Abstract
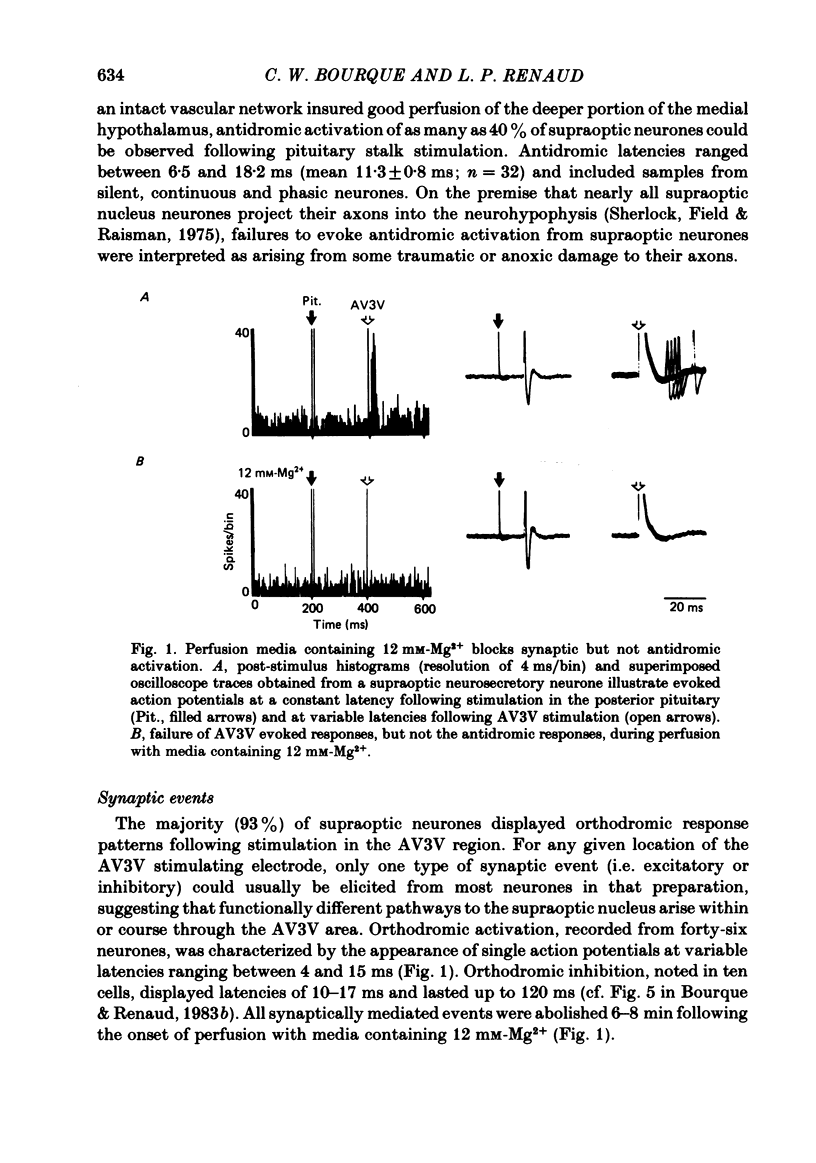
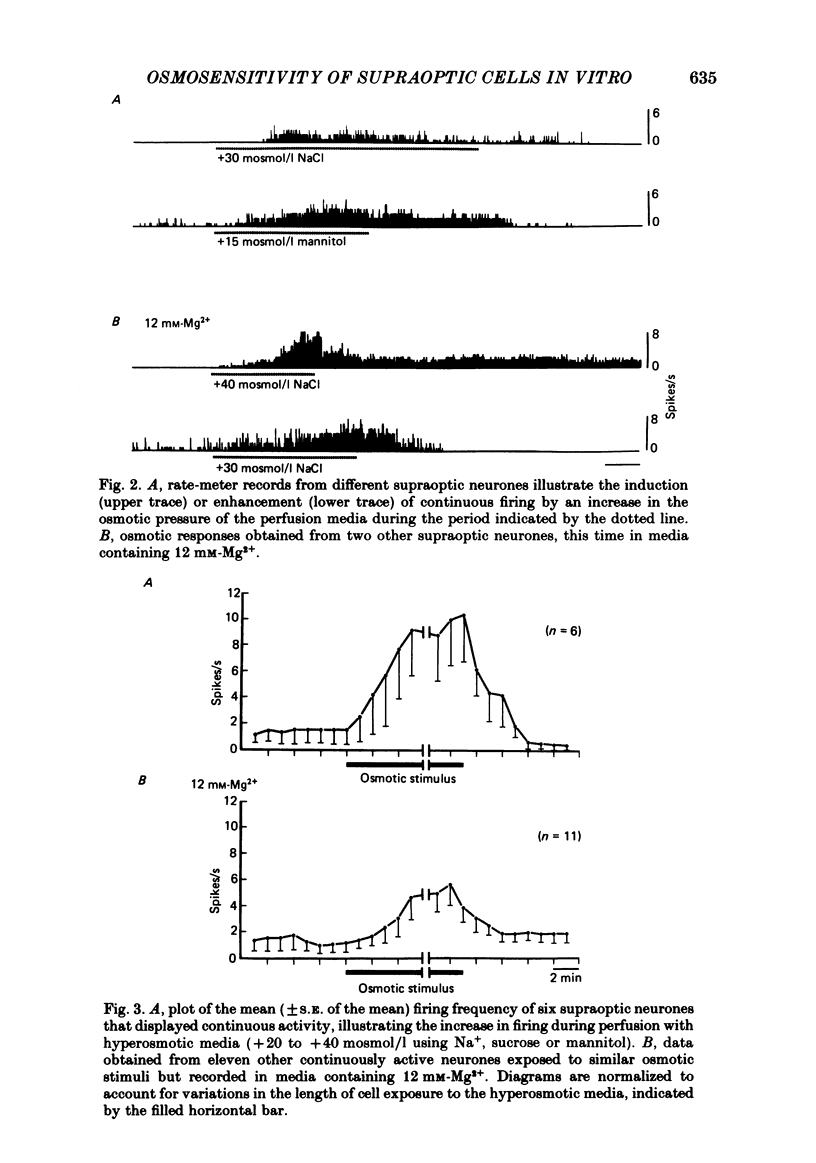
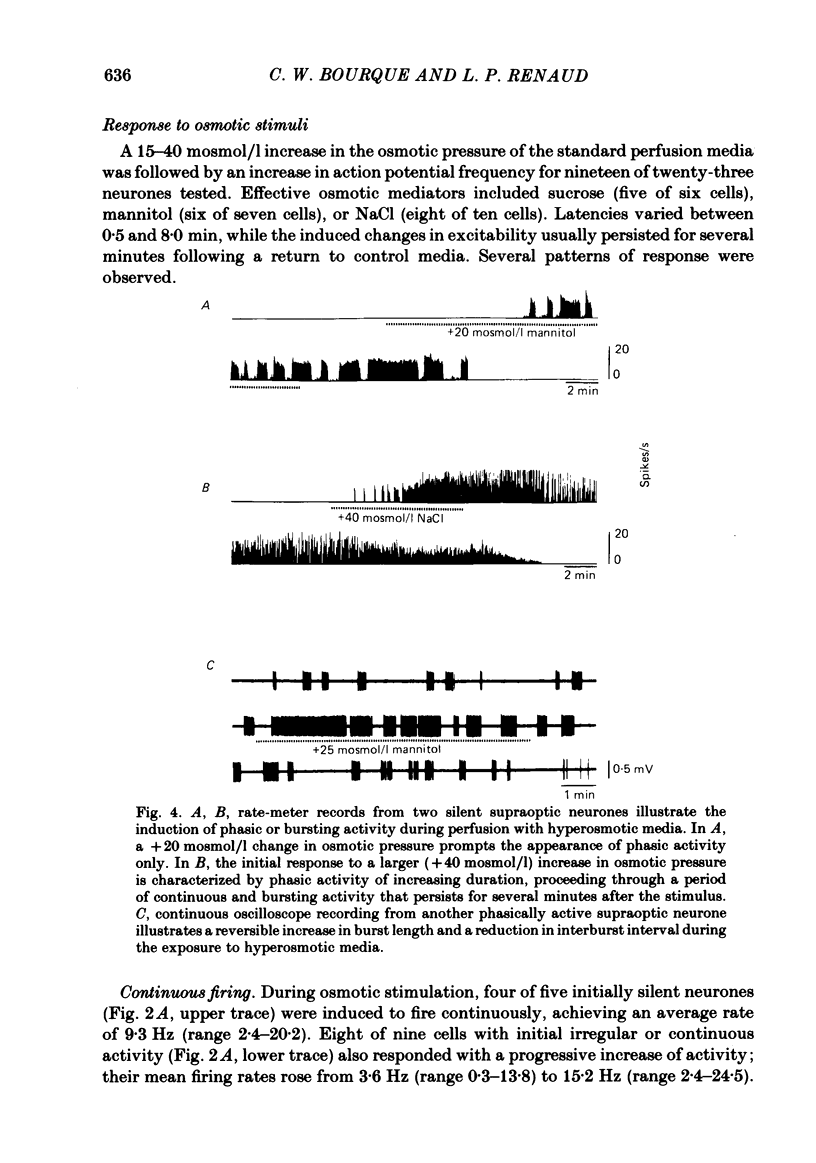
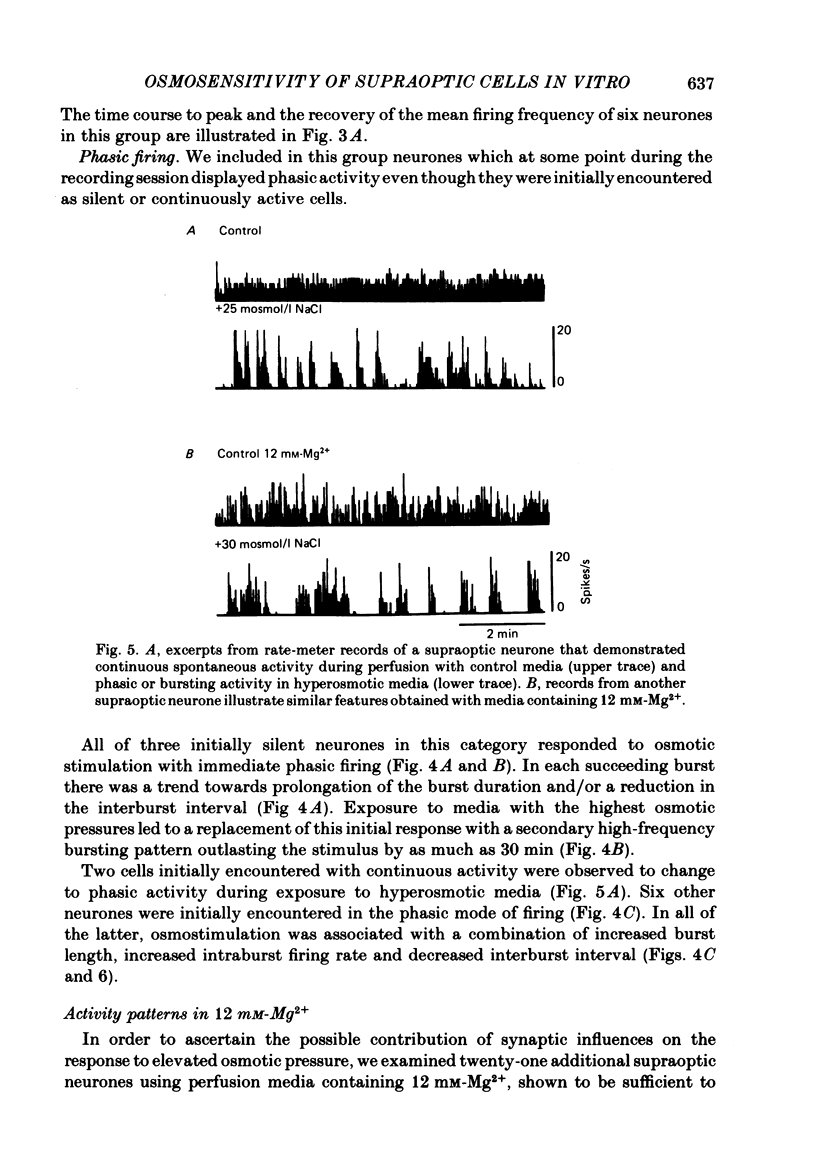
Extracellular recordings were obtained in vitro from supraoptic neurones in an explant of rat hypothalamus maintained viable through intravascular perfusion with artificial media. The spontaneous activity observed from 64% of cells included continuously active neurones (mean frequency 3.2 +/- 0.8 Hz) located throughout the nucleus, and phasically active neurones (8.6 +/- 0.6 Hz) located predominantly in the caudal half of the nucleus. Supraoptic neurones displayed antidromic activation (latency range 6.5-18.2 ms) following stimulation of the pituitary stalk and orthodromic excitatory or inhibitory responses following stimulation in the anteroventral third ventricular (AV3V) region. Synaptic responses were reversibly abolished during perfusion with media containing 12 mM-Mg2+. A 15-40 mosmol/l increase in the osmotic pressure of the perfusion media, through addition of NaCl, sucrose or mannitol, prompted nineteen of twenty-three cells to increase their discharge frequency. The patterns of response included the induction or simple increase in continuous firing frequency, the induction or enhancement of phasic or bursting activity and a change from continuous to phasic activity. Similar responses to an osmotic stimulus were obtained from thirteen of twenty-one supraoptic neurones, including two phasic neurones, where synaptic activity had been abolished during perfusion with 12 mM-Mg2+. Osmosensitivity appeared to be selective for supraoptic cells; no significant change in firing frequency was observed from