Abstract
Objective
Fulvic acid (FA), a humic substance, has various applications in agricultural (animal husbandry) and pharmaceutical industries. However, to the best of our knowledge, its antitumor effects remain unclear. This study aimed to elucidate the effects and underlying mechanisms of FA in ovarian cancer cells.
Methods
To determine the half-maximal inhibitory concentration (IC50) of FA, SK-OV-3 and OVCAR3 ovarian cancer cells were exposed to various concentrations of FA. The effects of FA and expression of cytochrome P450 family 1 subfamily A member 1 (CYP1A1) on cellular proliferation, migration, and invasion were evaluated using the Cell Counting Kit-8 and transwell assays for migration and invasion. Differentially expressed messenger ribonucleic acids (mRNAs) were identified via Illumina ribonucleic acid (RNA) sequencing and verified using fluorescent quantitative reverse transcription polymerase chain reaction (qRT-PCR). CYP1A1 protein levels were measured by western blotting.
Results
The IC50 values of FA for OVCAR3 and SK-OV-3 cells were 689.9 and 752.0 µg/ml, respectively. FA treatment suppressed cell proliferation, invasion, and migration. In FA-treated SK-OV-3 cells, 117 mRNAs were upregulated, and 342 mRNAs were downregulated, as identified by Illumina RNA sequencing. The qRT-PCR results revealed that FA upregulated CYP1A1 expression in both cell lines. CYP1A1 overexpression mimicked the effects of FA treatment on cell proliferation, invasion, and migration. Furthermore, CYP1A1 knockdown alleviated these effects induced by FA treatments.
Conclusion
FA suppressed cell proliferation, invasion, and migration and upregulated CYP1A1 expression in SK-OV-3 and OVCAR3 cells. Our results suggest that FA demonstrates antitumor effects in ovarian cancer cells through CYP1A1 regulation.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12672-025-02236-5.
Keywords: Fulvic acid, Ovarian cancer, Cytochrome P450 CYP1A1, RNA sequencing
Introduction
Fulvic acid (FA) is a natural organic compound and an important component of humic substances [1]. Humic substances are widely found in soil, water, sediments, and coal, and are primarily formed through the decomposition of plant and animal matter by microorganisms and geochemical processes [2]. FA mainly consists of four types of carbon: carbonyl, aromatic, alkyl and carbohydrate, enriched with diverse functional groups such as carboxyl (-COOH) and phenolic hydroxyl (-OH) moieties [3, 4]. These functional groups confer robust metal-chelating capabilities [3]. Due to its unique chemical structure and biological activity, FA has significant applications in agriculture, environmental science, medicine, and health [1, 5–10]. The existing literatures show that FA plays roles in immunomodulation, oxidative stress, and gut health [9, 10].
Despite these advancements, the antitumor effects of FA remain largely in the preliminary stage of research. FA has been shown to reduce endothelial adhesion of colorectal cancer cells when challenged by resistin [11]. Additionally, media conditioned by FA-stimulated RAW 264.7 cells has reduced the viability of various human cancer cell lines, including those from liver cancer, leukemia, and prostate cancer [12]. This effect may be attributed to the ability of FA to stimulate inducible nitric oxide and nitric oxide synthase production from RAW 264.7 cells through nuclear factor kappa B pathway activation [12]. In addition, FA has demonstrated the ability to lower cell viability, trigger apoptosis, and alter cell cycle distribution in A375 human melanoma cells [13], displaying broad spectrum of antitumor effects. Moreover, in vivo studies have reported that FA can inhibit tumor growth of non-small-cell lung cancer (NSCLC)-bearing nude mice [14]. Given these promising findings, further investigation into the therapeutic potential of FA across different cancer types is warranted.
Ovarian cancer is a malignant tumor originating from the ovaries and ranks third among gynecological tumor in terms of incidence [15]. The occurrence of ovarian cancer involves genetic, hormonal, and other factors [16, 17]. Ovarian cancer can occur in any age group, and its incidence is increasing annually [18]. Early detection is challenging due to the subtlety of initial symptoms, with most cases being diagnosed in advanced stages. Current treatment primarily involves comprehensive surgery. Despite advancements in treatment options, the 5-year survival rate for ovarian cancer remains < 50% [18, 19]. This underscores the urgent need for the development of new and more effective therapeutic agents. In this context, our study aimed to investigate the effects of FA on ovarian cancer cells and to elucidate the underlying mechanisms using Illumina ribonucleic acids (RNA) sequencing.
