Abstract
Introduction
Recurrent Clostridioides difficile infection (rCDI) is common, with symptoms ranging from diarrhea to life-threatening sepsis. This study aimed to assess the real-world outcomes of patients with rCDI in the United States (US) who received fecal microbiota, live-jslm (RBL), the first US Food and Drug Administration-approved microbiota-based therapy for the prevention of rCDI after antibiotic treatment.
Methods
Adults with rCDI who received RBL between July 2023 and August 2024 at home or in a clinic and had ≥ 8 weeks of follow-up or experienced CDI recurrence at any time after RBL administration were included. Treatment success, defined as no CDI recurrence within 8 weeks of RBL, was assessed overall and in subgroups stratified by age, number of prior CDI recurrences, duration of the antibiotic washout period, prior bezlotoxumab use, and RBL administration setting.
Results
Among 196 patients who received RBL, 176 had either ≥ 8 weeks of follow-up or had < 8 weeks of follow-up but experienced CDI recurrence during that period. The treatment success rate at 8 weeks was 83.0%. No significant differences were observed in treatment success rates among subgroups based on age (< 65 years old vs. ≥ 65 years old: 85.9% vs. 80.2%, p = 0.20), duration of the antibiotic washout period (24 h: 80.0%, 48 h: 84.5%, 72 h: 85.0%, p = 0.68), number of prior CDI recurrences (< 3 vs. ≥ 3: 82.5% vs. 83.1%, p = 0.60), or prior bezlotoxumab use (86.4% vs. 83.7%, p = 1.00). Patients receiving RBL at home had a higher treatment success rate compared to those receiving RBL in a clinic (87.3% vs. 62.5%, p < 0.01).
Conclusions
RBL was highly effective in preventing rCDI in a real-world setting, including at-home administration. The effectiveness was also observed among high-risk subgroups, such as patients ≥ 65 years old and those with ≥ 3 prior CDI recurrences.
Keywords: Fecal microbiota live-jslm, RBL, Recurrent Clostridioides difficile infection
Key Summary Points
| Why carry out this study? |
| Up to 35% of patients with Clostridioides difficile infection (CDI) treated with antibiotics for an initial episode will experience a recurrence, and among them, up to 65% will experience multiple recurrences. |
| Although effective in clinical trials, there is limited real-world evidence on the effectiveness of fecal microbiota, live-jslm (RBL), which is a first-in-class microbiome-based therapy approved for the prevention of recurrent CDI (rCDI) in adult patients following antibiotic treatment for rCDI. |
| What was learned from the study? |
| This retrospective study showed that RBL was 83.0% effective in preventing rCDI at 8 weeks in a real-world setting. |
| High treatment success rates were consistently observed among high-risk patient subgroups, including patients 65 years and older and those with three or more prior recurrences. |
| Findings indicate that RBL is effective in preventing rCDI, including among high-risk patient groups and regardless of whether it was administered at home or in a clinic. |
Introduction
Clostridioides difficile is an anaerobic Gram-positive, spore-forming, toxin-producing bacillus transmissible through the fecal–oral route that causes an infection of the large intestine [1]. The clinical presentation of Clostridioides difficile infection (CDI) ranges from mild diarrhea to severe debilitating disease, including life-threatening damage to the colon [2]. CDI, the most common healthcare-related infection in the United States (US), has a crude overall incidence rate of 121.2 cases per 100,000 persons [3].
The current standard of care for CDI recommended by the Infectious Disease Society of America and the Society for Healthcare Epidemiology of America involves the use of antibiotics, such as vancomycin and fidaxomicin [4, 5]. However, antibiotics disrupt the gut microbiota, increasing the risk for further recurrences. Up to 35% of patients treated for an initial episode of CDI experience at least one recurrence [6–9]. The risk of additional recurrences increases after the first and can be as high as 65% for three or more CDI episodes [6, 10]. Recurrent CDI (rCDI) often results in hospitalization and can lead to poorer outcomes, including sepsis, toxic megacolon, and death [11]. As such, rCDI poses a significant treatment challenge.
