger C., Baker R. Electron microscopic observations of axon collateral synaptic endings of cat oculomotor motoneurons stained by intracellular injection of horseradish peroxidase. Brain Res. 1982 Feb 25;234(2):423–429. doi: 10.1016/0006-8993(82)90881-2. [DOI] [PubMed] [Google Scholar]
- Stensaas L. J., Stensaas S. S. Light and electron microscopy of motoneurons and neuropile in the amphibian spinal cord. Brain Res. 1971 Aug 7;31(1):67–84. doi: 10.1016/0006-8993(71)90634-2. [DOI] [PubMed] [Google Scholar]
- Székely G. The morphology of motoneurons and dorsal root fibers in the frog's spinal cord. Brain Res. 1976 Feb 20;103(2):275–290. doi: 10.1016/0006-8993(76)90799-x. [DOI] [PubMed] [Google Scholar]
- Turner P. T. Effect of pentobarbital on uptake of horseradish peroxidase by rabbit cortical synapses. Exp Neurol. 1977 Jan;54(1):24–32. doi: 10.1016/0014-4886(77)90231-x. [DOI] [PubMed] [Google Scholar]
- Waxman S. G. Integrative properties and design principles of axons. Int Rev Neurobiol. 1975;18:1–40. doi: 10.1016/s0074-7742(08)60032-x. [DOI] [PubMed] [Google Scholar]
- Weakly J. N. Effect of barbiturates on 'quantal' synaptic transmission in spinal motoneurones. J Physiol. 1969 Sep;204(1):63–77. doi: 10.1113/jphysiol.1969.sp008898. [DOI] [PMC free article] [PubMed] [Google Scholar]