Materials and methods
Cells and FA
Human ovarian cancer cell lines SK-OV-3 (catalog number: CL-0215, Procell, Wuhan, China) and OVCAR3 (catalog number: CL-0178, Procell) were cultured according to the instructions provided by the supplier. All the cell lines were subjected to mycoplasma testing and short-tandem repeat identification. FA was obtained from Nongda Fertilizer Company (C176008, purity: > 95%, Tai’an, China) and dissolved in phosphate-buffered saline (PBS) to prepare the stock solution.
Small interfering RNA and overexpressing plasmid
Small interfering RNA (siRNA) targeting cytochrome P450 family 1 subfamily A member 1 (CYP1A1) (siCYP1A1, catalog number: stB0001414A, target sequence: CTGCAACGGGTGGAATTCA, RiboBio, Guangzhou, China) and negative control siRNA (siNC, catalog number: siN0000001-1-5, RiboBio) were synthesized. The coding sequence of CYP1A1 transcript variant 1 (NM_000499) was amplified using primers CYP1A1-BamHI-F (5’-cgcggatccgccaccATGCTTTTCCCAATCTCCATGTCGGCCAC-3’) and CYP1A1-XhoIR (5’-ccgctcgagCTAAGAGCGCAGCTGCATTTGGAAGTGCTCAC-3’). Amplification products were cloned into pcRNA3.1 to construct a CYP1A1-overexpressing plasmid (CYP1A1-OE).
Groups
To analyze the half-maximal inhibitory concentration (IC50) of FA, cells were cultured in culture medium containing 0, 25, 50, 100, 200, 300, 400, 500, and 1000 μg/mL of FA. To evaluate the effects of FA, SK-OV-3 and OVCAR3 cells were cultured in media with either 0 μg/mL (control) or 100 μg/mL of FA. To explore the role of CYP1A1, the cells were divided into four distinct experimental groups: pcDNA3.1 plasmid transfection (pcDNA), CYP1A1-OE transfection (CYP1A1-OE), FA treatment + siNC transfection (FA + siNC), and FA treatment + siCYP1A1 transfection (FA + siCYP1A1). Cells were transfected using Lipofectamine 2000 (11,668,030, Invitrogen, Carlsbad, CA, USA). The final amounts of plasmid DNA used in transfection were 100 ng per well for the 96-well plate, 500 ng per well for the 24-well plate, and 2500 ng per well for the 6-well plate. Additionally, the final concentration of siRNA was set to 50 nM.
Cell counting kit-8 assay
To determine the IC50 of FA, cells were seeded into 96-well plates at a density of 3000 cells per well and treated with various concentrations of FA (0, 25, 50, 100, 200, 300, 400, 500, and 1000 μg/mL) for 24 h. To analyze the effects of FA or CYP1A1 expression on cell proliferation, cells were also cultured in a 96-well culture plate (3000 cells/well) and exposed to different treatments for 24, 48, and 72 hous. Following treatment, 10 μL of Cell Counting Kit-8 solution (CK04, Dojindo, Kumamoto, Japan) was added to the culture medium. Following a 2-h incubation period, absorbance at 450 nm (A450) was measured using a Multiskan MK3 spectrophotometer (Thermo Scientific, Waltham, MA, USA). Cell viability was calculated based on the A450 value, and the IC50 was calculated using GraphPad Prism version 9 (GraphPad software, San Diego, CA, USA) according to the instructions available at https://www.graphpad.com/guides/prism/latest/curve-fitting/reg_absolute_ic50.htm.
Calculation of cell population doubling time
The population doubling time of the cells in each group was calculated using the A450 values at 24 h (A24) and 72 h (A72) measured in the CCK-8 assay. The calculation formula is as follows: .
Transwell migration and invasion assays
Cells from various treatment groups were resuspended in serum-free culture medium, with 3 × 104 cells in 200 μL of medium plated into in the upper chamber of a transwell insert (CLS3422, Corning Costar, Corning, NY, USA). For assessing cell invasion, the upper chamber was precoated with Matrigel matrix (354,230, Corning, Bedford, MA, USA). The lower chamber was filled with 600 μL of culture medium supplemented with 10% fetal bovine serum (35-081-CV, Corning). After a 24-h incubation period, the culture medium in the upper chamber was removed, and the cells adhering to the upper surface (those that did not pass through the polyester membrane) were removed. The chamber was then washed with PBS, fixed by immersion in 4% paraformaldehyde for 20 min, and stained with crystal violet dye for 10 min. Subsequently, the cells that had migrated through the polyester membrane were visualized under a microscope. Four distinct fields were captured, and the average number of cells in these fields was recorded as the measure of migration or invasion.
