Abstract
To assess the effect of gestational age and labor on the interleukin-8 (IL-8) concentration in whole cord blood and serum, IL-8 levels were determined simultaneously in cord blood serum and lysate in 134 infants. Following the elimination of some of the samples due to exclusion criteria, the data for 99 uninfected infants (71 term and 28 preterm) and 9 infants with neonatal bacterial infection delivered either vaginally or by elective or emergency cesarean section were analyzed. The effects of labor and gestational age were tested by analysis of variance. IL-8 was not detectable in the serum of 25 infants, whereas IL-8 levels in whole blood were measurable in all of the samples. The median IL-8 conncentrations in whole cord blood lysate were 106 pg/ml (range, 20 to 415 pg/ml) in preterm infants and 176 pg/ml (range, 34 to 1,667 pg/ml) in term infants. In contrast to the IL-8 levels in serum, IL-8 levels in whole blood were reduced after ECS. Gestational age had no independent effect on the IL-8 concentrations in either serum or whole blood; these concentrations increased in infected infants after labor. We conclude that the neonatal proinflammatory response to labor stress was more evident in the concentrations of IL-8 in whole blood than in serum. The levels of IL-8 in whole-blood lysate reflect proinflammatory stimulation in neonates and may be a useful diagnostic tool for the early diagnosis of neonatal infection.
Interleukin-8 (IL-8) belongs to the class of proinflammatory “CC” chemokines defined by the position of two cysteine groups and is synthesized predominantly by monocytes. Its active form effectively activates neutrophil granulocytes, advancing the chemotaxis and synthesis of myeloperoxidase, thus suggesting a critical role in host defense to infectious diseases (1, 2).
The IL-8 concentration in serum has been studied as a diagnostic marker of neonatal bacterial infection (NBI) (10, 11) and has been shown to be an early marker of neonatal bacterial infection, whereas the concentration of C-reactive protein (CRP) increases after 12 to 24 h in the course of systemic infectious disease. A “diagnostic gap “ exists between the decline of IL-8 after 4 to 6 h and the increase in C-reactive protein at 12 to 24 h, which is a well-established marker of confirmed bacterial infection. Due to the rapid serum clearing of IL-8, its value as a monitoring parameter of infectious disease may be limited. In vivo, a large proportion of IL-8 is associated with erythrocytes and leukocytes (polymorphonuclear leukocytes and peripheral blood mononuclear cells), and the concentration in serum represents only a small fraction of the total amount of IL-8. The measurement of cell-associated IL-8 reflects more quantitatively its production and adds information regarding the stage of the disease and the patient's inflammatory response (7, 9, 13, 14). Total IL-8 can be measured in whole blood after cell lysis with a good analytical test (16). In healthy adults, we previously observed an IL-8 peak level of <12 pg/ml with a median of 83 pg/ml (range, 45 to 230 pg/ml) in whole blood (16). IL-8 concentrations in whole blood grossly exceed those in serum in septic adult patients (ratio, 3 to 40) (13). In amniotic infection syndrome, increased maternal IL-8 levels in whole blood have been observed (16). The relevance of the IL-8 concentration in whole blood in the diagnosis and monitoring of neonatal infectious disease is not clear, since information on such levels in healthy neonates is limited. We determined the IL-8 concentrations in cord blood serum and in whole cord blood samples of term and preterm infants. Since stress-induced production of proinflammatory cytokines may be stimulated during labor (6), we considered the impact of mode of delivery in the analysis.
MATERIALS AND METHODS
The IL-8 levels in cord blood serum and in whole cord blood lysate were determined in 134 infants consecutively delivered at a tertiary care perinatal center. Of these, 35 infants were excluded according to the following criteria: presence of severe perinatal acidosis (umbilical arterial pH of <7.1), premature rupture of membranes (PROM) at >12 h after birth, signs of amniotic and/or maternal infection (either viral or bacterial), NBI at <72 h after birth, and incorrect sampling or processing of cord blood specimens. Infants were considered to have NBI if clinical criteria and the elevation of CRP (>10 mg/liter) or IL-6 (>100 pg/ml) in serum were evident within 72 h after birth. The clinical criteria included respiratory distress, gastrointestinal symptoms, temperature instability, hypotension, lethargy, and seizures. A total of 18 infants were excluded due to PROM or suspected amniotic infection.