any of six cells recorded in the lateral hypothalamus or thirteen cells recorded in the medial hypothalamus during exposure to a change in osmotic pressure of +20 to +40 mosmol/l, using either control media (seven cells) or media containing 12 mM-Mg2+ (twelve cells). These observations indicate that supraoptic neurones maintained in vitro can display spontaneous, antidromie , orthodromic and osmotically induced activity patterns identical to those observed with in vivo recordings. The persistence of a reduced osmosensitivity among supraoptic neurones in the absence of synaptic transmission indicates that although these cells can function as osmoreceptors, their osmosensitivity may be enhanced through synaptic input from adjacent neurones, possibly located in the AV3V area. The presence of phasic activity among supraoptic neurones maintained in media where synaptic transmission has been abolished suggests that the mechanisms responsible for such activity patterns are endogenous membrane properties of a subpopulation of supraoptic neurones.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe H., Ogata N. Ionic mechanism for the osmotically-induced depolarization in neurones of the guinea-pig supraoptic nucleus in vitro. J Physiol. 1982 Jun;327:157–171. doi: 10.1113/jphysiol.1982.sp014225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson B. Regulation of water intake. Physiol Rev. 1978 Jul;58(3):582–582. doi: 10.1152/physrev.1978.58.3.582. [DOI] [PubMed] [Google Scholar]
- Armstrong W. E., Sladek C. D. Spontaneous "phasic-firing' in supraoptic neurons recorded from hypothalamo-neurohypophysial explants in vitro. Neuroendocrinology. 1982 Jun;34(6):405–409. doi: 10.1159/000123336. [DOI] [PubMed] [Google Scholar]
