Abstract
Rivers serve crucial functions in the worldwide hydrological cycle. The industrial revolution, climate change, and urban development generated diverse water contaminants. This work aimed to assess the regional and seasonal distribution of some heavy metals (HMs) in the hot spot sites along the Great Cairo Sector, Nile River during 2021–2022. In addition, two commercial fish species (O. niloticus and C. gariepinus) were selected for assessing heavy metal content and human health risk. The results of heavy metals in water varied within; (1–7), (45–85.5), (19–84), (148–376), and (65–170) µg/l for Cd, Cu, Pb, Mn, and Zn, respectively over the study period. The heavy metal pollution index (HPI) results categorized the water status as unsuitable for drinking and aquatic life, but ideal for irrigation purposes. Based on Metal Index (MI) values, all examined sites were significantly at risk of metal contamination (> 1) over all uses. In the two investigated species, The results of Cd, Cu, Pb, Mn, and Zn varied in the edible part of fish species within (0.2–0.28), (2.01–5.41), (0.21–1.11), (12.1-15.25), (20.91–32.52) mg/g ww, respectively, for O. niloticus and within (0.2–0.35), (3.12–6.5), (1.52–3.62), (15.01–17.72), (15.12–26.93) mg/g ww, respectively, for C. gariepinus over the study period. The total annual daily intake of HMs was estimated to be 0.03625 and 0.03725 mg/kg.bw.day from the human consumption of O. niloticus and C. gariepinus, respectively. The Target Hazard Quotient (THQ) ranked in the order of Cd > Pb > Mn > Zn > Cu for O. niloticus and in the order of Pb > Cd > Mn > Cu > Zn for C. gariepinus with values lower than 1 that reported non-carcinogenic risk for consumers from the ingestion of investigated HMs seperately. Moreover, the Health Hazard Index (HI) slightly exceeded the threshold value of 1 of C. gariepinus, classified as moderate risk levels for consumers. Conversely, HI values were below 1 for O. niloticus, suggesting no risk from this species’ consumption. This study recommended an assertive water-quality monitoring strategy to mitigate health-related outbreaks and disruptions in aquatic ecosystems. The supplied data will undeniably assert environmental policymakers to implement sustainable pollution management and remediation measures.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-95308-z.
Keywords: Great Cairo Sector, Nile River, Target Hazard Quotient (THQ), Health Risk Index, O. niloticus, Heavy metal pollution index.
Subject terms: Ecology, Ecology, Environmental sciences
Introduction
Freshwater is vital for people’s existence, and its purity has become a worldwide concern1. The Nile River serves as Egypt´s essential source of fresh water, fulfilling practically all drinking and irrigation requirements2. The Nile water is affected by the hydrologic frameworks controlled by the stream´s canals, as well as land and water utilization, encompassing agricultural runoff, industrial discharges, municipal waste, and effluents from river vehicles3. The rise in industrial, agricultural, and recreational activities, along with inadequately designed drainage and sewage systems and the Ethiopian dam, poses a significant threat to both the amount and quality of Nile water4. Egypt´s annual allocation of the Nile River´s water is 55 billion cubic meters. Due to upstream dam developments on the Nile River, water consumption by adjacent nations, and population growth, Egypt’s Nile River has seen significant environmental alteration5. The Nile River supplies roughly 95% of the nation’s freshwater needs. Numerous branches, irrigation canals, and streams arise from the Nile, constituting an agricultural system that encompasses 31,000 km6. The Nile River serve mostly for drinking water, irrigation, industrial purposes, transportation, and fishing. Nonetheless, it is regarded as the principal receptor of wastewater and drainage produced by various activities4, resulting in substantial organic and inorganic waste, along with heavy metals, being discharged into the Nile and subsequently flowing into the Mediterranean Sea7. Heavy metals represent a compelling class of elements regarding aquatic pollution due to their detrimental impact on ecosystem balance, prolonged persistence, capacity for accumulation in water and sediments, and organism bioaccumulation8. Among these metals, cadmium (Cd) and lead (Pb) are potentially poisonous and lack any known biological function, but copper (Cu) and zinc (Zn) are vital9. However, excessive consumption of critical metals by aquatic species might lead to detrimental effects10. Elevated metal concentrations in aquatic systems can lead to significant water pollution issues, including possible health concerns for humans via the food chain2. Metal contamination in aquatic ecosystems is increasing at alarming rates, constituting a significant global issue. Metal contamination is often evaluated by measuring HMs levels in water, sediments, and biotic organisms11. It appears unsuitable to classify metals as fluxes in aquatic ecosystems just by water analysis12. Metal pollution in aquatic habitats poses a substantial risk to public health13. Prolonged exposure to metals is a significant health risk, potentially affecting all bodily organs, including respiratory ailments, neurological issues, carcinogenic effects, and osteoporosis14.
The deterioration of water’s purity is merely one detrimental consequence of water contamination, which is jeopardizing people’s health, ecological systems, financial growth, and society as a whole15,16. The potential for sustainability to maintain the integrity of the Nile’s water and its tourism appeal for the advantage of future generations must also be emphasized. Drinking water must adhere to various criteria and regulations, including World Health Organization (WHO), and Egyptian Water Quality Standard guidelines (EWQS), to guarantee that the water provided to the public is safe5.
Fish serve as bio-markers in the environment, providing valuable insights into the level of infection from HMs and the associated risks for human consumption17. The muscle tissue of fish is the primary portion ingested by customers. The muscle is recognized for its role in metal biotransformation and accumulation in fish18, which provides more significant insights into the pollution state than measuring their levels in water19. Excessive consumption of heavy metals impedes the functionality of biomolecules, including proteins and enzymes, by compromising their structures20. Fish is regarded as the primary and most economical source of protein in Egypt, with O. niloticus and C. gariepinus being the most frequently consumed species along the Nile River21. Estimation of the health risk exposed to humans through fish consumption is an important issue. Health risk assessments are based on assumptions that most chemicals with noncancer effects, exhibit a threshold respon22. The Target hazard quotient (THQ) has been employed for individual heavy metals contamination and the hazard index (HI) for combined cumulative effects. As Humans are often exposed to more than one pollutant and suffer combined or interactive effects, HI might overestimate the potential for noncancer health effects23. This is because the toxicological effects associated with exposure to multiple chemicals, often through different exposure pathways24.
