Abstract
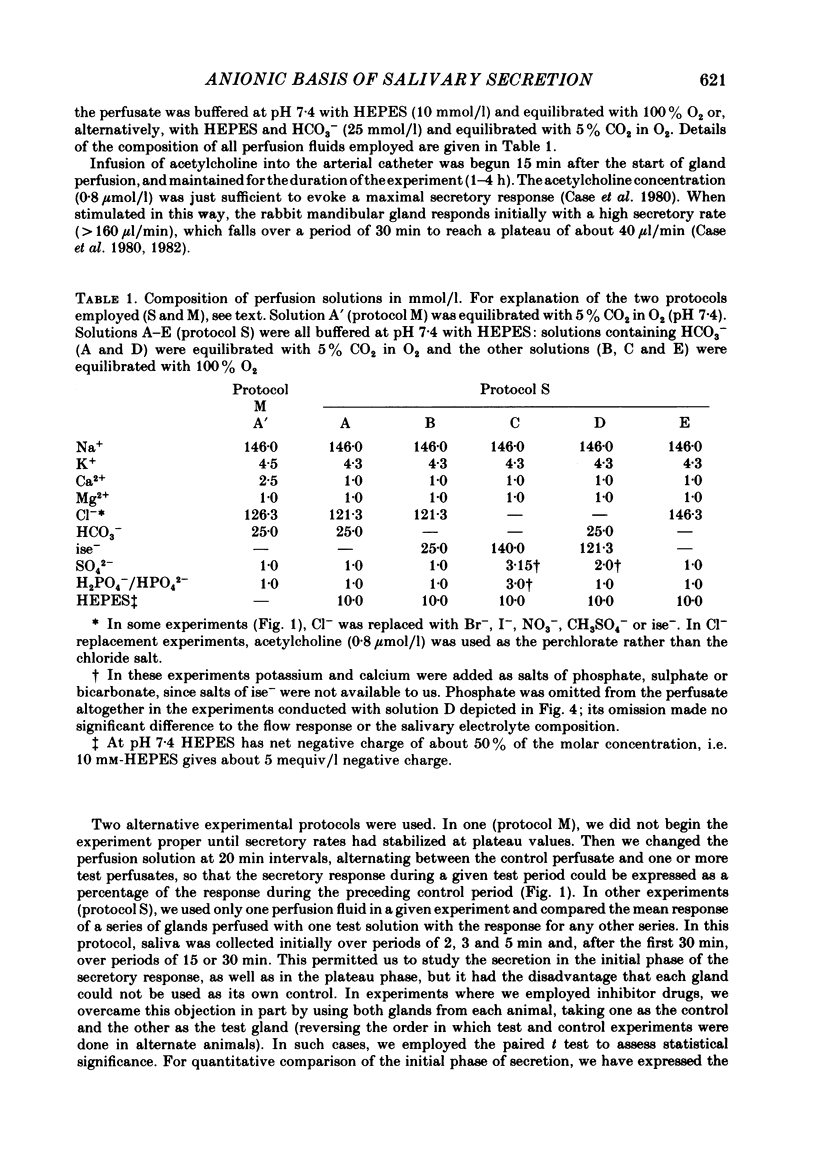
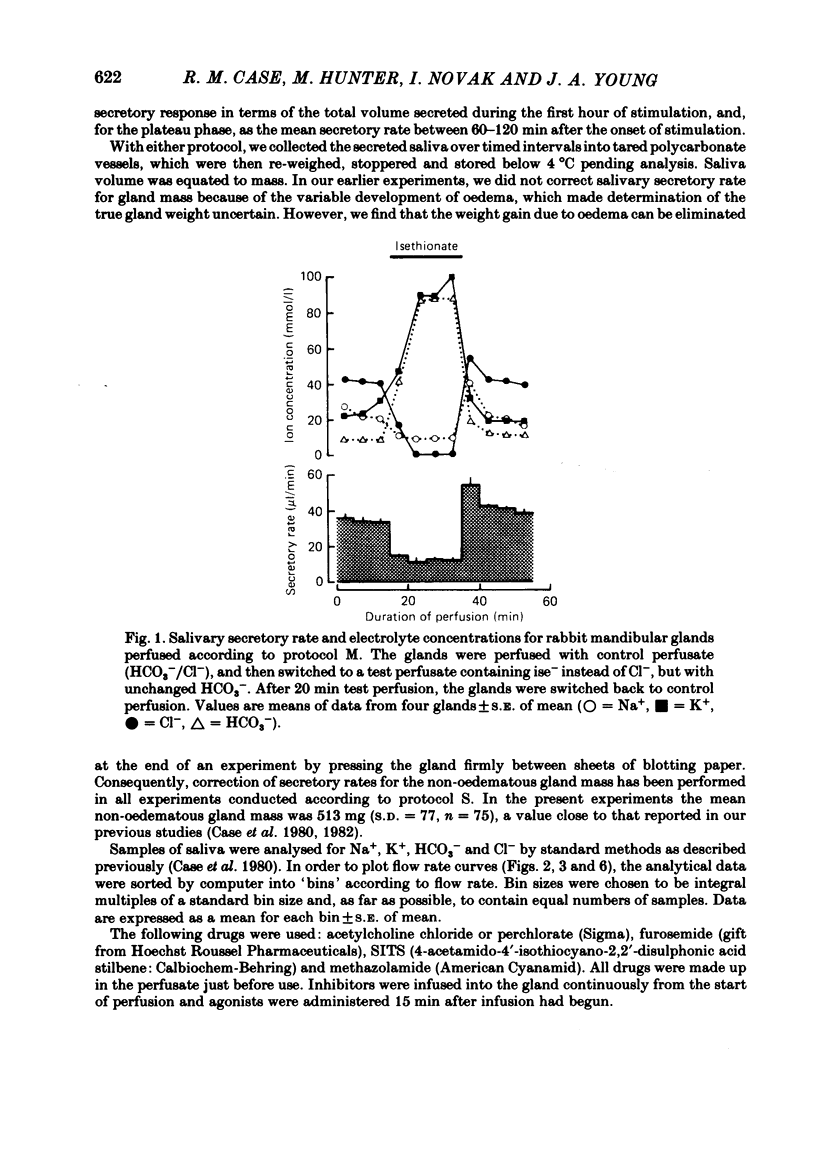
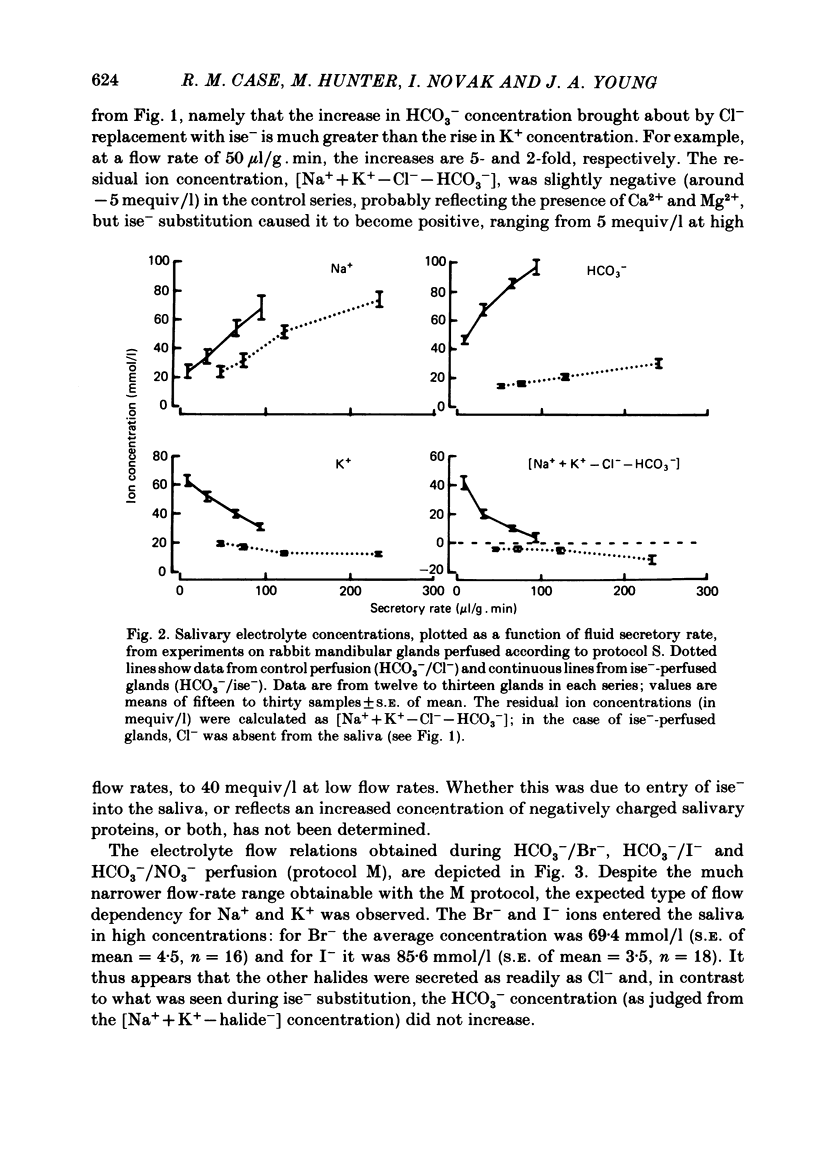
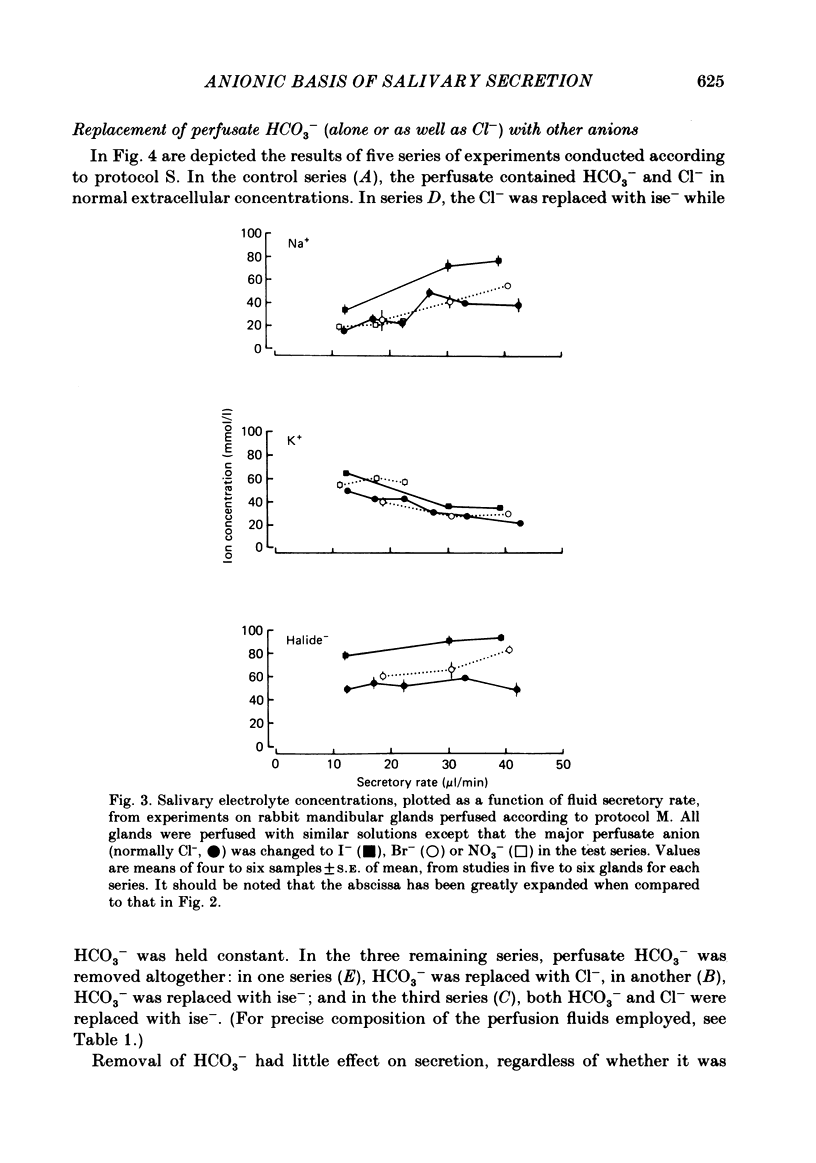
The role played by anions in salivary secretion has been studied in experiments on the isolated, perfused mandibular gland of the rabbit, in which perfusate Cl- and/or HCO3- were replaced by other anions. Replacement of Cl- with Br- had no significant effect on salivary secretion rate, but replacement with the other anions tested caused secretory rate to fall, by 38% (I-), 50% (NO3-), 61% (isethionate, ise -), and 66% ( CH3SO4 -), respectively. Replacement of perfusate Cl- with ise - or CH3SO4 - caused the salivary HCO3- concentration to rise up to 