The data obtained for 99 healthy (71 term and 28 preterm infants, median gestational age of 35 weeks [range, 28 to 36 weeks]) were analyzed. A total of 24 term infants and 18 preterm infants were delivered by elective cesarean section (ECS; term infants, n = 24; preterm infants, n = 18) or emergency cesarean section (EMCS; term infants, n = 12; preterm infants, n = 6) after onset of labor. A subset of 9 infants (term infants, n = 5; preterm infants, n = 4) diagnosed with NBI of the 18 infants excluded from the original study group due to suspected infection was analyzed separately.
IL-8 levels were determined in serum and the whole-blood lysate of blood samples obtained immediately after birth. The processing of samples was performed within 3 h of delivery. For determination of IL-8 levels in whole blood, 100 μl of EDTA-cord blood was incubated with 100 μl of a nonionic detergent (Cell Lysate Solution; Milenia Biotec GmbH, Bad Nauheim, Germany), vortexed, and frozen at −20°C until assay. EDTA-blood samples were then divided into aliquotes and centrifuged at 10,000 rpm for 10 min; the serum samples were then frozen at −20° until assay. IL-8 levels were determined by using an enzyme immunoassay (lower detection limit of 5 pg/ml; Immulite System; DPC Biermann GmbH, Bad Nauheim, Germany) within 2 weeks of sampling. The IL-8 levels in serum and whole blood were analyzed according to subgroups in relation to mode of delivery. Data were analyzed by using SPSS statistical software (SPSS for Windows, release 10.0.7; SPSS, Inc., Chicago, Ill.). Mode of delivery, gestational age, and NBI status were tested by univariate analysis of variance for independent effects. A P value of < 0.05 was considered significant.
RESULTS
Whereas IL-8 levels in whole cord blood lysate were detectable in all 99 samples, IL-8 levels in serum were undetectable in 20 term and 5 preterm infants. IL-8 was undetectable in serum in 13 (54%) of 24 term infants delivered by ECS. In the preterm infants, IL-8 was undetectable in the serum of 2 (11%) of 18 infants delivered by by ECS.
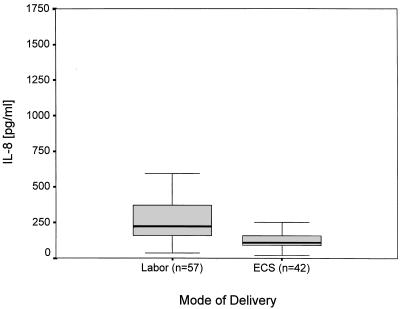
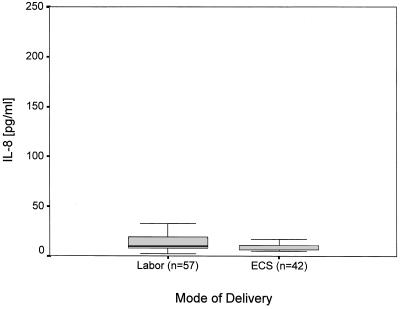
In the 99 healthy term and preterm infants the IL-8 levels in serum ranged between <5 and 270 pg/ml and <5 to 99 pg/ml, respectively. IL-8 levels in whole blood ranged from 34 to 1667 pg/ml (median, 176.0 pg/ml) in term infants and between 20 and 415 pg/ml (median, 106.0 pg/ml) in preterm infants (Table 1). We found no correlation of IL-8 levels in serum and whole blood (r = 0.34). Labor had an independent effect on IL-8 levels in whole blood (P < 0.01) but not on levels in serum (P = 0.42) (Fig. 1 and 2). Neither the IL-8 levels in serum nor those in whole blood were affected independently by gestational age (P = 0.76; P = 0.35).
TABLE 1.