Consequently, a thorough evaluation of the state of the surface and gaining expertise are imperative to guarantee proper and secure water utilization. Water quality monitoring establishes an empirical foundation for the sustainable management of water resources and assists governments in evaluating, regulating, and forecasting water pollution25. Employing water quality and heavy metal contamination indices constitutes a straightforward and efficient approach to evaluating water quality26. Assessing the human health risk from HMs intake via fish is a major issue. The purpose of this work is to (1) evaluate the potential sources of some HMs (Cd, Cu, Pb, Mn, and Zn) along the Great Cairo sector of the Nile River, (2) assess the extent of metal pollution using multiple indices [Pollution Index (PI), Heavy Metal Pollution Index (HPI), and Metal Index (MI)]; (3) analyze and compare the heavy metal content in the edible portions of two selected commercial fish species (O. niloticus and C. gariepinus), and (4) assess the bioaccumulation factor (BAF) and the associated health risk related to the fish intake by adults (70 years).
Results and discussion
Physiochemical parameters
Water temperature is a significant element that directly and indirectly impacts the water quality and aquatic life. It has an impact on aquatic species´ composition and the rate of chemical processes27. Water temperature fluctuated within (30–31.7), (24.1–26.9), (16.3–19.2), and (20.1–22.3) °C during summer, autumn, winter, and spring, respectively. One-way ANOVA analysis showed significant differences in water temperature values between seasons (P < 0.001) and sites (P < 0.05). The annual mean water temperature is significantly higher at site 3 than at the other sites, which may be attributed to the cooling system of the electric power station at Shubra. One crucial factor for assessing the water quality is its pH value. It relies on the balance between carbon dioxide and carbonate–bicarbonate. pH values varied from 7.5 at site 2 during winter to 8.5 at site 3 during summer. pH fluctuated significantly between the different seasons at p < 0.001 (One-Way ANOVA). The recent pH values were within the allowable criteria (6.5–9) for drinking water, recreation, agricultural, and aquatic life water use. Saad et al.28 reported the lowest pH values of 7.86 and 7.89 at El-Hawamdia and Helwan stations, respectively. Electrical Conductivity (EC) indicates the concentration of dissolved electrolyte ions in the water. However, significant increases in EC may be an indicator of polluting sources affecting the water. EC fluctuated between 239 µs/cm at site 3 during autumn and 642 µs/cm at site 1 during spring (Table S1 in the supplementary file). The annual mean values of EC were 439.83 ± 47.54, 295.5 ± 48.67, 368 ± 23.4, and 518.75 ± 97.65 µs/cm at sites 1, 2, 3, and 4 respectively. EC fluctuated significantly between seasons at p < 0.001. EC values were significantly higher at site 1 than S3 and S4 at p < 0.05 (Table S1). This may be attributed to high mineral salts discharged from the Iron and Steel Company at Helwan. Elsayed et al.29 recorded EC values varied in the ranges of 538–1176 and 371–516 µs/cm in the Rosetta and Damietta branches, respectively. Dissolved Oxygen (DO) is a critical variable that provides a healthy aquatic ecosystem. DO concentrations were higher than the acceptable level of 4 mg/l for aquatic life30; they were in the range of (6.04–6.63), (6.3–7.4), (7.3–8.5), and (6.4–7.1) mg/l during summer, autumn, winter, and spring, respectively, with significant differences between seasons (P < 0.05), Table 1. Site 4 recorded the highest DO concentration of 8.5 mg/l (Table S1).
Table 1.
Water quality parameters and HMs levels (µg/l) in the present study in relation to the established allowable limits for drinking water, irrigation, and aquatic life.
| Summer | Autumn | Winter | Spring | Drinking Water | Irrigation | Aquatic Life | ||
|---|---|---|---|---|---|---|---|---|
| EWQS43 | WHO44 | FAO 45 | USEPA 30 | |||||
| Temperature (°C) |
(30-31.7)a 30.70 ± 0.73 |
(24.1-26.92)b (25.33 ± 1.24) |
(16.3–19.2)d 17.40 ± 1.30 | (20.1–22.3)c 20.88 ± 1.01 | 12–25 | 8–28 | ||
| pH |
(7.75–7.81)a 7.78 ± 0.03 |
(7.12–7.45)c 7.29 ± 0.14 |
(8.6–9.23)b 8.92 ± 0.27 |
(7.95–8.01)b 7.98 ± 0.03 |
6.5–8.5 | 6.5–8.5 | 8.5 | |
| EC (µs/cm) |
(413–511)a, b 439.75 ± 47.59 |
(239–340)c 295.5 ± 48.67 |
(333–379)b, c 368 ± 23.40 |
(429–642)a 518.75 ± 97.65 | 2000 | 3000 | ||
| DO (mg/l) |
(6.04–6.63)b 6.34 ± 0.24 |
(6.29–7.4) b 6.77 ± 0.49 |
(7.3–8.5)a 7.93 ± 0.49 |
(6.38–7.06) b 6.65 ± 0.31 |
6 | 5.5 | ||
| BOD (mg/l) |
(4.02–6.5)a 5.03 ± 1.05 |
(4-5.2)a, b 4.50 ± 0.51 | (3.3–4.4)b 3.95 ± 0.51 |
(4.5–5.5)a 5.10 ± 0.43 |
3 | |||
| COD (mg/l) |
(11.2–16.4)a 14.05 ± 2.14 |
(5.2–11.9)b 8.60 ± 3.01 |
(7-9.5)b 8.28 ± 1.42 |
(8.5–11.1)b 9.78 ± 1.12 |
10 | 10 | ||
| Cd (µg/l) |
(2–7)a 4.5 ± 2.08 |
(2–4)a, b 3 ± 0.82 |
(1–4)b 2.5 ± 1.29 |
(2–6)a, b 3.75 ± 1.71 |
3 | 3 | 10 | 0.72 |
| Cu (µg/l) |
(56.2–85.5)a 67.5 ± 12.86 |
(56.3–69.8)a 63 ± 5.81 |
(45-56.3)a 51 ± 4.75 |
(45-56.2)a 51.19 ± 4.99 |
2000 | 2000 | 200 | 4 |
| Pb (µg/l) |
(21–86)a 64 ± 30.19 |
(19–48)b 32.75 ± 14.41 |
(22–35)b 29.5 ± 5.57 |
(23–43)b 30.75 ± 8.66 |
1 | 10 | 5000 | 2.5 |
| Mn (µg/l) |
(316–352)a 333 ± 17.70 |
(176–340)a, b 280 ± 74.69 |
(344–376)a 365 ± 14.38 |
(148–264)b 211 ± 48.15 |
100 | 100 | 200 | 100 |
| Zn (µg/l) |
(110–170)a 148.75 ± 26.58 |
(65–110)c 90.00 ± 19.58 |
(70–125)b, c 102.50 ± 23.98 |
(95–130)b 113.75 ± 16.52 |
500 | 3000 | 2000 | 120 |
Different letters refer to significant differences.