4-fold. Replacement with Br- or I- seemed to have little effect on salivary HCO3- concentration but, in contrast to ise -, Br- and I- entered the saliva in concentrations comparable to those of Cl- during control perfusion. In glands perfused with HCO3- and ise -, the addition of methazolamide, an inhibitor of carbonic anhydrase, caused a further 60% drop in secretory rate, but the saliva remained rich in HCO3-. Replacement of perfusate HCO3- with Cl- or ise - had no effect on salivary secretion or composition. Replacement of both HCO3- and Cl- in the perfusate with ise - reduced salivary secretion to less than 2% of control levels. In control glands (i.e. perfused with both HCO3- and Cl-), administration of furosemide, an inhibitor of Na+/Cl- co-transport, reduced the secretion rate and increased salivary HCO3- in a manner indistinguishable from that seen when perfusate Cl- was replaced with ise -. In control perfused glands, administration of SITS (4-acetamido-4'- isothio cyano-2,2'-disulphonic acid stilbene), an inhibitor of Cl-/HCO3- antiports , did not cause any change in salivary HCO3- concentration. Unexpectedly, it induced a significant increase in salivary secretory rate. The results show that salivary secretion depends on two independent transport systems. One is a Cl- -dependent, furosemide-sensitive system, probably a Na+/Cl- symport. The other is an HCO3- -dependent, methazolamide-sensitive system, and is probably an Na+/H+ antiport.