Fecal microbiota, live-jslm (REBYOTA® [RBL]; Ferring Pharmaceuticals Inc.), is a first-in-class microbiome-based therapy that was approved in 2022 by the US Food and Drug Administration (FDA) for the prevention of rCDI in adults following a washout period of 24–72 h after antibiotic treatment for rCDI [12]. The PUNCH CD3 clinical trial demonstrated that RBL was safe and effective in preventing the recurrence of CDI, showing 70.6% treatment success (defined as the absence of CDI-related diarrhea at 8 weeks) with RBL versus 57.5% with placebo [13]. Furthermore, 92.1% of patients who were successfully treated at 8 weeks remained free of CDI recurrence for 6 months [13].
While clinical trial data demonstrate the clear benefits of RBL in preventing rCDI, there is limited evidence on its effectiveness in non-clinical trial settings. Prior studies only evaluated the effectiveness of RBL administered in select outpatient settings and included small sample sizes [14–16]. To that end, this retrospective cohort study aimed to describe the demographic and clinical characteristics, prior medication use, prior healthcare resource utilization (HRU), and treatment success among patients with rCDI in the US who received RBL in a real-world setting, including at-home administration. Treatment success was also assessed among select high-risk patient subgroups.
Methods
Population and Setting
This retrospective cohort study analyzed de-identified data from adult patients with rCDI who received RBL through a network of infusion centers (i.e., Paragon Healthcare) either at home or in a clinic in the US. Paragon Healthcare offers a wide range of services, including home infusion therapy, infusion center site-of-care operations, specialty pharmaceuticals, and rare disease care. For home-based administration, the treatment may be delivered by either Paragon-affiliated nurses or nurses from an outside agency, depending on the location and availability. The analyses were conducted among patients with rCDI who received RBL between July 20, 2023 and August 26, 2024 (data cut-off date), and who had a minimum of 8 weeks of follow-up from the date of RBL administration to the data cut-off date or experienced a recurrence of CDI at any time after RBL administration. The study was considered exempt research under 45 CFR § 46.104(d)(4) as it involved only the secondary use of data that were de-identified in compliance with the Health Insurance Portability and Accountability Act (HIPAA), specifically, 45 CFR § 164.514.
Study Variables
Demographic and clinical data, including age, sex, geographic region, insurance type, RBL administration setting (i.e., home vs. clinic), and number of prior CDI recurrences, were collected at the time of RBL administration. Data on medication use prior to the current RBL administration were collected at the time of RBL administration, including on the list of antibiotics used and antibiotic washout period and prior use of gastric acid suppressants, fecal microbiota transplantation (FMT), RBL, and bezlotoxumab. HRU data included hospitalizations and emergency room visits related to prior CDI episodes.
The study’s outcome of interest was treatment success, which was defined as the absence of CDI recurrence within 8 weeks of RBL administration, consistent with the definition used in the PUNCH CD3 clinical trial [13]. Treatment success was evaluated at approximately 8 weeks post-treatment and assessed for the overall population, as well as within specific subgroups: RBL administration setting (home vs. clinic), age group (< 65 years vs. ≥ 65 years), duration of prior antibiotic washout (24, 48, or 72 h), number of prior CDI recurrences (< 3 or ≥ 3), and prior use of bezlotoxumab.
Statistical Analysis
Patient demographics, clinical characteristics, prior medication use, and prior HRU were summarized descriptively. Continuous variables were presented as medians and interquartile ranges (IQRs), while categorical variables were summarized using counts and percentages. Treatment success rates were reported as proportions and compared within each subgroup using chi-squared (or Fisher’s exact tests where the sample size was small). No analyses of treatment success rates adjusting for patient characteristics were conducted in this study. All statistical analyses were performed using R version 4.4.1.