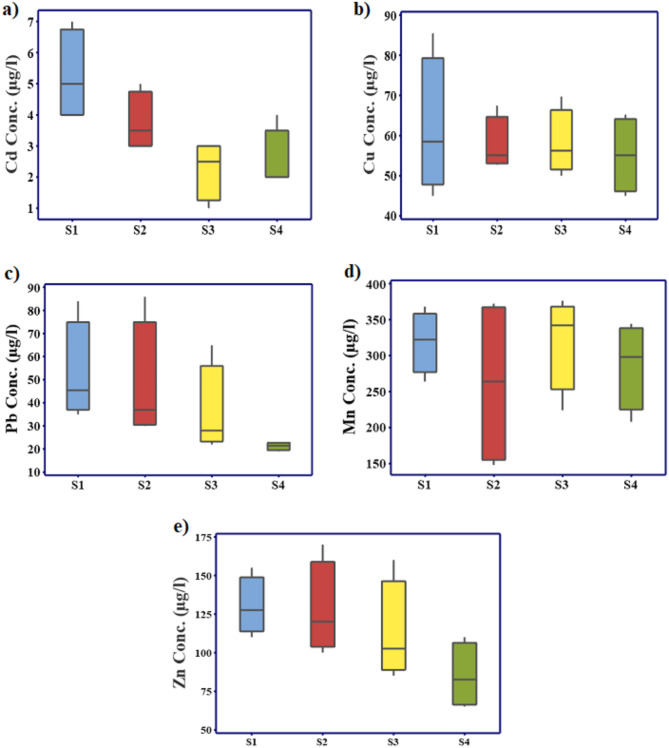
COD concentrations significantly varied between the sites within (7.4–7.8), (7.1–8.7), (7-8.5), and (7.5–8.3) mg/l; during summer, autumn, winter, and spring, respectively (ANOVA, p < 0.001). The maximum annual mean concentration of COD was 11.78 ± 3.3 mg/l at Site 3, affected by the drainage water of the Shubra electrical power station. However, Saad et al.28, Hussein et al.31, and Ali et al.32 recorded the maximum COD value at sites opposite to El-Hawamdia Company, affected by the effluent discharge from domestic sewage and agricultural discharge. BOD concentration ranged at the different sites within; (4–6.5), (4–5.2), (3.3–4.4), and (4.5–5.5) mg/l; during summer, autumn, winter, and spring, respectively, Fig. 1. All the sites showed BOD values within the acceptable limit (< 6 mg/l), except for site 3 during spring (Table S1). BOD recorded the lowest annual mean concentration at Site 4, El-Qanater El-Khayria, which agreed with the results of Ghannam33.
Fig. 1.
Box plot of (a) Water Temperature (°C), (b) EC (µS/cm), (c) pH, (d) DO (mg/l), (e) BOD (mg/l), and (f) COD (mg/l) concentrations in the Nile River water.
Heavy metals
Anthropogenic and natural processes simultaneously contribute to the environmental abundance of HMs34. High concentrations of both necessary (Cu, Mn, Zn) and unnecessary HMs (Cd, Pb) can have detrimental effects on aquatic ecosystems, biodiversity, wildlife, and people6. Cd is a bioaccumulative, and lethal HM that lasts in the environment for a long period35. Cd fluctuated in the different sites within the range of (2–7), (2–4), (1–4), and (2–6) µg/l during summer, autumn, winter, and spring, respectively (Table S2). Cd showed high temporal and spatial variation at p < 0.05 and < 0.001, respectively. Cd exceeded the acceptable limits of 3 µg/l for drinking water criteria and irrigation at some sites. Moreover, it exceeded the guideline values accepted for aquatic life (0.72 µg/l) according to USEPA30. Cd is a cytotoxic metal to aquatic organisms that is regulated by biotic factors, such as organic content, hardness, and pH4. The present results reported higher Cd levels than that recorded of the Nile River (Nd-3.96), and (Nd-5) µg/l reported by Hussein et al.31, and Elnazer et al.36, respectively, and that recorded of Rosetta branch (0.81–2.3), Damietta branch (0.2-2) µg/l, Great Cairo (0.01–0.09) µg/l, and Ismailia Canal (Nd-2.94) µg/l, by Al Afify & Abdel-Satar3, Tayel et al.37, Omar et al.38, and Goher et al.39, respectively. Comparatively, Cd varied within the range recorded of the Nile Islands (0.7–9.3) µg/l by Abdel Satar et al.40; Bahr Yusuf Canal (1.86–8.25) µg/l by Hassouna et al.41; Nile River (0.2–8.1) µg/l by Abdel-Satar et al.4; and El Bahr El pharaony (Nd-7) µg/l by Goher et al.42.
Cu is an essential metal, but its high concentration may cause adverse biological impacts. Cu had concentrations between (56.25–85.5), (56-69.75), (39.3-56.25), and (45-65.25) µg/l during summer, autumn, winter, and spring, respectively, with a nonsignificant variation between seasons. The Cu concentrations were within the level allowed for drinking water (2000 µg/l) and irrigation (200 µg/l). Meanwhile, they greatly exceeded the acceptable limit (4 µg/l) for aquatic ecosystems. S2 showed the highest annual mean Cu concentrations followed by S1 and S3. The current Cu concentrations were higher than the previous results (6.8–73), (1.1–20.30), (14–72), (3.04–13.94), (4.1-46.33), (10–51), (0-13.6), (2–21.24), and (2.19–8.89) µg/l of Nile Islands, Nile River, Rosetta Branch, Bahr Yusuf Canal, Damietta branch, Nile River, El Bahr El pharaony, Ismailia Canal, and Great Cairo by Abdel-Satar et al.40, Hussein et al.31, Al Afify & Abdel-Satar3, Hassouna et al.41, Tayel et al.37, Abdel-Satar et al. 20174, Goher et al.42, Goher et al.39, and Omar et al.38; respectively. However, they were lower than that recorded (Nd–170) µg/l of the Nile River by Elnazer et al.36.
Pb varied significantly between the different sites and seasons at p < 0.05. Pb had levels between (21–86), (19–48), (22–35), and (23–43) µg/l during summer, autumn, winter, and spring, respectively. The Pb concentrations greatly exceeded the guideline values for drinking water and aquatic life criteria, (Table 1). However, they were lower than the limit (5000 µg/l) reported for irrigation uses. Pb concentrations were significantly higher at S1 at p < 0.05. This study reported lower Zn levels than that recorded in the Nile River (163–402) µg/l by Elnazar et al.36 but higher than that recorded in the Nile Islands (3.5–66.5) µg/l, Nile.
