Abstract
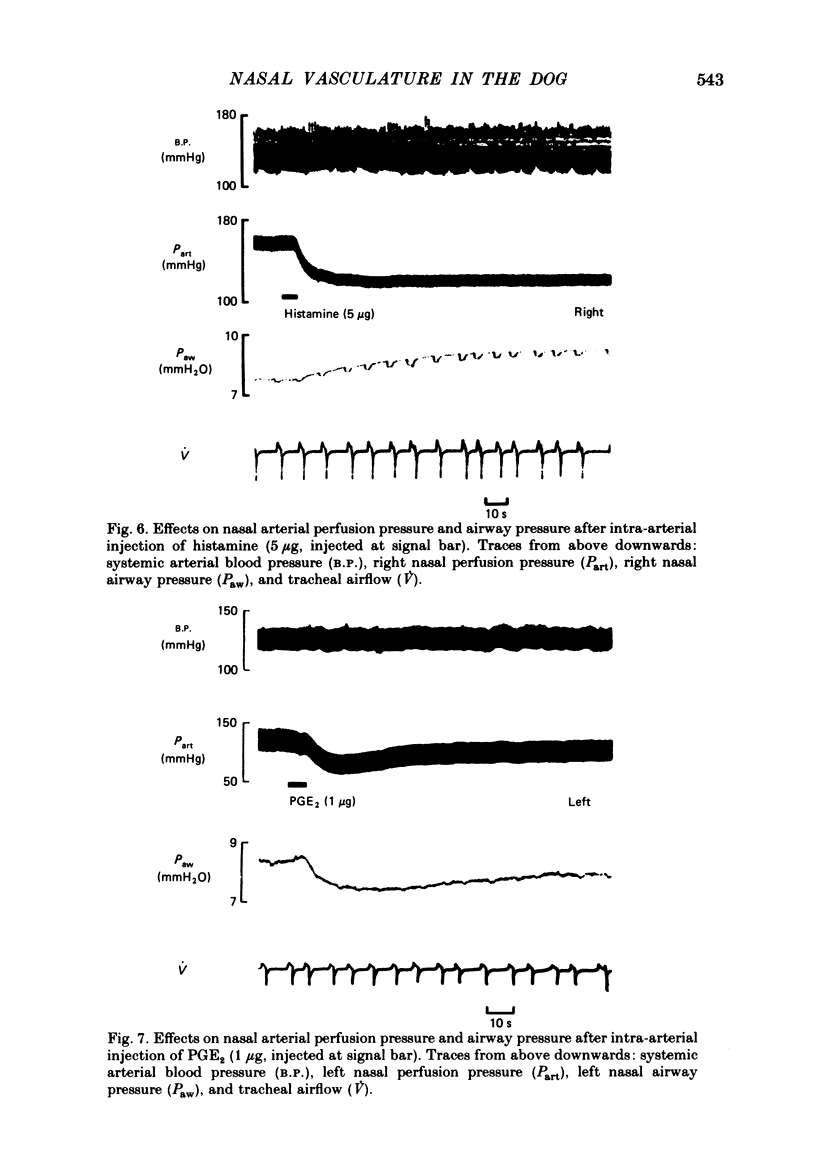
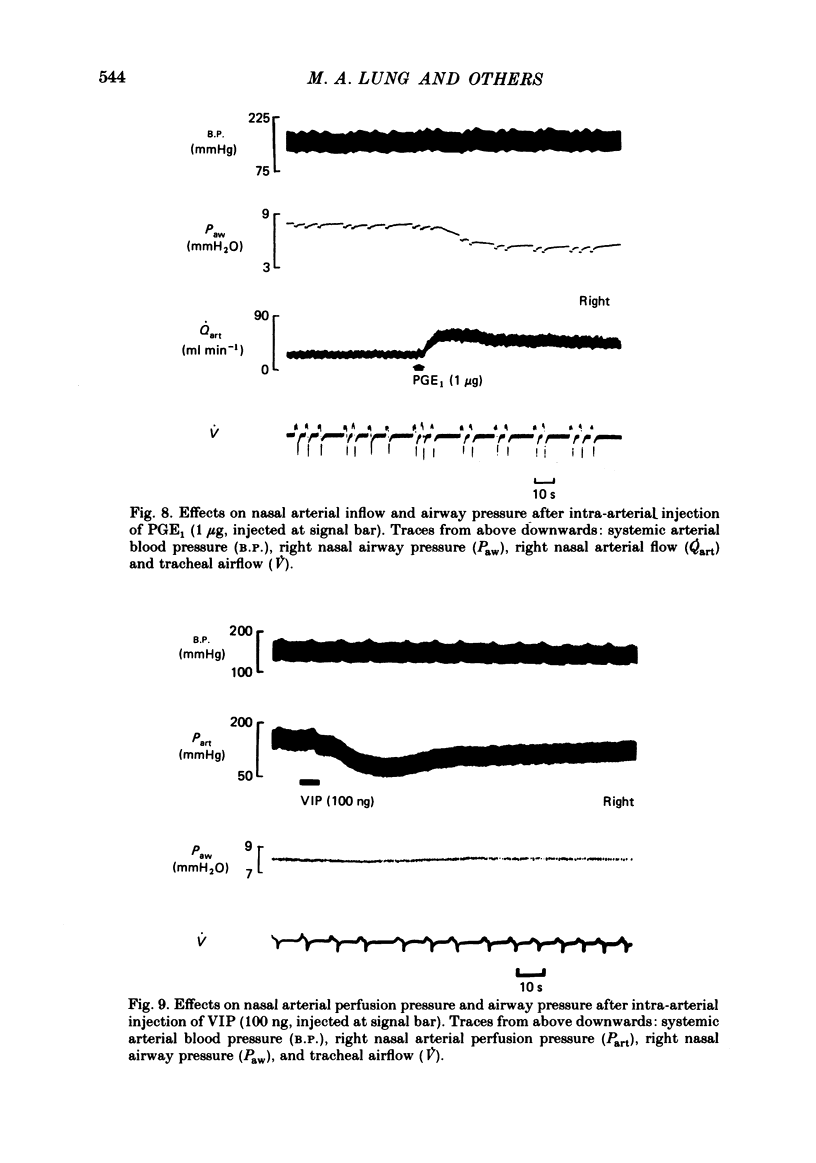
Nasal vascular and airflow resistances have been measured in dogs, simultaneously on both sides separately. Vascular resistance was measured either by constant flow perfusion of the terminal branch of the maxillary artery (which supplies, via the sphenopalatine artery, the nasal septum, most of the turbinates and the nasal sinuses) or by measuring blood flow through this artery, maintained by the dog's own blood pressure. Airflow resistance was assessed by inserting balloon-tipped endotracheal catheters into the back of each nasal cavity via the nasopharynx, and measuring transnasal pressure at constant airflow through each side of the nose simultaneously. Preliminary experiments indicated that there was 5-10% collateral anastomosis between the two sides. Close-arterial injection of drugs showed different patterns of response. Adrenaline, phenylephrine, chlorpheniramine and low doses of prostaglandin F2 alpha increased vascular resistance and lowered airway resistance. Salbutamol, methacholine and histamine lowered vascular resistance and increased airway resistance. Dobutamine decreased airway resistance with a small increase in vascular resistance. Prostaglandins E1, E2 and F2 alpha (high dose) decreased both vascular and airway resistances. Substance P, eledoisin-related peptide and vasoactive intestinal polypeptide lowered vascular resistance with little change in airway resistance. The results are interpreted in terms of possible drug actions on precapillary resistance vessels, sinusoids and venules, and arteriovenous anastomoses. It is concluded that nasal airway resistance cannot be correlated with vascular resistance or blood flow, since the latter has a complex and ill-defined relationship with nasal vascular blood volume.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y., Jackson R. T. The use of labeled microspheres to determine blood flow in the dog's nasal mucosa. Ann Otol Rhinol Laryngol. 1972 Feb;81(1):82–86. doi: 10.1177/000348947208100109. [DOI] [PubMed] [Google Scholar]
- Anggard A. The effect of prostaglandins on nasal airway resistance in man. Ann Otol Rhinol Laryngol. 1969 Jun;78(3):657–662. doi: 10.1177/000348946907800320. [DOI] [PubMed] [Google Scholar]