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burg M., Stoner L., Cardinal J., Green N. Furosemide effect on isolated perfused tubules. Am J Physiol. 1973 Jul;225(1):119–124. doi: 10.1152/ajplegacy.1973.225.1.119. [DOI] [PubMed] [Google Scholar]
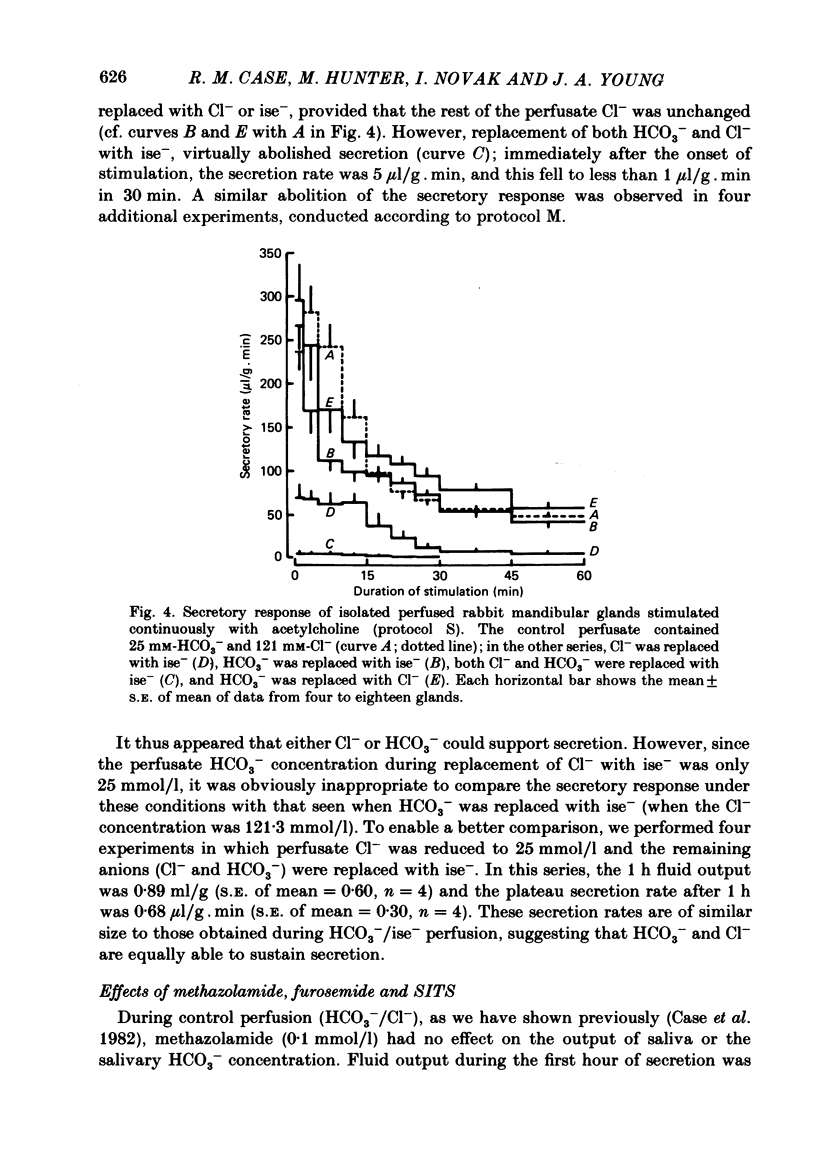
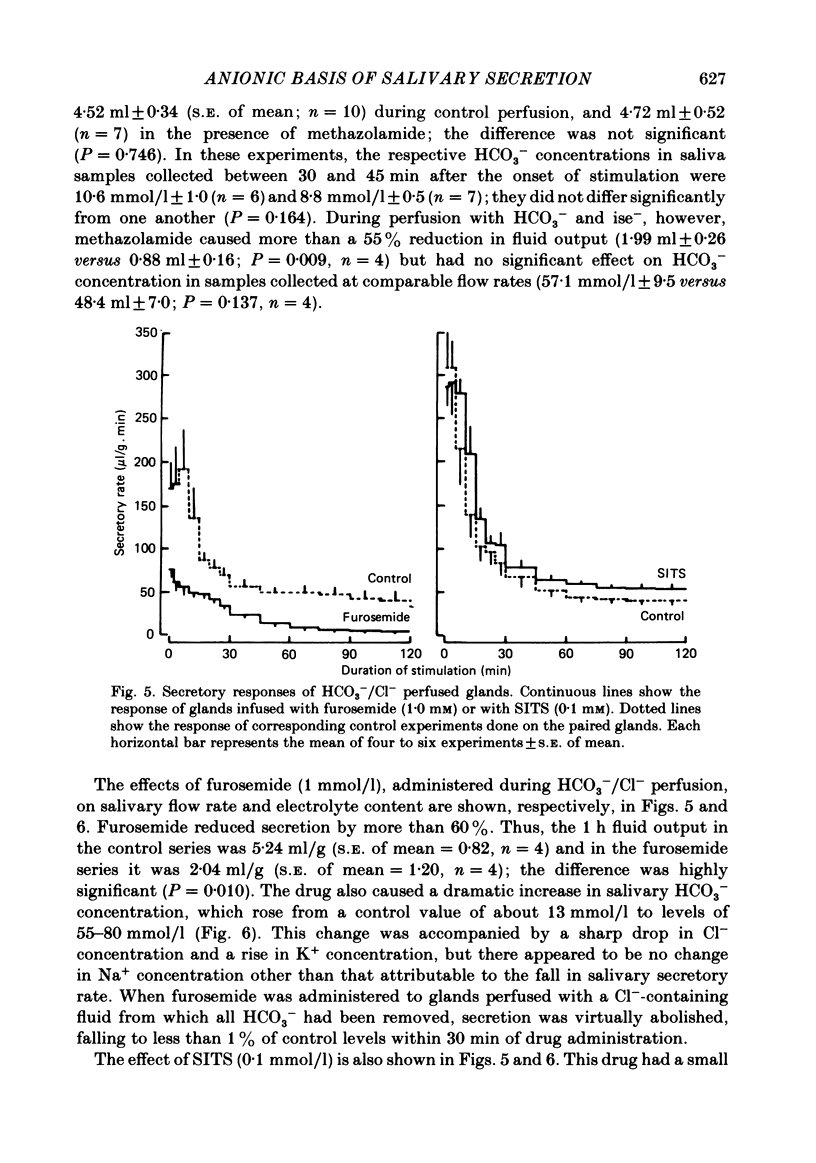
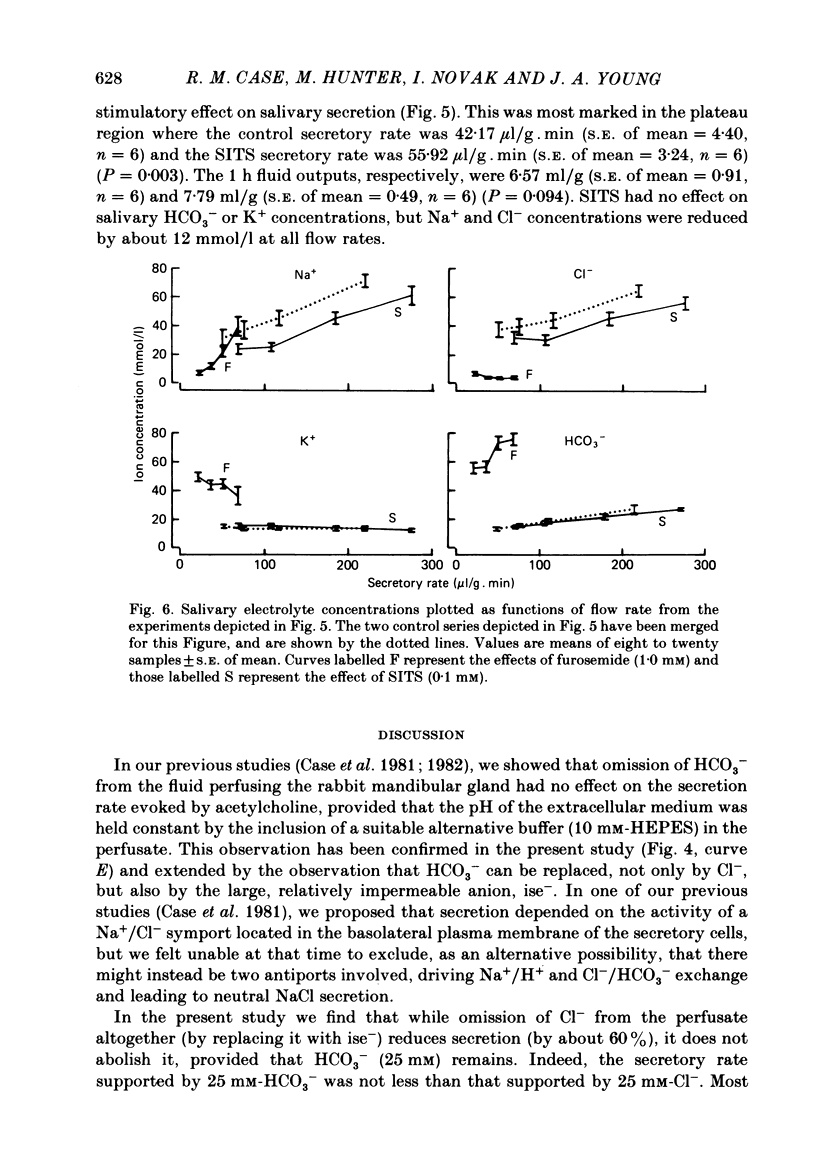
- Case R. M., Conigrave A. D., Favaloro E. J., Novak I., Thompson C. H., Young J. A. The role of buffer anions and protons in secretion by the rabbit mandibular salivary gland. J Physiol. 1982 Jan;322:273–286. doi: 10.1113/jphysiol.1982.sp014037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R. M., Conigrave A. D., Hunter M., Novak I., Thompson C. H., Young