River (6.8–42.80) µg/l, Rosetta Branch (9.3–67.9) µg/l, Bahr Yusuf Canal (17.62–49.62) µg/l, Nile River (5–50.4) µg/l, El Bahr El pharaony (0.2–31.8) µg/l, Ismailia Canal (10.55–34.01) µg/l, Great Cairo (0.03–2.07) µg/l by Abdel-Satar et al.40; Hussein et al.31; Al Afify & Abdel-Satar3; Hassouna et al.41; Abdel-Satar et al.4; Goher et al.42; Goher et al.39; and Omar et al.38; respectively. Mn varied significantly between seasons at (P < 0.05) and fluctuated within (316–352), (176–344), (344–376), and (148–264) µg/l during summer, autumn, winter, and spring, respectively. Zn showed significant temporal and spatial variations at p < 0.001. High Mn concentrations were reported in this study compared with some previous results of (21.8–247.7), (14.5–62.30), (40–220), (23.29–69.35), (7.3-138.4), (30–298), (0.36–4.45) µg/l by Abdel-.
Fig. 2.
Box plot of (a) Cd, (b) Cu, (c) Pb, (d) Mn, and (e) Zn concentrations in the Nile River water.
Satar et al.40, Hussein et al.31, Al Afify & Abdel-Satar3, Hassouna et al.41, Tayel et al.37, Abdel-Satar et al.4, Omar et al.38; respectively, but still lower than that reported of Ismailia Canal (20–483.4) µg/l, and El bahr El pharaony (1.6–720) µg/l by Goher et al.39,42, respectively. Zn varied significantly within (110–170), (65–110), (70–125), and (95–130) µg/l during summer, autumn, winter, and spring, respectively, Fig. (2). S4 was significantly lower than the other sites (p < 0.001, One-Way ANOVA) which may be attributed to its farness from the pollution sources. The maximum annual mean concentrations of Cd, Cu, Pb, and Zn were 4.85, 61.88, 52.5, and 130 µg/l, respectively at Site 1, affected by the drainage water from Iron and Steel Company (Table S2). On the other hand, Mn showed a higher annual mean concentration (321 µg/l) at site 3 affected by the cooling water discharged from the Shubra Electrical Power Station (Table S2). Zn concentrations were slightly greater than that reported of Nile Islands (16–140 µg/l) by Abdel Satar et al.40 and Rosetta branch (28.55–117.36 µg/l) by Al Afify & Abdel-Satar3 but lower than that reported of the Nile River (50–700 µg/l) by Elnazar et al.36 and El Bahr El pharaony (3–222 µg/l) by Goher et al.42. The annual mean concentration of HMs decreased in the rank; Mn > Zn > Pb > Cu > Cd over the study period. Hashem et al.46 ranked the HMs concentration in Nile water in the order of Zn > Mn > Cu > Pb and referred to the highest pollution sites affected by the El-Rahawy drain during different seasons. A comparison of the current metal concentrations with other rivers around the world was reported in Table (2). The current Cu, Pb, Mn, and Zn concentrations in the Great Cairo sector were much less than that reported of Halda River, Bangladesh by Dye et al.,47 although Cd showed similar concentrations. In addition, the results of Cd and Pb concentrations were lower in compared with the Pasig River, Philippines reported by Paronda48. On the other hand, this study evaluated higher Cd and Cu concentrations in contrary to the Pearl River, China, and the Orontes River, Turkey reported by Jiao49 and Kilic & Can50.
Table 2.
A comparison of the HMs results in the Nile River water with other previous studies in Egypt and other countries.
| Study Area | Cd | Cu | Pb | Mn | Zn | Ref. |
|---|---|---|---|---|---|---|
| Great Cairo, Nile River, Egypt | 1–7 | 45–85.5 | 19–84 | 148–376 | 65–170 | Present Study |
| Great Cairo, Nile River, Egypt | 0.01–0.09 | 2.19–8.89 | 0.03–2.07 | 0.36–4.45 | 20.48–55.81 | 38 |
| Nile River, Egypt | 0.2–8.1 | 10–51 | 5–50.4 | 30–298 | 10–108 | 4 |
| Nile River, Egypt | Nd–3.69 | 1.1–20.30 | 6.8–42.80 | 14.5–62.30 | 9.90–68.20 | 31 |
| Nile River, Egypt | Nd–5 | Nd–170 | 163–402 | – | 50–700 | 36 |
| Nile River, Rosetta Branch, Egypt | 0.81–2.3 | 14–72 | 9.3–67.9 | 40–220 | 21.1–133 | 3 |
| Damietta branch, Nile River, Egypt | 0.2–2 | 4.1–46.33 | – | 7.3–138.4 | 28.55–117.36 | 37 |
| Nile Islands, Egypt | 0.7–9.3 | 6.8–73 | 3.5–66.5 | 21.8–247.7 | 16–140 | 40 |
| Ismailia Canal, Nile River, Egypt | Nd–2.94 | 2–21.24 | 10.55–34.01 | 20–483.4 | 1.4–127.9 | 39 |
| Bahr Yusuf Canal, Egypt | 1.86–8.25 | 3.04–13.94 | 17.62–49.62 | 23.29–69.35 | 16.03–42.18 | 41 |
| El Bahr El pharaony, Nile River, Egypt | Nd-7 | 0–13.6 | 0.2–31.8 | 1.6–720 | 3–222 | 42 |
| Halda River, Bangladesh | 1–8 | – | 700–2600 | 175–1185 | 399–941 | 47 |
| Pasig River, Philippines | Nd-51 | Nd-63 | Nd-628 | – | – | 48 |