IL-8 concentration in serum and whole-blood lysate of infants
| Infant group (n) | Procedure (n)a | Median IL-8 concn (pg/ml) in:
|
|||
|---|---|---|---|---|---|
| Serumb
|
Whole-blood lysate
|
||||
| Median | Range | Median | Range | ||
| Noninfected infants | |||||
| Preterm (28) | ECS (18) | 9.5 | <5-99 | 95.5 | 20-340 |
| EMCS (6) | 12.5 | <5-42 | 170.0 | 75-373 | |
| VD (4) | 6.0 | <5-16 | 299.0 | 158-415 | |
| Term (71) | ECS (24) | 7.0 | <5-17 | 115.5 | 40-251 |
| EMCS (12) | 8.0 | <5-11 | 175.5 | 86-498 | |
| VD (33) | 16.0 | <5-270 | 223.0 | 34-1,667 | |
| VE (2) | 15.5 | <5-24 | 448.0 | 423-473 | |
| Infants with NBI | |||||
| Preterm (4) | EMCS (4) | 35.0 | 15-246 | 331.0 | 259-755 |
| Term (5) | EMCS (5) | 18.0 | 7-157 | 458.0 | 118-1,296 |
VD, vaginal delivery; VE, vacuum extraction.
Levels in serum were not detectable in 20 term and 5 preterm infants.
FIG. 1.
Effect of labor on IL-8 levels in whole cord blood lysate (P < 0.01; analysis of variance).
FIG. 2.
Effect of labor on IL-8 levels in cord blood serum (P = 0.42; analysis of variance).
In nine infants with NBI (five term infants and four preterm infants) delivered by emergency cesarean section, IL-8 was detected in serum and whole blood in all of the samples (Table 1). Analysis of variance revealed, compared to the data for noninfected infants, an impact of NBI on the IL-8 levels in whole-blood lysate (P < 0.01) and, less significantly, in serum (P = 0.049).
DISCUSSION
Methods with sufficient analytical performance for the processing of blood samples and for the determination of IL-8 concentrations in whole blood have been established previously (16). Therefore, the IL-8 levels measured in whole cord blood in our study reliably represent total IL-8 levels in whole blood in ex vivo conditions. However, considering the capacity of vascular endothelium in synthesis and the binding of IL-8, even levels determined in whole blood lysate may not represent the total in vivo concentrations.
IL-8 levels in whole blood exceeded those determined previously in healthy adults (16). IL-8 levels in serum were undetectable in a large proportion of infants, predominantly after ECS. We suggest that a physiological role of IL-8 in labor, as has been proposed for other proinflammatory cytokines (6), may explain this observation.
Considerable interindividual variation of the total IL-8 concentration in cord blood was observed in healthy term infants, as well as in preterm infants. The differences of IL-8 levels in whole-blood assays may reflect variations of intracellular synthesis and/or membrane-binding capacity, since the levels in serum and WBL were not correlated. Decreased IL-8 levels in whole blood in preterm infants may result from diminished IL-8 transcription in monocytes of these infants (17). Levels of other proinflammatory cytokines, such as IL-6, are negatively correlated with the duration of gestation (19). In relation to gestational age, increased anti-inflammatory mediator levels, such as those of IL-10, have been observed in the cord blood of preterm infants, as well as decreased levels of other proinflammatory cytokines in serum (5, 8, 19). In our study group we were not able to detect an independent effect of gestational age. Preterm infants in this study were of relatively advanced gestational age, and no extremely premature infants of <28 weeks' gestational age were included, which may have had a considerable impact on our results.
Decreased IL-8 levels in the infants delivered by ECS support observations stressing the role of labor as an important trigger of neonatal proinflammatory cytokine production (6). Recently, Jokic et al. (12) found no relation between cytokine levels in cord blood serum and the mode of delivery. However, in our study the total IL-8 concentration in whole blood was affected by the occurrence of labor. Buoncuore et al. (6) demonstrated elevated levels of IL-6 in serum in infants after vaginal delivery compared to infants delivered by ECS. The newborn immune response to the stress of delivery is a possible mechanism that results in elevated levels of IL-1β, IL-6, and tumor necrosis factor alpha in serum during the perinatal period (15).