- Baertschi A. J., Vallet P. G. Osmosensitivity of the hepatic portal vein area and vasopressin release in rats. J Physiol. 1981 Jun;315:217–230. doi: 10.1113/jphysiol.1981.sp013743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bie P. Osmoreceptors, vasopressin, and control of renal water excretion. Physiol Rev. 1980 Oct;60(4):961–1048. doi: 10.1152/physrev.1980.60.4.961. [DOI] [PubMed] [Google Scholar]
- Bourque C. W., Renaud L. P. A perfused in vitro preparation of hypothalamus for electrophysiological studies on neurosecretory neurons. J Neurosci Methods. 1983 Mar;7(3):203–214. doi: 10.1016/0165-0270(83)90002-x. [DOI] [PubMed] [Google Scholar]
- Bourque C. W., Renaud L. P. In vitro neurophysiology of identified rat hypothalamic 'neuroendocrine' neurons. Neuroendocrinology. 1983 Feb;36(2):161–164. doi: 10.1159/000123453. [DOI] [PubMed] [Google Scholar]
- Brimble M. J., Dyball R. E. Characterization of the responses of oxytocin- and vasopressin-secreting neurones in the supraoptic nucleus to osmotic stimulation. J Physiol. 1977 Sep;271(1):253–271. doi: 10.1113/jphysiol.1977.sp011999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brimble M. J., Dyball R. E., Forsling M. L. Oxytocin release following osmotic activation of oxytocin neurones in the paraventricular and supraoptic nuclei. J Physiol. 1978 May;278:69–78. doi: 10.1113/jphysiol.1978.sp012293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreifuss J. J., Harris M. C., Tribollet E. Excitation of phasically firing hypothalamic supraoptic neurones by carotid occlusion in rats. J Physiol. 