| Pearl River, China | 0.05–0.75 | 3–5 | 0.06–1.09 | – | 17–61 | 49 |
| Orontes River, Turkey | Nd-0.16 | 2.01–20.9 | 0.3–3.3 | 13–1555 | 1–87 | 50 |
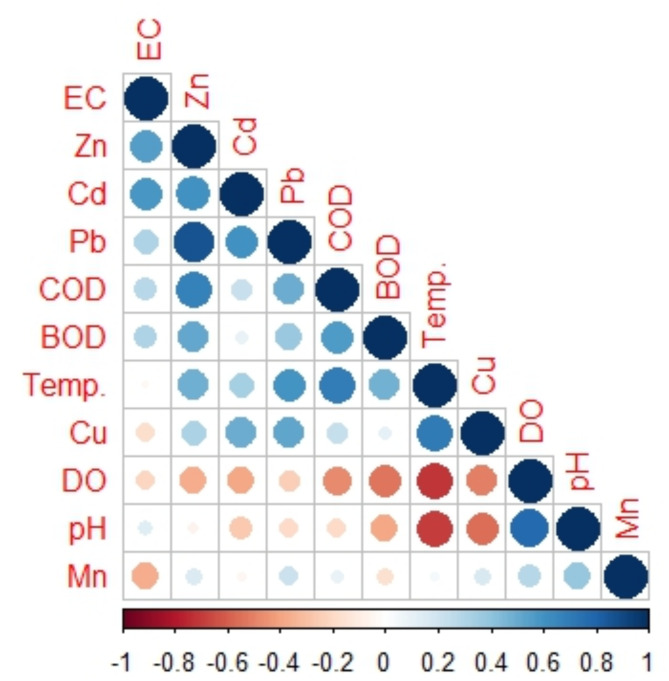
The results of the Pearson correlation coefficient showed a strong negative correlation between DO and temperature (r = −0.73), which reflects low DO solubility at high water temperature values during summer (Fig. 3). Zn was positively correlated with EC (r = 0.51), COD (r = 0.69), BOD (r = 0.54), Cd (r = 0.52), and Pb (r = 0.85) but negatively correlated with DO (r=−0.35). The positive correlation reflects the potential sources of Cd, Pb, and Zn from the wastewater drains coupled with the increase of organic matter induced by COD and BOD concentrations and hence a rise in EC values and a decrease in DO consumed by high organic matter discharged. Moreover, Cu was negatively correlated with pH and DO (r= −0.69 and − 0.61), respectively. This reflects its source from the wastewater drains. Cu, Pb, and Zn were positively correlated with water temperature (r = 0.68, 0.50, 0.41), which reflects their higher levels during summer than the other seasons. That may be attributed to the liberation of metals from bottom sediment at high temperatures during the fermentation process51. This assumption agreed with the findings outlined by Goher et al.,39,42; Bahnasawy et al.52, Nwabueze and Oghenevwairhe53, Ibrahim and Omar54. On the other hand, Mn showed an elevated seasonal mean during winter, which may be the result of water-carrying soil from neighboring coasts, given the abundance of Mn in the earth´s outermost layers55.
Fig. 3.
A correlogram illustrating the relationships between the variables under investigation in water samples. The circles’ color and size correspond to the correlation coefficients. Red color presents negative correlations while blue refers to positive correlations with different shades. Large-sized circles refer to a strong correlation and small- sized circles present a weak correlation. Correlations with p-value greater than 0.05 are deemed insignificant and are indicated by an empty white space.
Pollution index
The PI is determined by the concentration of each metal, which allows us to predict the single effect of the pollution degree of each HM. Table 3 shows the PI values and categories of Cd, Cu, Pb, Mn, and Zn in the Nile River water based on standard values for drinking, irrigation, and aquatic life30,43–45. The PI values of Cd, Cu, Pb, Mn, Zn varied within; 0.53–1.3, 0.021–0.029, 1.49–4.55, 2-2.26, 0.02–0.03 according to EWQS43 drinking water criteria and within 0.53 - 1.34, 0.021–0.029, 14.92–45.54, 2-2.26, 0.13–0.20 according to WHO44 drinking water criteria. Cu and Zn showed PI values < 1 that showed a negligible effect on drinking water according to EWQS43 and WHO44 drinking water criteria. Cd had no effect on all sites except site 1 which was slightly affected according to EWQS43 and WHO44 drinking water criteria. Otherwise, Mn was moderately affected at all sites according to EWQS43 and WHO44 drinking water criteria. Pb varied from slightly affected at S4 (El Qanater) to strongly affected at the other sites according to EWQS43. Furthermore, Pb was seriously affected at all sites according to WHO44 drinking water criteria. For irrigation utilization, Cd, Cu, Pb, Mn, and Zn showed PI values of 0.16–0.4, 0.2–0.24, 0.003–0.009, 1–1.13, and 0.03–0.05, respectively according to FAO45. Cd, Cu, Pb, and Zn had PI values < 1 at all sites, exhibited no pollution effect, while Mn was slightly affected at all sites for irrigation uses. The PI values of Cd, Cu, Pb, Mn, and Zn for aquatic life varied within 2.2 - 5.6, 9.91–12.08, 5.97–18.22, 2-2.26, and 0.53–0.82, respectively, at the different sites according to USEPA30. Cd varied from moderately affected at S3 to strongly affected at S2 and S4, then seriously affected at S1. Furthermore, Cu and Pb showed PI values > 5, indicating a serious effect on the aquatic ecosystems at all sites according to USEPA30 criteria guidelines. Zn was slightly affected in the aquatic ecosystems at all sites, while Mn was moderately affected at all sites. Goher et al.39 documented a serious effect of Zn along the Ismailia Canal based on drinking and aquatic life standards while indicating slight to severe pollution by Pb consequences at all stations for drinking and aquatic life use.
Table 3.
PI values of the investigated HMs in the Nile River water.