Results
Study Population
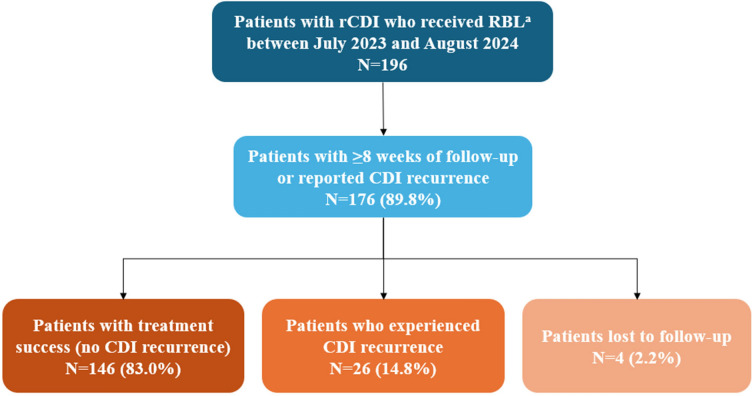
A total of 196 patients received RBL between July 2023 and August 2024 (Fig. 1). Of these, 176 patients were included in the analysis because they either had at least 8 weeks of follow-up or had less than 8 weeks of follow-up but experienced a CDI recurrence following RBL administration. The median age of patients was 65.5 years (IQR: 52.0, 77.0), and the majority (71.6%) were women (Table 1). More than half of patients were insured either through commercial or private insurance (54.0%) and 44.3% were insured through Medicare. RBL was primarily administered in a home setting (80.7%). More than three-quarters of patients had experienced three or more prior CDI recurrences before receiving RBL (77.3%).
Fig. 1.
Sample selection and treatment success of patients with rCDI who received RBL. CDI Clostridioides difficile infection, RBL fecal microbiota, live-jslm, rCDI recurrent Clostridioides difficile infection. aRBL was administered by healthcare professionals affiliated with Paragon Healthcare
Table 1.
Patient demographic and clinical characteristics
| Study sample | |
|---|---|
| N = 176 | |
| Demographic characteristics | |
| Age (years), median (IQR) | 65.5 (52.0, 77.0) |
| Women, n (%) | 126 (71.6) |
| Geographic region, n (%) | |
| South | 100 (56.8) |
| West | 43 (24.4) |
| Northeast | 17 (9.7) |
| Midwest | 16 (9.1) |
| Patient insurance type, n (%) | |
| Commercial/private insurance | 95 (54.0) |
| Medicare | 78 (44.3) |
| Medicaid | 3 (1.7) |
| Administration setting, n (%) | |
| Home | 142 (80.7) |
| Clinic | 32 (18.2) |
| Missing | 2 (1.1) |
| Clinical characteristics | |
| Prior CDI recurrences, n (%) | |
| ≥ 3 prior CDI recurrences | 136 (77.3) |
| Prior medication use | |
| Antibiotic washout period, n (%) | |
| 24 h | 50 (28.4) |
| 48 h | 71 (40.3) |
| 72 h | 40 (22.7) |
| Other | 7 (4.0) |
| Missing | 8 (4.5) |
| Gastric acid suppressant use, n (%) | 26 (14.8) |
| Prior FMT, n (%) | 11 (6.3) |
| Missing | 7 (4.0) |
| Prior RBL, n (%) | 4 (2.3) |
| Missing | 7 (4.0) |
| Prior bezlotoxumab, n (%) | 22 (12.5) |
| Missing | 7 (4.0) |
| Prior HRU | |
| Prior hospitalizations due to CDI, n (%) | 57 (32.4) |
| Missing | 8 (4.5) |
| Prior ER visits due to CDI, n (%) | 60 (34.1) |
| Missing | 9 (5.1) |
CDI Clostridioides difficile infection, ER emergency room, FMT fecal microbiota transplantation, HRU healthcare resource utilization, IQR interquartile range, RBL fecal microbiota, live-jslm, rCDI recurrent Clostridioides difficile infection
Prior Medication Use and HRU
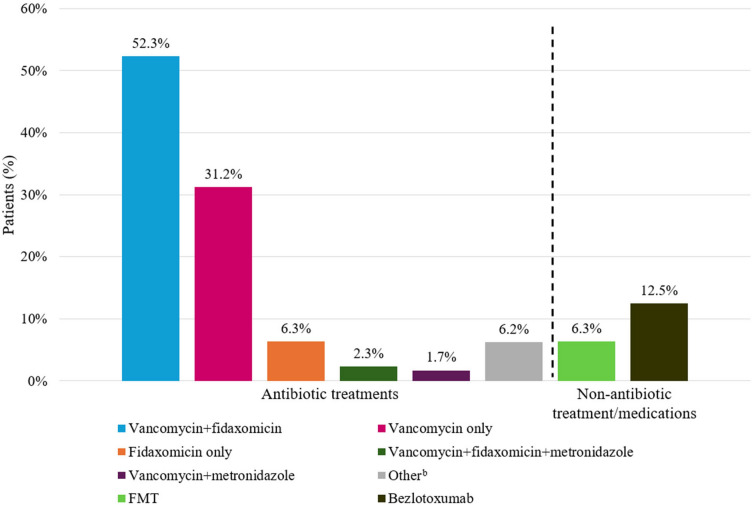
Prior to receiving RBL, the majority of patients had been treated with antibiotics for rCDI (Fig. 2). Specifically, 31.2% had used vancomycin only, 6.3% had used fidaxomicin only, 52.3% had used both vancomycin and fidaxomicin, and 2.3% had used vancomycin, fidaxomicin, and metronidazole. Most patients (91.4%) received RBL within 24–72 h following antibiotic treatment. Other prior treatments included gastric acid suppressants (14.8%), FMT (6.3%), RBL (2.3%), and bezlotoxumab (12.5%). Approximately a third of patients (32.4%) were previously hospitalized due to CDI and 34.1% of patients had prior emergency room visits due to CDI.