Illumina RNA sequencing and differentially expressed gene analysis
SK-OV-3 cells (1 × 106) were treated with or without 100 μg/mL of FA for 48 h and harvested for RNA sequencing analysis. RNA extraction, complementary DNA (cDNA) library construction, and RNA sequencing were conducted by Beijing Biomarker Biological Technology Co., Ltd (Beijing, China). Once the library was verified for quality, sequencing was performed using the PE150 mode on the Illumina NovaSeq 6000 platform (Illumina Inc., San Diego, CA, USA). After obtaining the sequencing data, bioinformatics analysis was performed using the tools and procedures provided by BMKCloud (www.biocloud.net). Differential gene expression was determined based on a fold change of ≥ 2, which was set as the criterion for identifying significant gene differences.
Fluorescent reverse transcription quantitative polymerase chain reaction (qPCR)
Total RNA was isolated using Trizol (15596026CN, Invitrogen, Carlsbad, CA, USA). After RNA extraction, cDNA was synthesized using EasyScript First-Strand cDNA Synthesis SuperMix (AE301-02, TransGen Biotech, Beijing, China). Subsequently, fluorescent quantitative PCR was performed using AceQ qPCR synergetic binding reagent (SYBR) Green Master Mix (Q111-02, Vazyme, Nanjing, China) to measure gene expression levels. The relative RNA expression levels of CYP1A1, secreted and transmembrane 1 (SECTM1), SH3 domain binding glutamate rich protein (SH3BGR), keratin 81 (KRT81), ankyrin repeat domain 1 (ANKRD1), and olfactomedin like 3 (OLFML3) were calculated using the 2(−ΔΔCT) method, with 18S ribosomal RNA as the reference gene. The primer sequences used for the PCR are detailed in Table 1.
Table 1.
Primer sequence in fluorescent quantitative reverse transcription polymerase chain reaction
| Primer name | Primer sequence (5'–3') |
|---|---|
| CYP1A1-F | TGGGCAAGCGGAAGTGTAT |
| CYP1A1-R | AGGCATGCTTCATGGTTAGC |
| SECTM1-F | ACAAGTCACGCTGGAGGTTT |
| SECTM1-R | CCTGTACCAGGCGAACATGA |
| SH3BGR-F | CCAGACTCAAAGGGATCAGA |
| SH3BGR-R | GGCTATTTCTTCCGTCTCTTC |
| KRT81-F | AATGAGCTGAACCGCATGAT |
| KRT81-R | CCAATGCCTTCACATAGCCT |
| ANKRD1-F | GAGTGCGCGGAGCATCTTAT |
| ANKRD1-R | TTGAGATCCGCGCCATACA |
| OLFML3-F | GCATCCAGTGCTCCTTTGA |
| OLFML3-R | AGCCATCATCCCAGGCATA |
| 18S-F | CCTGGATACCGCAGCTAGGA |
| 18S-R | GCGGCGCAATACGAATGCCCC |
R: reverse primer; F: forward primer; CYP1A1: cytochrome P450 family 1 subfamily A member 1; SECTM1: secreted and transmembrane 1; SH3BGR: SH3 domain binding glutamate rich protein; KRT81: keratin 81; ANKRD1: ankyrin repeat domain 1; OLFML3: olfactomedin like 3; 18S: 18S ribosomal RNA
Western blotting
Total protein from cells in the pcDNA, CYP1A1-OE, FA + siNC, and FA + siCYP1A1 groups was extracted and quantified. A sample of 30 µg of protein was separated using sodium dodecyl sulfate–polyacrylamide gel electrophoresis. The proteins were then transferred to a polyvinylidene difluoride membrane (Catalog No. 03010040001, Roche, Basel, Switzerland). To block non-specific binding, the membrane was incubated with 5% non-fat dried milk, followed by probing with a diluted goat polyclonal antibody to CYP1A1 (1:500; ab126887, Abcam, Cambridge, MA, USA) or goat polyclonal antibody to glyceraldehyde-3-phosphate dehydrogenase (GAPDH; 1:2000; ab157156, Abcam). After washing with Tris-buffered saline containing 0.1% Tween 20 (TBST), the membrane was incubated with a diluted horseradish peroxidase (HRP)-conjugated rabbit F(ab')2 anti-goat IgG (H + L) secondary antibody (1:5000; 6020–05, Southern Biotech, Birmingham, AL, USA). Following additional TBST washes, HRP activity was detected using the SuperSignal™ West Pico PLUS Chemiluminescence Substrate (Catalog No. 34577, Thermo Scientific, Rockford, IL, USA). The resulting chemiluminescent signals were captured on X-ray film for analysis.