| Site | Drinking 43 | Drinking 44 | Irrigation 45 | Aquatic life 30 | ||||
|---|---|---|---|---|---|---|---|---|
| Cd | PI | Effect | PI | Effect | PI | Effect | PI | Effect |
| S1 | 1.34 | Slight | 1.34 | Slight | 0.40 | No | 5.60 | Serious |
| S2 | 0.97 | No | 0.97 | No | 0.29 | No | 4.05 | Strong |
| S3 | 0.53 | No | 0.53 | No | 0.16 | No | 2.20 | Moderate |
| S4 | 0.75 | No | 0.75 | No | 0.22 | No | 3.11 | Strong |
| Cu | ||||||||
| S1 | 0.02 | No | 0.02 | No | 0.24 | No | 12.08 | Serious |
| S2 | 0.02 | No | 0.02 | No | 0.21 | No | 10.71 | Serious |
| S3 | 0.02 | No | 0.02 | No | 0.21 | No | 10.73 | Serious |
| S4 | 0.02 | No | 0.02 | No | 0.20 | No | 9.91 | Serious |
| Pb | ||||||||
| S1 | 4.55 | Strong | 45.50 | Serious | 0.009 | No | 18.20 | Serious |
| S2 | 4.55 | Strong | 45.54 | Serious | 0.009 | No | 18.22 | Serious |
| S3 | 3.43 | Strong | 34.31 | Serious | 0.007 | No | 13.72 | Serious |
| S4 | 1.49 | Slight | 14.92 | Serious | 0.003 | No | 5.97 | Serious |
| Mn | ||||||||
| S1 | 2.26 | Moderate | 2.26 | Moderate | 1.13 | Slight | 2.26 | Moderate |
| S2 | 2.00 | Moderate | 2.00 | Moderate | 1.00 | Slight | 2.00 | Moderate |
| S3 | 2.19 | Moderate | 2.19 | Moderate | 1.09 | Slight | 2.19 | Moderate |
| S4 | 2.01 | Moderate | 2.01 | Moderate | 1.00 | Slight | 2.01 | Moderate |
| Zn | ||||||||
| S1 | 0.03 | No | 0.19 | No | 0.05 | No | 0.79 | Slight |
| S2 | 0.03 | No | 0.20 | No | 0.05 | No | 0.82 | Slight |
| S3 | 0.03 | No | 0.18 | No | 0.05 | No | 0.75 | Slight |
| S4 | 0.02 | No | 0.13 | No | 0.03 | No | 0.53 | Slight |
Metal index (MI)
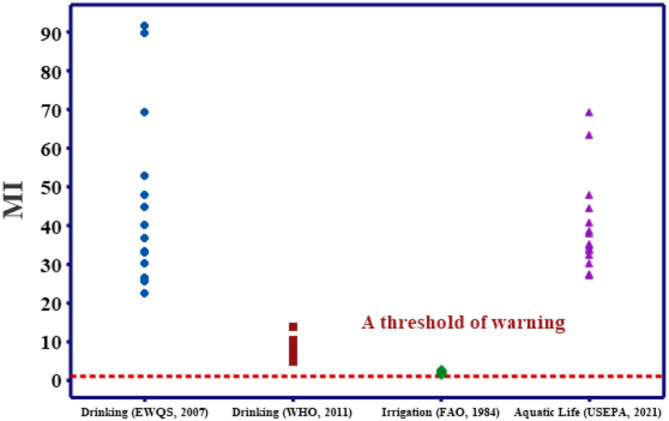
A further indicator for determining the HMs contamination in the Nile River water for various uses is the Metal Index (MI). It takes into account all the measured HMs. MI values ranged within 22.59 -91.56 and 5.38–13.99 in the different seasons for drinking water according to EWQS43 and WHO44, respectively and 1.48–2.8 for irrigation according to FAO45 and 26.85–69.15 for aquatic life utilizations according to USEPA30. As MI values > 1, metal contamination poses a major risk to all of the sites under investigation for all purposes, Fig. 4. This may be due to the release of municipal, industrial, and irrigation waste products discharged into the surface river water56. Site 1 was the most contaminated site over the study period by the investigated HMs. Site 1 is directly affected by the drainage water from the iron and Steel Company. This effluent carries huge quantities of different metals. According to Goher et al.39, MI was greater than 1 along the Ismailia Canal and Nile River at all the sites and posed a major HMs danger for drinking water and aquatic life use. Moreover, Hussein et al.31 recorded MI ≥ 1 at all the selected sites along the Nile River that are extremely endangered by metal contamination for use in drinking water and aquatic life use. Abdel Satar et al. reported MI values in the Nile water exceeding the critical limit for drinking water according to EWQS Guidelines, indicating great contamination by HMs. Hassouna et al. recorded MI values in the Bahr Yusuf Canal exceeding the acceptable limit for aquatic life and pointed out the great risk exposed to aquatic organisms living there. Al-Afify et al. reported that some metals in the Nile water surpassed the adaptability for aquatic life before and after the pollution source. Previous researchers linked the industries and agrochemicals as the primary sources of HMs in the Nile River water.
Fig. 4.
Individual plot of the MI values in the Nile River water.
Heavy metal pollution index (HPI)
Table 4 represents the HPI values of the Nile River. HPI exceeded 100 during the different seasons for drinking water according to EWQS43 and WHO44, and for the protection of aquatic life according to USEPA30. All sites were classified as extremely contaminated. On the other hand, HPI for irrigation fluctuated between 18 and 72, classified as low contaminated, and hence was excellent and suitable for irrigation use according to FAO45 (Table 4). The HPI results classified the water status of the investigated sites as undesirable for drinking and aquatic life, but it is excellent for irrigation utilizations.
Table 4.
HPI values of the investigated HMs in the Nile River water.
| Season | Site | Drinking 43 | Drinking44 | Irrigation 45 | Aquatic life 30 | ||||
| HPI | Classification | HPI | Classification | HPI | Classification | HPI | Classification | ||
| Summer | S1 | 6300.88 | Extreme Contamination | 2074.23 | Extreme Contamination | 72.33 | No Contamination | 1571.49 | Extreme Contamination |
| S2 | 6433.23 | Extreme Contamination | 2070.04 | Extreme Contamination | 54.68 | No Contamination | 1345.05 | Extreme Contamination | |
| S3 | 4848.22 | Extreme Contamination | 1521.80 | Extreme Contamination | 27.14 | No Contamination | 866.18 | Extreme Contamination | |
| S4 | 1595.60 | Extreme Contamination | 580.30 | Extreme Contamination | 44.76 | No Contamination | 724.89 | Extreme Contamination | |
| Autumn | S1 | 3601.60 | Extreme Contamination | 1188.60 | Extreme Contamination | 44.94 | No Contamination | 934.92 | Extreme Contamination |
| S2 | 3146.45 | Extreme Contamination | 1025.01 | Extreme Contamination | 32.37 | No Contamination | 780.04 | Extreme Contamination | |
| S3 | 1661.79 | Extreme Contamination | 578.24 | Extreme Contamination | 36.37 | No Contamination | 666.25 | Extreme Contamination | |
| S4 | 1430.17 | Extreme Contamination | 484.21 | Extreme Contamination | 25.79 | No Contamination | 535.10 | Extreme Contamination | |
| Winter | S1 | 2636.08 | Extreme Contamination | 896.70 | Extreme Contamination | 45.74 | No Contamination | 820.39 | Extreme Contamination |
| S2 | 2404.97 | Extreme Contamination | 804.20 | Extreme Contamination | 36.72 | No Contamination | 692.64 | Extreme Contamination | |
| S3 | 2165.60 | Extreme Contamination | 686.67 | Extreme Contamination | 18.68 | No Contamination | 473.38 | Extreme Contamination | |