Fig. 2.
Treatments and medications received prior to RBL administrationa. CDI Clostridioides difficile infection, FMT fecal microbiota transplantation, RBL fecal microbiota, live-jslm. aAll treatments and medications were received for any prior CDI episodes; bother or missing antibiotic treatment history
Treatment Success
At week 8, the overall treatment success rate was 83.0%, 14.8% of patients experienced a CDI recurrence, and 2.2% of patients had missing CDI recurrence status (Fig. 3).
Fig. 3.
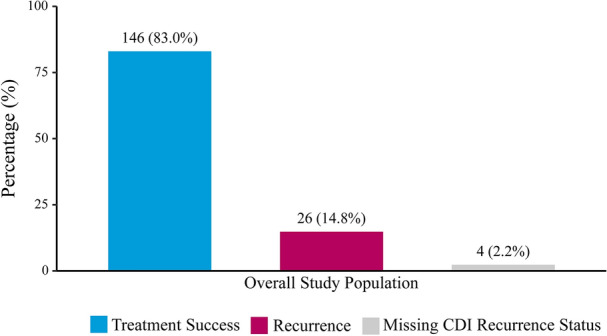
RBL treatment success rate in the overall population, n (%). CDI Clostridioides difficile infection, RBL fecal microbiota, live-jslm
Patients who received RBL at home had a higher treatment success rate compared to those who received RBL in a clinic (87.3% vs. 62.5%, p < 0.01, Fig. 4). No significant differences were observed in treatment success rates based on the age (< 65 years: 85.9%, ≥ 65 years 80.2%, p = 0.20), antibiotic washout period (24 h: 80.0%, 48 h: 84.5%, 72 h: 85.0%, p = 0.68), number of prior CDI recurrences (< 3 vs. ≥ 3: 82.5% vs. 83.1%, p = 0.60), or prior bezlotoxumab use (86.4% vs. 83.7%, p = 1.00).
Fig. 4.
RBL treatment success rate by subgroup, % (n). CDI Clostridioides difficile infection, RBL fecal microbiota, live-jslm
Discussion
This is the first real-world use study to assess the efficacy of RBL administered both at home and in clinics, expanding upon prior research that focused primarily on outpatient clinic administration, and including patients who could have been excluded from clinical trials. Additionally, this study included a larger sample size (N = 176), addressing the limitations of previous studies with fewer than 100 patients [14, 16]. Study patients exhibited diverse demographic and clinical characteristics, as well as varying prior medication use and HRU. The overall treatment success rate was 83.0% at 8 weeks following RBL administration. Subgroup analyses revealed consistently high treatment success rates regardless of age or the number of prior CDI recurrences. Factors such as prior treatments, including bezlotoxumab, or the duration of the antibiotic washout period, did not impact the treatment success rate of RBL. Patients who received RBL at home also achieved a high treatment success rate. These findings provide valuable real-world evidence supporting the effectiveness of RBL in preventing rCDI irrespective of treatment history, including among patients with high-risk characteristics and those who received RBL at home.