Detailed analyses of blood cell populations have demonstrated IL-6 release by fetal monocytes during spontaneous labor at term but not in preterm delivery. Different mechanisms may induce term and preterm labor, and fetal IL-6 may have an important role in promoting term labor (18). Monocytes are the primary source of IL-8 in cord blood. Increased IL-8 levels in whole blood in the term infants in this study, which may have resulted from the activation of myelomonocytic cells, are consistent with the observation of increased IL-6 concentrations at term. In our study, in the labor group IL-8 levels in serum were undetectable in 18% of the infants. In contrast, IL-8 concentrations in whole blood indicated an inflammatory response to labor. These results support the relevance of the IL-8 concentration in whole blood as a sensitive marker of cytokine production, which may be superior to the measurement of serum levels. Dexamethasone inhibits lipopolysaccharide-induced IL-6 production, as well as IL-8 release from mononuclear and/or neutrophil cells in neonates (4, 20). We were not able to assess the effect of antenatal steroids on spontaneous IL-8 production in relation to labor because of the relatively small number of spontaneously delivered preterm infants. General anesthesia and epidural anesthesia may have an impact on cytokine release, and increased levels of IL-6 have been observed in neonates following anesthesia (3). We did not assess the impact of narcotic drugs on fetal cytokine production. However, the decreased IL-8 levels of study infants delivered by ECS might have been mitigated. The actual number of erythrocytes and leukocytes/100 μl of cord blood may influence the total concentration of IL-8. These levels were not normalized for cell numbers but, presumably, this would have affected the results of all of the groups equally. Thus, the differences between delivery groups may not have been affected. Cell numbers of infected infants were not considered in this small pilot group but may be crucial in further studies on the concentrations of IL-8 in whole blood in subjects with NBI. To date, the physiological role of proinflammatory cytokines in the modulation of neonatal host defense and the induction of labor has not been clarified completely.
Our data extend the information of neonatal immune response in relation to the mode of delivery and confirm previous findings, indicating a labor-induced perinatal proinflammatory response. Measurement of the IL-8 levels in whole blood may be more sensitive than measuring IL-8 levels in serum in the early diagnosis of NBI, a hypothesis which could not be confirmed in the small subgroup of infected infants exclusively delivered by emergency cesarean section in this study. Longitudinal observations are necessary to verify the significance of the IL-8 present in whole-blood lysate in the diagnosis of NBI.
Acknowledgments
We thank A. Thiel for technical assistance.
REFERENCES
- 1.Atta-ur-Rahman, K. Harvey, and R. A. Siddiqui. 1999. Interleukin-8: an autocrine inflammatory mediator. Curr. Pharmacol. Des. 5:241-253. [PubMed] [Google Scholar]
- 2.Baggiolini, M., P. Loetscher, and B. Moser. 1995. Interleukin-8 and the chemokine family. Int. J. Immunopharmacol. 17:103-108. [DOI] [PubMed] [Google Scholar]
- 3.Bessler, H., A. Kuperman, B. Beilin, G. Klinger, N. Gurary, C. Mozes, and L. Sirota. 1998. Labor affects cytokine production in newborns. Am. J. Reprod. Immunol. 39:27-32. [DOI] [PubMed] [Google Scholar]
- 4.Bessler, H., C. Mendel, R. Straussberg, N. Gurary, D. Aloni, and L. Sirota. 1999. Effects of dexamethasone on IL-1beta, IL-6, and TNF-alpha production by mononuclear cells of newborns and adults. Biol. Neonate 75:225-233. [DOI] [PubMed] [Google Scholar]
- 5.Blanco-Quiros, A., E. Arranz, G. Solis, A. Villar, A. Ramos, and D. Coto. 2000. Cord blood interleukin-10 levels are increased in preterm newborns. Eur. J. Pediatr. 159:420-423. [DOI] [PubMed] [Google Scholar]