1976 May;257(2):337–354. doi: 10.1113/jphysiol.1976.sp011372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn F. L., Brennan T. J., Nelson A. E., Robertson G. L. The role of blood osmolality and volume in regulating vasopressin secretion in the rat. J Clin Invest. 1973 Dec;52(12):3212–3219. doi: 10.1172/JCI107521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyball R. E., Prilusky J. Responses of supraoptic neurones in the intact and deafferented rat hypothalamus to injections of hypertonic sodium chloride. J Physiol. 1981 Feb;311:443–452. doi: 10.1113/jphysiol.1981.sp013596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller E. W., Wakerley J. B. Electrophysiological studies of paraventricular and supraoptic neurones recorded in vitro from slices of rat hypothalamus. J Physiol. 1980 May;302:347–362. doi: 10.1113/jphysiol.1980.sp013247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamamura M., Shibuki K., Yagi K. Amygdalar inputs to ADH-secreting supraoptic neurones in rats. Exp Brain Res. 1982;48(3):420–428. doi: 10.1007/BF00238618. [DOI] [PubMed] [Google Scholar]
- Harris M. C. Effects of chemoreceptor and baroreceptor stimulation on the discharge of hypothalamic supraoptic neurones in rats. J Endocrinol. 1979 Jul;82(1):115–125. doi: 10.1677/joe.0.0820115. [DOI] [PubMed] [Google Scholar]
- Hatton G. I., Doran A. D., Salm A. K., Tweedle C. D. Brain slice preparation: hypothalamus. Brain Res Bull. 1980 Jul-Aug;5(4):405–414. doi: 10.1016/s0361-9230(80)80010-4. [DOI] [PubMed] [Google Scholar]
- Hatton G. I. Phasic bursting activity of rat paraventricular neurones in the absence of synaptic transmission. J Physiol. 1982 Jun;327:273–284. doi: 10.1113/jphysiol.1982.sp014231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward J. N. Functional and morphological aspects of hypothalamic neurons. Physiol Rev. 1977 Jul;57(3):574–658. doi: 10.1152/physrev.1977.57.3.574. [DOI] [PubMed] [Google Scholar]