The high treatment success rate observed in this study is consistent with the results of prior studies conducted involving distinct yet smaller cohorts of patients with rCDI. For example, a retrospective analysis by Feuerstadt et al. of 64 patients who received RBL under enforcement discretion found that approximately 83% (n = 53) remained recurrence-free at 8 weeks following RBL administration, regardless of the number of doses received [14]. Among those who achieved treatment success at 8 weeks following administration, 88.7% (n = 47) experienced sustained clinical response through 6 months [14]. Similarly, a retrospective study of 67 patients who received RBL in a network of physicians’ offices showed that 78% (n = 52) maintained efficacy at 8 weeks after treatment [16]. In another recent two-center study, all 22 patients with rCDI who received RBL via colonoscopy achieved treatment success at 8 weeks, and among those who had 24 weeks of follow-up (n = 16), a clinical cure rate of 93.8% (15/16) was reported [15]. Although differences in eligibility criteria, study design, and study populations limit direct comparisons between these real-world studies and clinical trials, the treatment success rates observed in real-world settings appear comparable or higher than those in the phase III PUNCH CD3 trial [13].
The current study provides reassuring evidence that expands upon these prior findings by evaluating the effectiveness of RBL in key patient subgroups at high risk of poor outcomes, such as patients aged 65 years or older and patients with three or more prior CDI recurrences. Advanced age has been extensively documented as the most frequently reported risk factor for rCDI, with the likelihood of recurrence approximately doubling among patients aged 65 years or older relative to younger cohorts [17, 18]. Furthermore, the risk of rCDI has been consistently shown to increase with each subsequent recurrence, reaching up to 65% after three or more CDI episodes [6, 10]. This study provides important evidence showing that the RBL treatment success rate at 8 weeks was comparable between patients under 65 years and those aged 65 years or older (85.9% vs. 80.2%, p = 0.20), as well as between patients with fewer than three prior CDI recurrences and those with three or more (82.5% vs. 83.1%, p = 0.60). These findings highlight the effectiveness of RBL in preventing rCDI among high-risk populations.
Regarding the antibiotic washout period, patients who received RBL 24 h, 48 h, and 72 h after completing antibiotic treatment also had comparable treatment success rates (80.0%, 84.5%, and 85.0%, p = 0.68). This finding suggests that all patients receiving RBL within the indicated time frame after completion of antibiotics benefit similarly. Additionally, the study demonstrated that a high treatment success rate (87.3%) was achievable for patients who receive RBL in a home administration setting. However, these findings should be interpreted with caution as the results were not adjusted for baseline differences between patients who received RBL at home and those who received it in a clinic. Nevertheless, home administration of RBL stands to be a convenient and effective option for preventing rCDI.
CDI and rCDI continue to pose a significant healthcare burden due to their transmissibility and the associated clinical and economic impact on patients and the healthcare system [9, 10, 19, 20]. In line with the substantial burden previously documented, 32.4% of patients in this study had previously been hospitalized for CDI and 34.1% had previously visited the emergency room due to CDI before receiving RBL. These findings underscore the need for an effective treatment to prevent CDI recurrence and reduce HRU in this population. Although FMT can be administered under enforcement discretion for the prevention of rCDI, it is not an FDA-approved drug like RBL; instead, it is recognized by the FDA as an investigational medical procedure for severe and fulminant CDI [21]. As of November 2024, one of the largest providers of FMT temporarily paused distribution for non-severe CDI under FDA enforcement discretion, following recent guidance [21, 22]. In 2023, the FDA also approved the oral fecal microbiota spores, live-brpk (Vowst™; Seres Therapeutics), for the prevention of rCDI following antibiotic treatment [23]. Recently, a two-center study comparing the effectiveness of RBL via colonoscopy, conventional FMT, and Vowst showed a superior efficacy of RBL in preventing rCDI at 8 weeks [15]. Although more robust long-term data (e.g., extended follow-up beyond 8 weeks after RBL administration and long-term CDI recurrence status) are needed to better understand the long-term outcomes of RBL in real-world settings, the available evidence, along with findings from the present study, currently supports RBL as an effective treatment option for the prevention of rCDI across a variety of settings.