| S4 | 1653.56 | Extreme Contamination | 553.31 | Extreme Contamination | 26.87 | No Contamination | 497.26 | Extreme Contamination | |
| Spring | S1 | 3246.17 | Extreme Contamination | 1124.59 | Extreme Contamination | 61.21 | No Contamination | 1035.47 | Extreme Contamination |
| S2 | 2262.98 | Extreme Contamination | 779.13 | Extreme Contamination | 40.72 | No Contamination | 773.59 | Extreme Contamination | |
| S3 | 2032.40 | Extreme Contamination | 688.25 | Extreme Contamination | 33.45 | No Contamination | 663.63 | Extreme Contamination | |
| S4 | 1726.85 | Extreme Contamination | 572.77 | Extreme Contamination | 23.90 | No Contamination | 518.13 | Extreme Contamination | |
HMs in fish
Aquatic organisms are impacted by HM contamination at the cellular level, leading to ecological discrepancies that may threaten the food chain. Fish are thought to be one of the best indicators of HMs toxicity in the aquatic environment59. Cd concentrations varied in the edible part of fish between (0.22 - 0.28) mg/g ww for O. niloticus and between (0.2–0.35) mg/g ww for C. gariepinus. These concentrations exceeded the permissible levels of (0.2, 0.1, 0.2, and 0.05) set by ANZECC/ARMCANZ60, EU61, MAFF62, and EC63, respectively. However, they were still lower than the FAO64 guideline value of 0.5 mg/g. The recent Cd concentrations were within the level recorded of Mango tilapia (Nd-0.3) mg/g ww, Nile tilapia (Nd-2) mg/g ww, and Tilapia Zillii (Nd-1.1) mg/g ww in the Rayahs, Nile River by Ghannam et al.65, but less than that recorded of O. niloticus (2.6–14.7) mg/g ww in the Damietta branch by Tayel et al.37. Cu concentrations varied between 3.12 and 6.5 mg/g ww in C. gariepinus and between 2.01 and 5.41 mg/g ww in O. niloticus. These concentrations were lower than the guideline values of (30, 30, 30, 10, 20 mg/g) set by FAO66, FAO/WHO64; WHO67; EU61, and MAFF62 respectively. In comparison, the current results of Cu were similar to those recorded of O. niloticus and C. gariepinus at the Great Cairo sector, Nile River by El Haddad et al.,68. Pb concentration fluctuated between (0.21–1.11) and (1.52–3.62) mg/g ww in the edible part of O. niloticus and C. gariepinus, respectively. The annual mean concentration of Pb in O. niloticus (0.638 ± 0.38 mg/g) exceeded the guideline values of (0.5, 0.5, 0.2, and 0.1) reported by FAO3, FAO/WHO64, EU61, and EC63, respectively. But it was still below the 2 mg/g allowable limits set by ANZECC/ARMCANZ60 and MAFF65. On the other hand, the annual mean concentration of Pb in C. gariepinus (2.665 ± 0.97 mg/g ww) exceeded all the stipulated permissible limits, (Table 5). Pb is known to be detrimental to human health regardless of the exposure rate, causing neurotoxicity and nephrotoxicity rapid behavioral and metabolic disorders, and growth retardation in humans22. The current Pb concentrations exceeded those documented of O. niloticus and C. gariepinus, in the Great Cairo, Nile River by El-Haddad et al.68. Mn concentrations varied within (12.1–15.25) and (15.01–17.72) mg/g ww in O. niloticus and C. gariepinus, respectively. These concentrations exceeded the WHO67 guideline value of 1 mg/g; nonetheless, they were below the permitted thresholds of 20 mg/g set by Dural et al.69. Zn concentrations fluctuated between 20.91 and 32.51 mg/g ww in O. niloticus and between 15.12 and 26.92 mg/g ww in C. gariepinus in the different seasons. The annual mean concentration of Zn was 27.29 ± 4.81 and 21.63 ± 5.14 for O. niloticus and C. gariepinus, respectively. It was lower than the permissible level allowed of (30, 40, 100) mg/g by FAO66, FAO/WHO64, and WHO67; respectively. This study inferred higher Zn levels than that recorded of Oncorhynchus mykiss trout (6.2–11.2 mg/g) in the North and Southeast Rivers, Iran, by Khammar et al.13.
Table 5.
Comparison of the HMs results (mg/g ww) in the muscles of O. niloticus and C. gariepinus with other species in different areas and the international standard permissible limits.
| Study Area | Fish Sp. | Cd | Cu | Pb | Mn | Zn | Ref. |
|---|---|---|---|---|---|---|---|
| Great Cairo, Nile River | Oreochromis niloticus | 0.2–0.28 | 2.01–5.41 | 0.21–1.11 | 12.1–15.25 | 20.91–32.52 | Present Study |
| C. gariepinus | 0.2–0.35 | 3.12–6.5 | 1.52–3.62 | 15.01–17.72 | 15.12–26.92 | ||
| Great Cairo, Nile River | Oreochromis niloticus | 1.62–7.71 | 0.02–0.26 | 0.2–1.89 | 4.93–47.17 | 68 | |
| S. galilaeus | 2.55–8.34 | 0.03–1.37 | 0.29–2.28 | 6.66–53.71 | |||
| Damietta branch, Nile River | Oreochromis niloticus | 2.6–14.7 | 7.8–25.3 | – | 20.8–75.2 | 25.85–67.5 | 37 |
| Rayahs, Nile River | Nile tilapia | Nd-2 | Nd-18.6 | Nd-15.4 | 1.1–56.90 | 8.4–98.4 | 65 |
| Mango tilapia | Nd-0.3 | Nd-26.2 | Nd-19.4 | Nd-35.16 | Nd-64.4 | ||
| Tilapia Zillii | Nd-1.1 | Nd-26.4 | Nd-16.3 | Nd-41 | 4.7–96.29 | ||
| El Bahr El pharaony | Oreochromis niloticus | 0.021–0.037 | 0.82–3.65 | 1.72–5.64 | 0.24–2.52 | 5.35–16.56 | 42 |
| North and Southeast Rivers, Iran | Oncirhynchus mykiss trout | – | 5.8–8.4 | 3.2–6.6 | – | 6.2–11.2 | 13 |
| Standard Permissible level | 30 | 0.5 | 30 | 66 | |||
| 0.5 | 30 | 0.5 | 40 | 64 | |||
| 30 | 1 | 100 | 68 | ||||
| 0.2 | 2 | 60 | |||||
| 0.2 | 20 | 2 | 50 | 62 | |||
| 0.1 | 10 | 0.1 | 61 | ||||
| 0.05 | 0.2 | 63 | |||||
| 20 | 69 | ||||||
Elevated concentrations of critical metals (e.g., Cu, Mn, and Zn) in the fish muscles may lead to diarrhea, abdominal cramps, tremors, anemia, alopecia, and facial muscle spasms22. Moreover, nonessential elements like Pb and Cd, irrespective of their amounts pose significant dangers, and can induce acute toxicity to human biological systems22. Prolonged exposure to these elements from seafood sources may induce mutagenic, teratogenic, and carcinogenic health effects. This encompasses neurological and nervous system impairment, cardiovascular, renal, and liver failure, cancers, vascular trauma, telangiectasias, hormonal and endocrine dysfunction, infertility, digestive gland damage, and other systems, depending on the extent and pattern of uptake and the exposure route22,23.