- Leng G. Lateral hypothalamic neurones: osmosensitivity and the influence of activating magnocellular neurosecretory neurones. J Physiol. 1982 May;326:35–48. doi: 10.1113/jphysiol.1982.sp014175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng G., Mason W. T., Dyer R. G. The supraoptic nucleus as an osmoreceptor. Neuroendocrinology. 1982 Jan;34(1):75–82. doi: 10.1159/000123280. [DOI] [PubMed] [Google Scholar]
- Leng G. Rat supraoptic neurones: the effects of locally applied hypertonic saline. J Physiol. 1980 Jul;304:405–414. doi: 10.1113/jphysiol.1980.sp013332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng G. The effects of neural stalk stimulation upon firing patterns in rat supraoptic neurones. Exp Brain Res. 1981;41(2):135–145. doi: 10.1007/BF00236603. [DOI] [PubMed] [Google Scholar]
- Llinás R., Yarom Y., Sugimori M. Isolated mammalian brain in vitro: new technique for analysis of electrical activity of neuronal circuit function. Fed Proc. 1981 Jun;40(8):2240–2245. [PubMed] [Google Scholar]
- Mason W. T. Electrical properties of neurons recorded from the rat supraoptic nucleus in vitro. Proc R Soc Lond B Biol Sci. 1983 Jan 22;217(1207):141–161. doi: 10.1098/rspb.1983.0003. [DOI] [PubMed] [Google Scholar]
- Mason W. T. Supraoptic neurones of rat hypothalamus are osmosensitive. Nature. 1980 Sep 11;287(5778):154–157. doi: 10.1038/287154a0. [DOI] [PubMed] [Google Scholar]