Limitations
The results of this study should be interpreted in light of certain limitations. First, only limited baseline patient characteristics were collected in the current data. For example, factors like the nature of previous rCDI episodes, patient comorbidities, and whether medications (e.g., antibiotics) observed prior to RBL administration were used for the current rCDI episode or previous CDI recurrences are not captured or characterized in the available data. To fully understand the impact of these characteristics on treatment outcomes, future studies utilizing real-world data with more comprehensive baseline patient characteristics are warranted. Second, the analyses in this study did not adjust for any external confounding factors and may be subject to confounding bias. Third, this study did not include a control arm, as data were only collected for patients who received RBL; future studies are warranted that incorporate a comparator arm and provide comparative effectiveness data on recurrence rates for available therapies. Fourth, given the non-randomized nature of this study, there is potential for selection bias. For example, we did not have further information on healthcare access across patients, so unknown differences between patients who choose to receive treatment at home versus in a clinic may have impacted the treatment outcomes. Finally, patients included in this study received RBL from healthcare professionals affiliated with a network of infusion centers, with the majority located in the South or West regions of the US. This may limit the generalizability of the findings to broader patient populations and other healthcare settings.
Conclusions
This retrospective study supports the effectiveness of RBL in preventing rCDI after 8 weeks of administration in real-world clinical practices, including among high-risk subgroups such as older patients and those with multiple prior recurrences. These findings are consistent with prior real-world studies of RBL and indicate that RBL can be effective when administered either at home or in a clinic for the prevention of rCDI, with the potential to reduce the substantial disease-related clinical and economic burden.
Acknowledgments
Medical Writing, Editorial and Other Assistance
Medical writing assistance was provided by professional medical writer Loraine Georgy, PhD, MWC, an employee of Analysis Group, Inc., which received payment from Ferring Pharmaceuticals, Inc. for participating in this research.
Author Contributions
Conceptualization and study design: Sahil Khanna, Sanghyuk Seo, Min Yang, Viviana Garcia-Horton, and Amy Guo. Statistical analysis: Viviana Garcia-Horton, Yipeng Gao, Hannah H. Kim, Loren Ormenaj. Writing, review, and editing of the manuscript: Sahil Khanna, Sanghyuk Seo, Min Yang, Viviana Garcia-Horton, Yipeng Gao, Hannah H. Kim, Loren Ormenaj, and Amy Guo.
Funding
This study was funded by Ferring Pharmaceuticals, Inc, which also funded the journal’s Rapid Service Fees.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available for privacy reasons.
Declarations
Conflict of Interest
Amy Guo and Sanghyuk Seo are employees of Ferring Pharmaceuticals, Inc. Min Yang, Viviana García-Horton, Yipeng Gao, Hannah Kim, and Loren Ormenaj are employees of Analysis Group, Inc., which received payment from Ferring Pharmaceuticals, Inc. for participating in this research. Sahil Khanna has received research grants from Ferring Pharmaceuticals, Inc., Finch, Pfizer, Seres Therapeutics, and Vedanta, and served as an advisor/consultant for Probio Tech, LLC, Rise, and Takeda.
Ethical Approval
The study was considered exempt research under 45 CFR § 46.104(d)(4) as it involved only the secondary use of data that were de-identified in compliance with the Health Insurance Portability and Accountability Act (HIPAA), specifically, 45 CFR § 164.514.
Footnotes
Prior Publication: Part of the material in this manuscript was presented at the Academy of Managed Care Pharmacy (AMCP) Nexus 2024 held October 14–17 in Las Vegas, NV, IDWeek 2024 held October 16–19 in Los Angeles, CA, and the American College of Gastroenterology (ACG) Annual Scientific Meeting 2024 held October 25–30 in Philadelphia, PA as poster presentations.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sandhu BK, McBride SM. Clostridioides difficile. Trends Microbiol. 2018;26(12):1049–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Bella S, et al. Clostridioides difficile infection: history, epidemiology, risk factors, prevention, clinical manifestations, treatment, and future options. Clin Microbiol Rev. 2024;37(2):e00135-e223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) Emerging infections program Healthcare-Associated Infections-Community Interface report Clostridioides difficile infection. Atlanta: Centers for Disease Control and Prevention (CDC) 2019; Available from: https://www.cdc.gov/hai/eip/Annual-CDI-Report-2019.html. Accessed 8 Oct 2024.