Statistical analysis
Data were analyzed using GraphPad Prism version 9.0.0 (GraphPad Software). To evaluate statistical significance between two groups, an unpaired t-test was employed. A one-way analysis of variance was used for comparisons among multiple groups. A p-value less than 0.05 was deemed statistically significant.
Results
IC50 of FA
To investigate the impact of FA on ovarian cancer cells, we first evaluated the viability of OVCAR3 and SK-OV-3 cells after treatment with various concentrations of FA. At 25 and 50 μg/mL, FA exhibited minimal inhibitory effects on either OVCAR3 or SK-OV-3 cells, as illustrated in Fig. 1a, b. However, at concentrations ranging from 100 to 1000 μg/mL, FA markedly reduced cell viability in both cell lines, as demonstrated in Fig. 1a, b. The calculated IC50 of FA at 24 h for OVCAR3 cells was 689.9 μg/mL and for SK-OV-3 cells was 752.0 μg/mL, as shown in Fig. 1c, d
Fig. 1.
Determination of half-maximal inhibitory concentration (IC50) of fulvic acid (FA). a, b The viability of OVCAR3 and SK-OV-3 cells following treatment with FA at various concentrations, including 25, 50, 100, 200, 300, 400, 500, and 1000 μg/mL. c, d The fitting curve used to determine the IC50 is calculated with GraphPad Prism version 9.0.0
Effect of FA on cell proliferation, migration, and invasion
To explore the role of FA in ovarian cancer cells, we investigated its effects on the proliferation, migration, and invasion of OVCAR3 and SK-OV-3 cells. To maintain cell viability during FA treatment, we chose a concentration of 100 μg/mL, at which the cell survival rate was over 80%. FA treatment for 24, 48, and 72 h reduced cell proliferation rate in both cell lines (Fig. 2a, b). Calculation revealed that FA treatment prolonged the population doubling time of OVCAR3 (from 42.4 h to 48.8 h) and SK-OV-3 (from 41.1 h to 46.8 h) cells. Additionally, FA treatment significantly decreased the number of migrated or invaded cells in Transwell cell migration and invasion assays (Fig. 2c–f). These results demonstrate that 100 μg/mL FA treatment can effectively suppress cell growth and metastasis capability of ovarian cancer cells in vitro.
Fig. 2.
Treatment with fulvic acid (FA) inhibits proliferation, migration, and invasion. a, b The Cell Counting Kit-8 (CCK-8) assay is utilized to assess cell proliferation. c–f Cell migration and invasion are evaluated through transwell migration and invasion assays. c, e show representative images for each group. d, f present the statistical results for the number of migrated and invaded cells. *indicates a p-value of < 0.05