- Poulain D. A., Ellendorff F., Vincent J. D. Septal connections with identified oxytocin and vasopressin neurones in the supraoptic nucleus of the rat. An electrophysiological investigation. Neuroscience. 1980;5(2):379–387. doi: 10.1016/0306-4522(80)90113-x. [DOI] [PubMed] [Google Scholar]
- Poulain D. A., Wakerley J. B. Electrophysiology of hypothalamic magnocellular neurones secreting oxytocin and vasopressin. Neuroscience. 1982 Apr;7(4):773–808. doi: 10.1016/0306-4522(82)90044-6. [DOI] [PubMed] [Google Scholar]
- Sherlock D. A., Field P. M., Raisman G. Retrograde transport of horseradish peroxidase in the magnocellular neurosecretory system of the rat. Brain Res. 1975 May 9;88(3):403–414. doi: 10.1016/0006-8993(75)90653-8. [DOI] [PubMed] [Google Scholar]
- Sladek C. D., Joynt R. J. Cholinergic involvement in osmotic control of vasopressin release by the organ-cultured rat hypothalamo-neurohypophyseal system. Endocrinology. 1979 Aug;105(2):367–371. doi: 10.1210/endo-105-2-367. [DOI] [PubMed] [Google Scholar]
- Sladek C. D., Joynt R. J. Role of angiotensin in the osmotic control of vasopressin release by the organ-cultured rat hypothalamo-neurohypophyseal system. Endocrinology. 1980 Jan;106(1):173–178. doi: 10.1210/endo-106-1-173. [DOI] [PubMed] [Google Scholar]
- Sladek C. D., Knigge K. M. Osmotic control of vasopressin release by rat hypothalamo-neurohypophyseal explants in organ culture. Endocrinology. 1977 Dec;101(6):1834–1838. doi: 10.1210/endo-101-6-1834. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Sawchenko P. E. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- Thomson A. M. Responses of supraoptic neurons to electrical stimulation of the medial amygdaloid nucleus. Neuroscience. 1982;7(9):2197–2205. doi: 10.1016/0306-4522(82)90130-0. [DOI] [PubMed] [Google Scholar]