- Anggård A., Edwall L. The effects of sympathetic nerve stimulation on the tracer disappearance rate and local blood content in the nasal mucosa of the cat. Acta Otolaryngol. 1974 Jan-Feb;77(1):131–139. doi: 10.3109/00016487409124608. [DOI] [PubMed] [Google Scholar]
- Bedwani J. R., Eccles R., Jones A. S. The isolation of prostaglandin E from pig nasal mucosa. Clin Otolaryngol Allied Sci. 1983 Jun;8(3):159–163. doi: 10.1111/j.1365-2273.1983.tb01420.x. [DOI] [PubMed] [Google Scholar]
- Bentley A. J., Jackson R. T. Changes in the patency of the upper nasal passage induced by histamine and antihistamines. Laryngoscope. 1970 Dec;80(12):1859–1870. doi: 10.1002/lary.5540801207. [DOI] [PubMed] [Google Scholar]
- Cohen B. M. The nasal respiratory handicap of expiratory airflow disease: the response to bronchodilator aerosols. Respiration. 1970;27(4):406–416. doi: 10.1159/000192697. [DOI] [PubMed] [Google Scholar]
- DAWES J. D., PRICHARD M. M. Studies of the vascular arrangements of the nose. J Anat. 1953 Jul;87(3):311–322. [PMC free article] [PubMed] [Google Scholar]
- Eccles R., Eccles K. S. Sympathetic innervation of the nasal mucosa of the pig. Res Vet Sci. 1981 May;30(3):349–352. [PubMed] [Google Scholar]
- Eccles R., Lee R. L. Nasal vasomotor oscillations in the cat associated with the respiratory rhythm. Acta Otolaryngol. 1981 Sep-Oct;92(3-4):357–361. doi: 10.3109/00016488109133272. [DOI] [PubMed] [Google Scholar]
- Eccles R., Wilson H. The autonomic innervation of the nasal blood vessels of the cat. J Physiol. 1974 May;238(3):549–560. doi: 10.1113/jphysiol.1974.sp010542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall L. J., Jackson R. T. Effects of alpha and beta adrenergic agonists on nasal blood flow. Ann Otol Rhinol Laryngol. 1968 Dec;77(6):1120–1130. doi: 10.1177/000348946807700610. [DOI] [PubMed] [Google Scholar]
- Hiley C. R., Wilson H., Yates M. S. Identification of beta-adrenoceptors and histamine receptors in the cat nasal vasculature. Acta Otolaryngol. 1978 May-Jun;85(5-6):444–448. [PubMed] [Google Scholar]
- Jackson R. T. A new in vitro method of drug assay of nasal blood vessels. Arch Otorhinolaryngol. 1979;225(1):33–38. doi: 10.1007/BF00455873. [DOI] [PubMed] [Google Scholar]
- Jackson R. T. Pharmacological responsiveness of the nasal mucosa. Ann Otol Rhinol Laryngol. 1970 Jun;79(3):461–467. doi: 10.1177/000348947007900305. [DOI] [PubMed] [Google Scholar]
- Jackson R. T., Rooker D. W. Stimulation and section of the vidian nerve in relation to autonomic control of the nasal vasculature. Laryngoscope. 1971 Apr;81(4):565–569. doi: 10.1288/00005537-197104000-00007. [DOI] [PubMed] [Google Scholar]