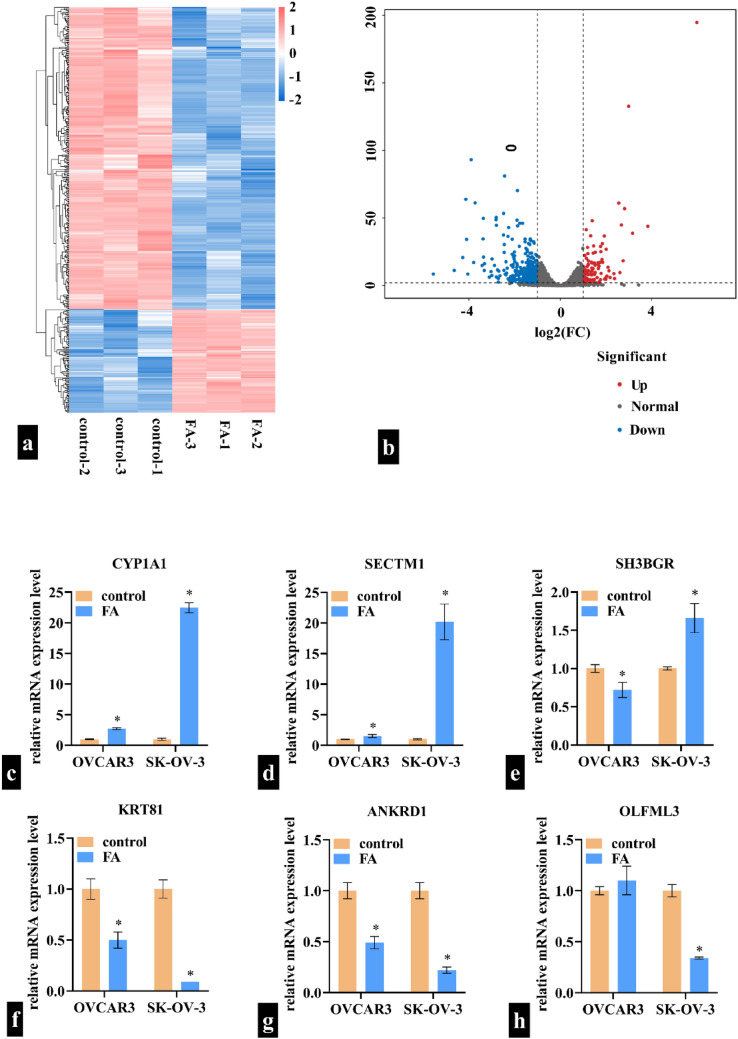
CYP1A1 is differentially expressed following FA treatment
To explore the regulatory mechanism of FA, differentially expressed mRNAs were identified using Illumina RNA sequencing. The sequencing data have been submitted to the Gene Expression Omnibus under accession number GSE253366. In total, 117 mRNAs were upregulated and 342 mRNAs were downregulated in FA-treated SK-OV-3 cells. The details of all the differentially expressed mRNAs are shown in Supplementary File 1. The heat map and volcano plot of differentially expressed genes are shown in Fig. 3a, b respectively. We also assessed how FA influenced the expression levels of the top three upregulated and the top three downregulated mRNAs in both OVCAR3 and SK-OV-3 cells. In OVCAR3 cells, FA upregulated CYP1A1 and SECTM1, downregulated SH3BGR, KRT81, and ANKRD1, and exhibited no effect on OLFML3. In SK-OV-3 cells, FA upregulated CYP1A1, SECTM1, and SH3BGR and downregulated KRT81, ANKRD1, and OLFML3 (Fig. 3c–h). Notably, CYP1A1 showed the most significant change in both cell lines. Therefore, we further analyzed the role of CYP1A1 in ovarian cancer cells to confirm whether FA exerts its effects through regulating CYP1A1.
Fig. 3.
Differentially expressed mRNAs induced by fulvic acid (FA) treatment are identified using Illumina RNA sequencing. a Heat map of differentially expressed gene clusters. b Volcano map of differentially expressed genes. c–h: Expression levels of CYP1A1, SECTM1, SH3BGR, KRT81, ANKRD1, and OLFML3 after FA treatment. *indicates a p-value of < 0.05
Effect of CYP1A1 overexpression on cell proliferation, migration, and invasion
To explore the role of CYP1A1, we constructed CYP1A1-OE and transfected it into OVCAR3 and SK-OV-3 cells. As shown in Fig. 4a, b, the protein level of CYP1A1 was significantly higher in the CYP1A1-OE group compared to the pcDNA group, indicating that CYP1A1 was successfully overexpressed in OVCAR3 and SK-OV-3 cells. After transfection with CYP1A1-OE for 24, 48, and 72 h, we observed decreased cell proliferation rate in both OVCAR3 and SK-OV-3 cells compared to that in the cells transfected with pcDNA (Fig. 5a, b). The transfection of CYP1A1-OE prolonged the population doubling time of OVCAR3 (from 40.2 h to 50.6 h) and SK-OV-3 (from 43.8 h to 62.5 h) cells. Additionally, CYP1A1-OE transfection reduced the number of migrated or invaded cells in Transwell cell migration and invasion assays (Fig. 5c–f). These findings indicate that overexpression of CYP1A1 can suppress the cell growth and metastasis capability of ovarian cancer cells in vitro.
Fig. 4.