- 4.McDonald LC, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):e1–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson S, et al. Clinical practice guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 focused update guidelines on management of Clostridioides difficile infection in adults. Clin Infect Dis. 2021;73(5):e1029–44. [DOI] [PubMed] [Google Scholar]
- 6.Tsigrelis C. Recurrent Clostridioides difficile infection: recognition, management, prevention. Cleve Clin J Med. 2020;87(6):347–59. [DOI] [PubMed] [Google Scholar]
- 7.Alrahmany D, et al. Risk factors for recurrence of Clostridioides difficile in hospitalized patients. J Infect Public Health. 2021;14(11):1642–9. [DOI] [PubMed] [Google Scholar]
- 8.Guh AY, et al. Comparison of the risk of recurrent Clostridioides difficile infections among patients in 2018 versus 2013. Open Forum Infect Dis. 2022;9(9):ofac422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lessa FC, et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372(9):825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nelson WW, et al. Health care resource utilization and costs of recurrent Clostridioides difficile infection in the elderly: a real-world claims analysis. J Manag Care Spec Pharm. 2021;27(7):828–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Depestel DD, Aronoff DM. Epidemiology of Clostridium difficile infection. J Pharm Pract. 2013;26(5):464–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferring Pharmaceuticals. Ferring receives U.S. FDA approval for REBYOTA™ (fecal microbiota, live-jslm) – A novel first-in-class microbiota-based live biotherapeutic. 2022; Available from: https://www.ferring.com/ferring-receives-u-s-fda-approval-for-rebyota-fecal-microbiota-live-jslm-a-novel-first-in-class-microbiota-based-live-biotherapeutic/. Accessed 8 Oct 2024.
- 13.Khanna S, et al. Efficacy and safety of RBX2660 in PUNCH CD3, a Phase III, randomized, double-blind, placebo-controlled trial with a Bayesian primary analysis for the prevention of recurrent Clostridioides difficile infection. Drugs. 2022;82(15):1527–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feuerstadt P, et al. Retrospective analysis of the safety and efficacy of fecal microbiota, live-jslm (REBYOTA(TM)) administered under enforcement discretion to patients with Clostridioides difficile infection. Open Forum Infect Dis. 2023;10(5):ofad171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen L, et al. Real-world outcomes of fecal microbiota-based therapies to prevent recurrent Clostridioides difficile infections up to 24 weeks, in American College of Gastroenterology Annual Scientific Meeting: October 25–30, 2024. Philadelphia, Pennsylvania.
- 16.Ritter TE, et al. Real-world experience with fecal microbiome replacement for the prevention of recurrent Clostridioides difficile infection, in American College of Gastroenterology Annual Scientific Meeting: October 25-30, 2024. Philadelphia, Pennsylvania.
- 17.Kelly CP. Can we identify patients at high risk of recurrent Clostridium difficile infection? Clin Microbiol Infect. 2012;18(Suppl 6):21–7. [DOI] [PubMed] [Google Scholar]
- 18.Song JH, Kim YS. Recurrent Clostridium difficile infection: risk factors, treatment, and prevention. Gut Liver. 2019;13(1):16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodrigues R, Barber GE, Ananthakrishnan AN. A comprehensive study of costs associated with recurrent Clostridium difficile infection. Infect Control Hosp Epidemiol. 2017;38(2):196–202. [DOI] [PubMed] [Google Scholar]
- 20.Feuerstadt P, Theriault N, Tillotson G. The burden of CDI in the United States: a multifactorial challenge. BMC Infect Dis. 2023;23(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Enforcement Discretion for Investigational FMT Extended for Severe and Fulminant CDI. OpenBiome; Available from: https://openbiome.org/fmt-distribution-through-december-31/. Accessed 8 Oct 2024.
- 22.Wang Y, et al. A comparison of currently available and investigational fecal microbiota transplant products for recurrent Clostridioides difficile infection. Antibiotics (Basel). 2024;13(5):436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seres Therapeutics. Seres Therapeutics and Nestlé Health Science announce FDA approval of VOWST™ (fecal microbiota spores, live-brpk) for prevention of recurrence of C. difficile infection in adults following antibacterial treatment for recurrent CDI. 2023; Available from: https://ir.serestherapeutics.com/news-releases/news-release-details/seres-therapeutics-and-nestle-health-science-announce-fda. Accessed 8 Oct 2024.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available for privacy reasons.