- Karim S. M., Adaikan P. G., Kunaratnam N. Effect of 17 phenyl PGF2 alpha on nasal patency in man. Prostaglandins Med. 1979 Jul;3(1):33–37. doi: 10.1016/0161-4630(79)90013-2. [DOI] [PubMed] [Google Scholar]
- Karim S. M., Adaikan P. G., Kunaratnam N. Effect of topical prostaglandins on nasal patency in man. Prostaglandins. 1978 Mar;15(3):457–462. doi: 10.1016/0090-6980(78)90129-6. [DOI] [PubMed] [Google Scholar]
- Krausz S. A pharmacological study of the control of nasal cooling in the dog. Pflugers Arch. 1977 Dec 12;372(2):115–119. doi: 10.1007/BF00585324. [DOI] [PubMed] [Google Scholar]
- Malm L. Beta-adrenergic receptors in the vessels of the cat nasal mucosa. Acta Otolaryngol. 1974 Sep-Oct;78(3-4):242–246. doi: 10.3109/00016487409126350. [DOI] [PubMed] [Google Scholar]
- Malm L. Responses of resistance and capacitance vessels in feline nasal mucosa to vasoactive agents. Acta Otolaryngol. 1974 Jul-Aug;78(1-2):90–97. doi: 10.3109/00016487409126331. [DOI] [PubMed] [Google Scholar]
- Malm L. Stimulation of sympathetic nerve fibres to the nose in cats. Acta Otolaryngol. 1973 Jun;75(6):519–526. doi: 10.3109/00016487309139783. [DOI] [PubMed] [Google Scholar]
- Malm L., Sundler F., Uddman R. Effects of vasoactive intestinal polypeptide on resistance and capacitance vessels in the nasal mucosa. Acta Otolaryngol. 1980 Sep-Oct;90(3-4):304–308. doi: 10.3109/00016488009131730. [DOI] [PubMed] [Google Scholar]
- Matson C. J., Welter A. N., Kvam D. C. An experimental non-invasive animal technique for measuring nasal airway resistance: I. Adrenergic and antihistaminic agents. Arch Int Pharmacodyn Ther. 1978 Mar;232(1):68–78. [PubMed] [Google Scholar]
- McCaffrey T. V., Kern E. B. Response of nasal airway resistance to hypercapnia and hypoxia in the dog. Acta Otolaryngol. 1979 May-Jun;87(5-6):545–553. doi: 10.3109/00016487909126463. [DOI] [PubMed] [Google Scholar]
- McLean J. A., Mathews K. P., Ciarkowski A. A., Brayton P. R., Solomon W. R. The effects of topical saline and isoproterenol on nasal airway resistance. J Allergy Clin Immunol. 1976 Nov;58(5):563–574. doi: 10.1016/0091-6749(76)90202-5. [DOI] [PubMed] [Google Scholar]
- Okazki T., Reisman R. E., Arbesman C. E. Prostaglandin E in the secretions of allergic rhinitis. Prostaglandins. 1977 Apr;13(4):681–690. doi: 10.1016/0090-6980(77)90239-8. [DOI] [PubMed] [Google Scholar]