Cytochrome P450 family 1 subfamily A member 1 (CYP1A1) expression levels. a Western blot image is shown. b Statistical analysis of CYP1A1 expression, normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH), is provided. CYP1A1-OE: CYP1A1 overexpression plasmid; pcDNA: empty vector pcDNA3.1; siCYP1A1: siRNA targeting CYP1A1; siNC: negative control siRNA; FA: fulvic acid
Fig. 5.
Cytochrome P450 family 1 subfamily A member 1 (CYP1A1 overexpression inhibits proliferation, migration, and invasion. CYP1A1 overexpression plasmid (CYP1A1-OE) and empty vector pcDNA3.1 (pcDNA) are transfected into OVCAR3 and SK-OV-3 cells, respectively. a, b Cell proliferation is measured via the Cell Counting Kit-8 assay. c–f Cell migration and invasion are measured using transwell migration and invasion assays. Panels c and e show representative images for each group. d, f present the statistical results for the number of migrated and invaded cells. *indicates a p-value of < 0.05
CYP1A1 knockdown alleviates the effects of FA
To further verify the role of CYP1A1 in the regulatory effect of FA, we transfected cells with siCYP1A1 to block the FA-induced upregulation of CYP1A1. As shown in Fig. 4a, b, the CYP1A1 protein level was significantly lower in the FA + siCYP1A1 group compared to the FA + siNC group, indicating that CYP1A1 was successfully knocked down in FA-treated OVCAR3 and SK-OV-3 cells. In both OVCAR3 and SK-OV-3 cells, the cell proliferation rate was increased in the FA + siCYP1A1 group compared to the FA + siNC group (Fig. 6a, b). Transfection with siCYP1A1 shortened the population doubling time of FA-treated OVCAR3 (from 55.0 to 43.0 h) and SK-OV-3 (from 58.9 to 42.0 h) cells. In addition, siCYP1A1 transfection increased the number of migrated (Fig. 6c, d) and invaded (Fig. 6e, f) cells in FA-treated OVCAR3 and SK-OV-3 cells in Transwell migration and invasion assays. These findings suggest that the knockdown of CYP1A1 mitigated the inhibitory effects of FA on cell growth and metastasis.
Fig. 6.
Cytochrome P450 family 1 subfamily A member 1 (CYP1A1) knockdown alleviates the effects of fulvic acid (FA) on proliferation, migration, and invasion. siRNA targeting CYP1A1 (siCYP1A1) and negative control siRNA (siNC) are transfected into the FA-treated OVCAR3 and SK-OV-3 cells, respectively. a, b Cell proliferation is measured via the Cell Counting Kit-8 assay. c–f Cell migration and invasion are measured using transwell migration and invasion assays. Panels c and e show representative images for each group. d, f present the statistical results for the number of migrated and invaded cells. * indicates a p-value of < 0.05
Discussion
The findings of this study demonstrated that FA effectively inhibited the proliferation, migration, and invasion of SK-OV-3 and OVCAR3 ovarian cancer cells. To our knowledge, this is the first study to document the antitumor effects of FA in the context of ovarian cancer. These results establish a crucial experimental foundation for considering FA as a potential therapeutic agent for ovarian tumors.
Currently, studies on the roles of FA in cancers are still limited, with the cancer types involved including NSCLC, colorectal cancer, hepatic cancer, leukemia, prostate cancer, and human melanoma [11–14, 20]. These studies and our current study, using in vitro cell experiments or animal models, only suggest the potential antitumor effects of FA, and its clinical translation value requires cautious evaluation. Firstly, while cell experiments are a necessary starting point for drug development (such as screening effective concentrations and exploring mechanisms), they cannot simulate the complex pharmacokinetics processes in the human body (such as metabolism, absorption, distribution, and excretion). This may lead to significant differences between the effective concentrations in vitro and the actual clinical needs. Therefore, conclusions drawn at the cellular level must be further validated and supplemented through animal experiments and clinical trials. Secondly, only one study has reported the antitumor effect of FA in an NSCLC animal model. In A549 cell xenograft nude mice, FA at doses of 25, 50, and 100 mg/kg significantly inhibited tumor growth [14]. Although this finding provides preliminary support for the antitumor activity of FA, more independent experiments are needed to verify its universality and dose–effect relationship.