- 6.Buonocore, G., M. De Filippo, D. Gioia, E. Picciolini, E. Luzzi, V. Bocci, and R. Bracci. 1995. Maternal and neonatal serum cytokine levels in relation to mode of delivery. Biol. Neonate 68:104-110. [DOI] [PubMed] [Google Scholar]
- 7.Cavaillon, J. M., C. Munoz, C. Fitting, B. Misset, and Carlet, J. 1992. Circulating cytokines: the tip of the iceberg? Circ. Shock 38:145-152. [PubMed] [Google Scholar]
- 8.De Jongh, R. F., M. Puylaert, E. Bosmans, W. Ombelet, M. Maes, and R. Heylen. 1999. The fetomaternal dependency of cord blood interleukin-6. Am. J. Perinatol. 16:121-128. [DOI] [PubMed] [Google Scholar]
- 9.Flach, R., M. Majetschak, T. Heukamp, V. Jennissen, S. Flohe, J. Borgermann, U. Obertacke, and F. U. Schade. 1999. Relation of ex vivo stimulated blood cytokine synthesis to post-traumatic sepsis. Cytokine 11:173-178. [DOI] [PubMed] [Google Scholar]
- 10.Franz, A. R., G. Steinbach, M. Kron, and F. Pohlandt. 1999. Reduction of unnecessary antibiotic therapy in newborn infants using interleukin-8 and C-reactive protein as markers of bacterial infections. Pediatrics 104(Pt. 1):447-453. [DOI] [PubMed] [Google Scholar]
- 11.Franz, A. R., M. Kron, F. Pohlandt, and G. Steinbach. 1999. Comparison of procalcitonin with interleukin 8, C-reactive protein and differential white blood cell count for the early diagnosis of bacterial infections in newborn infants. Pediatr. Infect. Dis. J. 18:666-671. [DOI] [PubMed] [Google Scholar]
- 12.Jokic, M., B. Guillois, B. Cauquelin, J. D. Giroux, J. L. Bessis, R. Morello, G. Levy, and J. J. Ballet. 2000. Fetal distress increases interleukin-6 and interleukin-8 and decreases tumour necrosis factor-alpha cord blood levels in noninfected full-term neonates. BJOG 107:420-425. [DOI] [PubMed] [Google Scholar]
- 13.Marie, C., C. Fitting, C. Cheval, M. R. Losser, J. Carlet, D. Payen, K. Foster, and J. M. Cavaillon. 1997. Presence of high levels of leukocyte-associated interleukin-8 upon cell activation and in patients with sepsis syndrome. Infect. Immun. 65:865-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marie, C., C. Fitting, J. Muret, D. Payen, and J. M. Cavaillon. 2000. Interleukin-8 production in whole blood assays: is interleukin-10 responsible for the downregulation observed in sepsis? Cytokine 12:55-61. [DOI] [PubMed] [Google Scholar]
- 15.Protonotariou, E., A. Malamitsi-Puchner, G. Giannaki, D. Rizos, I. Phocas, and A. Sarandakou. 1999. Patterns of inflammatory cytokine serum concentrations during the perinatal period. Early Hum. Dev. 56:31-38. [DOI] [PubMed] [Google Scholar]
- 16.Reinsberg, J., C. J. Dembinski, Dorn, D. Behrendt, P. Bartmann, and H. van Der Ven. 2000. Determination of total interleukin-8 in whole blood after cell lysis. Clin. Chem. 46:1387-1394. [PubMed] [Google Scholar]
- 17.Schibler, K. R., M. S. Trautman, K. W. Liechty, W. L. White, G. Rothstein, and R. D. Christensen. 1993. Diminished transcription of interleukin-8 by monocytes from preterm neonates. J. Leukoc. Biol. 53:399-403. [DOI] [PubMed] [Google Scholar]
- 18.Steinborn, A., C. Sohn, C. Sayehli, A. Baudendistel, D. Huwelmeier, C. Solbach, E. Schmitt, and M. Kaufmann. 1999. Spontaneous labour at term is associated with fetal monocyte activation. Clin. Exp. Immunol. 117:147-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weimann, E., S. Rutkowski, and G. Reisbach. 1998. G-CSF, GM-CSF, and IL-6 levels in cord blood: diminished increase of G-CSF and IL-6 in preterms with perinatal infection compared to term neonates. J. Perinat. Med. 26:211-218. [DOI] [PubMed] [Google Scholar]
- 20.Zentay, Z., M. Sharaf, M. Qadir, D. Drafta, and D. Davidson. 1999. Mechanism for dexamethasone inhibition of neutrophil migration upon exposure to lipopolysaccharide in vitro: role of neutrophil interleukin-8 release. Pediatr. Res. 46:406-410. [DOI] [PubMed] [Google Scholar]