- Proctor D. F. The upper airways. I. Nasal physiology and defense of the lungs. Am Rev Respir Dis. 1977 Jan;115(1):97–129. doi: 10.1164/arrd.1977.115.1.97. [DOI] [PubMed] [Google Scholar]
- Rao S., Potdar A. Nasal airflow with body in various positions. J Appl Physiol. 1970 Feb;28(2):162–165. doi: 10.1152/jappl.1970.28.2.162. [DOI] [PubMed] [Google Scholar]
- Salem H., Clemente E. A new experimental method for evaluating drugs in the nasal cavity. Arch Otolaryngol. 1972 Dec;96(6):524–529. doi: 10.1001/archotol.1972.00770090802005. [DOI] [PubMed] [Google Scholar]
- Salman S. D., Proctor D. F., Swift D. L., Eveering S. A. Nasal resistance: description of a method and effect of temperature and humidity changes. Ann Otol Rhinol Laryngol. 1971 Oct;80(5):736–743. doi: 10.1177/000348947108000516. [DOI] [PubMed] [Google Scholar]
- Stovall R., Jackson R. T. Prostaglandins and nasal blood flow. Ann Otol Rhinol Laryngol. 1967 Dec;76(5):1051–1059. doi: 10.1177/000348946707600517. [DOI] [PubMed] [Google Scholar]
- Svensson G., Hegardt B., Löfkvist T. Effects of topical use of beta-adrenoceptor stimulants of nasal mucosa. Rhinomanometric evaluations in experiments with terbutaline and KWD 2131. Acta Otolaryngol. 1980 Sep-Oct;90(3-4):297–303. doi: 10.3109/00016488009131729. [DOI] [PubMed] [Google Scholar]
- TOH C. C., MOHIUDDIN A. Vasoactive substances in the nasal mucosa. Br J Pharmacol Chemother. 1958 Jun;13(2):113–117. doi: 10.1111/j.1476-5381.1958.tb00204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi Y., Proctor D. F., Salman S., Evering S. Effects of cold air and carbon dioxide on nasal air flow resistance. Ann Otol Rhinol Laryngol. 1969 Feb;78(1):40–48. doi: 10.1177/000348946907800104. [DOI] [PubMed] [Google Scholar]
- Temesrékási D. Mikroskopischer Bau und Funktion des Schwellgewebes der Nasenmuschel des Menschen. Z Mikrosk Anat Forsch. 1969;80(2):219–229. [PubMed] [Google Scholar]
- Uddman R., Alumets J., Densert O., Håkanson R., Sundler F. Occurrence and distribution of VIP nerves in the nasal mucosa and tracheobronchial wall. Acta Otolaryngol. 1978 Nov-Dec;86(5-6):443–448. doi: 10.3109/00016487809107524. [DOI] [PubMed] [Google Scholar]
- Uddman R., Malm L., Sundler F. The origin of vasoactive intestinal polypeptide (VIP) nerves in the feline nasal mucosa. Acta Otolaryngol. 1980 Jan-Feb;89(1-2):152–156. doi: 10.3109/00016488009127121. [DOI] [PubMed] [Google Scholar]