Exploring the underlying molecular regulatory mechanisms is essential to fully illustrate drug effects. To comprehensively understand the regulatory mechanism of FA in ovarian cancer, we analyzed the differentially expressed mRNAs using Illumina RNA sequencing. These findings offer a valuable resource for researchers investigating the regulatory mechanism of FA. Our results showed that FA upregulated CYP1A1 mRNA expression. In addition, CYP1A1 knockdown attenuated the inhibitory effects of FA. These results suggest that FA exerts its antitumor effects through CYP1A1 regulation. However, FA can also induce the abnormal expression of other genes. Therefore, it is certain that other mechanisms underlie the antitumor effects of FA in ovarian cancer.
The CYP1A1 gene encodes a protein that belongs to the cytochrome P450 superfamily of enzymes. These cytochrome P450 enzymes are monooxygenases that play key roles in drug metabolism as well as the synthesis of cholesterol, steroids, and other lipids [21–23]. It is increasingly recognized that CYP1A1 plays a crucial role in detoxifying environmental carcinogens and in the metabolic activation of compounds that have cancer-preventive properties [24]. In addition, polymorphisms in CYP1A1 are associated with the risk of numerous cancers, such as esophageal, colorectal, and cervical cancer [25–28]. In this study, we found that overexpression of CYP1A1 suppresses cell proliferation, migration, and invasion in the OVCAR3 and SK-OV-3 cells. These results suggest that CYP1A1 may act as a tumor suppressor gene in the development of ovarian cancer. CYP1A1 could be a potential target for molecular targeted therapy in ovarian cancer. The role of CYP1A1 in ovarian cancer still needs to be further verified through clinical and animal studies.
Our current study has several limitations. First, we did not conduct in vivo studies to confirm the potential antitumor effects of FA in ovarian cancer. Second, we did not perform a dose–response analysis to fully explore the range of the effects of FA. Third, our research was limited to only two ovarian cancer cell lines, leaving the impact of FA on other tumor types unexplored. Lastly, while we focused on the relationship between FA and CYP1A1, this alone is insufficient to fully elucidate the regulatory mechanisms of FA. Future research should aim to identify potential signaling pathways and gene regulation networks associated with FA.
Conclusions
FA inhibited cell proliferation, migration, and invasion, while enhancing the expression of CYP1A1 in SK-OV-3 and OVCAR3 cells. Our study indicated that FA exerts antitumor effects in ovarian cancer by regulating CYP1A1. These insights provide a new experimental foundation for exploring FA as a potential therapeutic option in the treatment of ovarian cancer. Future research we will focus on further elucidating the molecular mechanisms of FA, conducting systematic animal experiments and integrating pharmacokinetic studies to optimize drug administration, ultimately providing evidence for its clinical application in cancer treatment.
Supplementary Information
Acknowledgements
None.
Author contributions
M. C., Mg.Cg., Y.T. and F.D. designed the experiments. M.C., and Mg. Cg. performed the experiments. C.S. and W. L. contributed data collection and analysis. M. C. and Mg.Cg. wrote the draft manuscript. Y.T. and F.D. critically revised the manuscript. All authors read and approved the final manuscript.
Funding
None.
Data availability
Sequence data that support the findings of this study have been deposited in the Gene Expression Omnibus with the primary accession number GSE253366.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Min Chen and Ming Cheng contributed equally to this study.
Contributor Information
Yi Tang, Email: tyck288@sdau.edu.cn.
Fangjun Ding, Email: dfj401@163.com.