J. A. Secretion of saliva by the rabbit mandibular gland in vitro: the role of anions. Philos Trans R Soc Lond B Biol Sci. 1981 Dec 18;296(1080):179–192. doi: 10.1098/rstb.1981.0181. [DOI] [PubMed] [Google Scholar]
- Case R. M., Conigrave A. D., Novak I., Young J. A. Electrolyte and protein secretion by the perfused rabbit mandibular gland stimulated with acetylcholine or catecholamines. J Physiol. 1980 Mar;300:467–487. doi: 10.1113/jphysiol.1980.sp013173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case R. M., Scratcherd T., Wynne R. D. The origin and secretion of pancreatic juice bicarbonate. J Physiol. 1970 Sep;210(1):1–15. doi: 10.1113/jphysiol.1970.sp009193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren T. H. Current status of membrane-bound carbonic anhydrase. Ann N Y Acad Sci. 1980;341:246–258. doi: 10.1111/j.1749-6632.1980.tb47176.x. [DOI] [PubMed] [Google Scholar]
- Martin C. J., Frömter E., Gebler B., Knauf H., Young J. A. The effects of carbachol on water and electrolyte fluxes and transepithelial electrical potential differences of the rabbit submaxillary main duct perfused in vitro. Pflugers Arch. 1973 Jun 26;341(2):131–142. doi: 10.1007/BF00587320. [DOI] [PubMed] [Google Scholar]
- Schuurmans Stekhoven F., Bonting S. L. Transport adenosine triphosphatases: properties and functions. Physiol Rev. 1981 Jan;61(1):1–76. doi: 10.1152/physrev.1981.61.1.1. [DOI] [PubMed] [Google Scholar]
- Silva P., Stoff J., Field M., Fine L., Forrest J. N., Epstein F. H. Mechanism of active chloride secretion by shark rectal gland: role of Na-K-ATPase in chloride transport. Am J Physiol. 1977 Oct;233(4):F298–F306. doi: 10.1152/ajprenal.1977.233.4.F298. [DOI] [PubMed] [Google Scholar]
- Turnberg L. A., Bieberdorf F. A., Morawski S. G., Fordtran J. S. Interrelationships of chloride, bicarbonate, sodium, and hydrogen transport in the human ileum. J Clin Invest. 1970 Mar;49(3):557–567. doi: 10.1172/JCI106266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnock D. G., Eveloff J. NaCl entry mechanisms in the luminal membrane of the renal tubule. Am J Physiol. 1982 Jun;242(6):F561–F574. doi: 10.1152/ajprenal.1982.242.6.F561. [DOI] [PubMed] [Google Scholar]