References
- 1.Olk DC, et al. Environmental and agricultural relevance of humic fractions extracted by alkali from soils and natural waters. J Environ Qual. 2019;48(2):217–32. [DOI] [PubMed] [Google Scholar]
- 2.Hriciková S, et al. Humic substances as a versatile intermediary. Life. 2023;13(4):858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Song C, et al. Applying fulvic acid for sediment metals remediation: Mechanism, factors, and prospect. Front Microbiol. 2022;13:1084097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu JG, et al. Structure analysis of the fulvic acids extracted from composted corn stalk residue. Chin J Analytical Chem. 1999;27:933–7. [Google Scholar]
- 5.Tang WW, et al. Impact of humic/fulvic acid on the removal of heavy metals from aqueous solutions using nanomaterials: a review. Sci Total Environ. 2014;468–469:1014–27. [DOI] [PubMed] [Google Scholar]
- 6.Fan L, et al. Photoinduced reduction of high concentration hg(ii) to hg(2)cl(2) from acid wastewater with the presence of fulvic acid under anaerobic conditions. Chemosphere. 2018;198:13–20. [DOI] [PubMed] [Google Scholar]
- 7.Feng P, et al. Effects of fulvic acid on growth performance, serum index, gut microbiota, and metabolites of xianju yellow chicken. Front Nutrit. 2022;9:963271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ozfidan-Konakci C, et al. The humic acid-induced changes in the water status, chlorophyll fluorescence and antioxidant defense systems of wheat leaves with cadmium stress. Ecotoxicol Environ Safet. 2018;155:66–75. [DOI] [PubMed] [Google Scholar]
- 9.Winkler J, et al. Therapeutic potential of fulvic acid in chronic inflammatory diseases and diabetes. J Diabet Res. 2018;2018:5391014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu C, et al. Fulvic acid attenuates atopic dermatitis by downregulating ccl17/22. Molecules. 2023;28:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang WS, et al. Fulvic acid attenuates resistin-induced adhesion of hct-116 colorectal cancer cells to endothelial cells. Int J Mol Sci. 2015;16(12):29370–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jayasooriya R, et al. Fulvic acid promotes extracellular anti-cancer mediators from raw 2647 cells, causing to cancer cell death in vitro. Int Immunopharmaco: 2016;36:241–8. [DOI] [PubMed] [Google Scholar]
- 13.Salehi M, et al. Activation of apoptosis and g0/g1 cell cycle arrest along with inhibition of melanogenesis by humic acid and fulvic acid: Bax/bcl-2 and tyr genes expression and evaluation of nanomechanical properties in a375 human melanoma cell line. Iran J Basic Med Sci. 2022;25(4):489–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xin P, et al. Natural fulvic acids inhibit non-small-cell lung cancer through the cox-2/pge2/ep4 axis: In silico and in vivo assessments. Heliyon. 2023;9(6): e17080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuroki L, et al. Treatment of epithelial ovarian cancer. BMJ. 2020;371: m3773. [DOI] [PubMed] [Google Scholar]
- 16.Koushik A, et al. Hormonal and reproductive factors and the risk of ovarian cancer. Cancer Causes Control. 2017;28(5):393–403. [DOI] [PubMed] [Google Scholar]
- 17.Pullen RL Jr. Ovarian cancer. Nursing. 2024;54(6):17–28. [DOI] [PubMed] [Google Scholar]
- 18.Webb PM, et al. Epidemiology of epithelial ovarian cancer. Best Pract Res Clin Obstet Gynaecol. 2017;41:3–14. [DOI] [PubMed] [Google Scholar]
- 19.Arnaoutoglou C, et al. Epithelial ovarian cancer: a five year review. Medicina. 2023;59(7):1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pant K, et al. Anti-proliferative and anticancer properties of fulvic acid on hepatic cancer cells. J Clin Exp Hepatol. 2015;5:S2. [Google Scholar]
- 21.Tervahauta T, et al. Metabolism of ropinirole is mediated by several canine cyp enzymes. Veterin Med Sci. 2023;9(4):1584–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Surichan S, et al. Tangeretin inhibits the proliferation of human breast cancer cells via cyp1a1/cyp1b1 enzyme induction and cyp1a1/cyp1b1-mediated metabolism to the product 4’ hydroxy tangeretin. Toxicol Vitro. 2018;50:274–84. [DOI] [PubMed] [Google Scholar]
- 23.Guengerich FP, et al. Recent structural insights into cytochrome p450 function. Trends Pharmacol Sci. 2016;37(8):625–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Androutsopoulos VP, et al. Cytochrome p450 cyp1a1: Wider roles in cancer progression and prevention. BMC Cancer. 2009;9:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaya Z, et al. Association between cyp1a1 polymorphisms and esophageal cancer susceptibility: a case-control study. In Vivo. 2023;37(2):868–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng T, et al. Polymorphisms in pah metabolising enzyme cyp1a1 in colorectal cancer and their clinicopathological correlations. Pathol Res Pract. 2022;231:153801. [DOI] [PubMed] [Google Scholar]
- 27.Barek MA, et al. Assessment of the association of cyp1a1 gene polymorphisms with the susceptibility of cervical cancer: a case-control study and meta-analysis. Heliyon. 2023;9(7): e17712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rajagopal T, et al. Impact of xenobiotic-metabolizing gene polymorphisms on breast cancer risk in south indian women. Breast Cancer Res Treat. 2021;186(3):823–37. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Sequence data that support the findings of this study have been deposited in the Gene Expression Omnibus with the primary accession number GSE253366.