The toxicity of HMs encompasses numerous factors. These HMs generate free radicals that lead to the oxidative degradation of biomolecules (DNA, proteins, and lipids)70. They can specifically target and relate to DNA strands to produce apotosis, tumor growth, and alterations in the cell cycle. They impede metabolic activities, tissue repair, and cellular and organ detoxification71. However, each metal possesses its unique toxicity mechanism70.
Bioaccumulation factor (BAF)
Fish might bioaccumulate HMs directly by consuming water and the food chain or from the sediment particles via the digestive system across biological membranes, including gills and muscles. Bioaccumulation of HMs in the different fish organs is greatly interspecific72. The BAF values of Cd, Cu, Pb, Mn, and Zn in the edible part varied within 61.33 - 88, 39.41–80.18, 6.82–21.99, 33.15–61.66, and 204-316.89, respectively, for O. niloticus and within 53.33-90, 60.95–96.3, 34.59-122.71, 46.44–71.14, and 147.51-269.44, respectively, for C. gariepinus, Fig. 5. No significant variations between the BAF measurements of all investigated HMs between the two fish species (unpaired t-test). BAF values of the investigated HMs were lower than 1000, so they indicated no probability of accumulation based on Olayinka–Olagunju et al. classification73. The BAF of the investigated HMs followed the order of Zn > Mn > Cd > Cu > Pb in O. niloticus and Zn > Pb > Cd > Cu > Mn in C. gariepinus. The variance in the accumulation levels of various fish species can primarily be ascribed to variances in the regulatory ability, behavior, and feeding habits74. In comparison, this accumulation pattern was slightly different from the pattern reported in O. niloticus muscles (Zn > Mn > Cu > Pb > Cd) in Great Cairo sector and the Nile rayahs discussed by Ghannam et al.65, and Taleb et al.75.
Fig. 5.
Boxplot of BAF values of Cd, Cu, Pb, Mn and Zn in the edible part of O. niloticus and C. gariepinus.
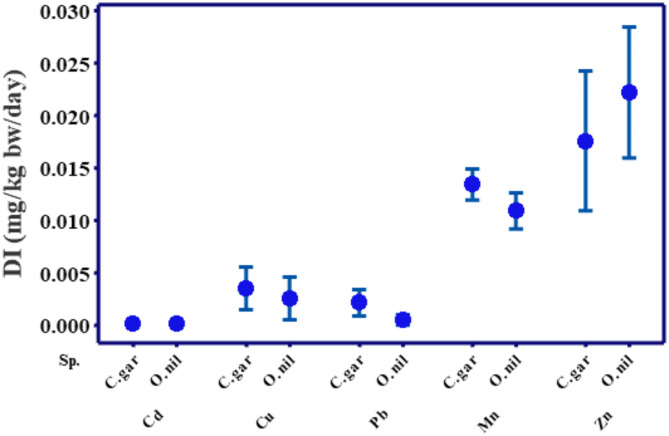
Daily intake (DI)
Fish, positioned at the apex of the food structure, are the main target for the biomagnification of HMs and likely serve as channels for transfer to humans76. O. niloticus and C. gariepinus are the most common commercial fish species for human consumption in the Great Cairo Sector. Based on the consumption of 57 g of fish tissues by a 70 kg Egyptian person per day, the annual DI of Cd, Cu, Pb, Mn, and Zn from O. niloticus consumption was 0.0222, 0.0026, 0.0109, 0.0002, and 0.0005, mg/kg bw/day, respectively. Meanwhile, the DI.
of Cd, Cu, Pb, Mn, and Zn from C. gariepinus consumption was 0.0176, 0.0035, 0.0134, 0.0002, and 0.0022 mg/kg bw/day, respectively, Table 6. Moreover, the total DI values of all investigated HMs for O. niloticus were estimated to be 0.044, 0.037, 0.029, and 0.035 mg/kg bw/day during summer, autumn, winter, and spring, respectively. Meanwhile, they were; 0.044, 0.040, 0.032, and 0.033 mg/kg bw/day for C. gariepinus during summer, autumn, winter, and spring, respectively. Among the investigated HMs, Zn, and Mn constituted the highest estimated DI than Cu, Pb, and Cd (Fig. 6).
Table 6.
The DI (mg/kg bw/day), THQ, and HI values of HMs from the consumption of O. niloticus and C. gariepinus muscles collected from the Great Cairo sector, the Nile River.
| O. niloticus | C. gariepinus | |||||||
|---|---|---|---|---|---|---|---|---|
| DI (mg/kg bw/day) | Summer | Autumn | Winter | Spring | Summer | Autumn | Winter | Spring |
| Cd | 0.00023 | 0.00016 | 0.00018 | 0.00019 | 0.00029 | 0.00022 | 0.00017 | 0.00016 |
| Cu | 0.00441 | 0.00252 | 0.00164 | 0.00173 | 0.00529 | 0.00368 | 0.00265 | 0.00254 |
| Pb | 0.00090 | 0.00059 | 0.00042 | 0.00017 | 0.00181 | 0.00269 | 0.00295 | 0.00124 |
| Mn | 0.01242 | 0.01084 | 0.00985 | 0.01059 | 0.01443 | 0.01328 | 0.01380 | 0.01222 |
| Zn | 0.02647 | 0.02322 | 0.01703 | 0.02216 | 0.02192 | 0.01975 | 0.01231 | 0.01646 |
 (mg/kg bw/day) (mg/kg bw/day) |
0.044 | 0.037 | 0.029 | 0.035 | 0.044 | 0.040 | 0.032 | 0.033 |
| THQ | Summer | Autumn | Winter | Spring | Summer | Autumn | Winter | Spring |
| Cd | 0.23 | 0.16 | 0.18 | 0.19 | 0.29 | 0.22 | 0.17 | 0.16 |
| Cu | 0.11 | 0.06 | 0.04 | 0.04 | 0.13 | 0.09 | 0.07 | 0.06 |
| Pb | 0.26 | 0.17 | 0.12 | 0.05 | 0.52 | 0.77 | 0.84 | 0.35 |
| Mn | 0.09 | 0.08 | 0.07 | 0.08 | 0.10 | 0.09 | 0.10 | 0.09 |
| Zn | 0.09 | 0.08 | 0.06 | 0.07 | 0.07 | 0.07 | 0.04 | 0.05 |
| HI | 0.77 | 0.55 | 0.47 | 0.43 | 1.11 | 1.24 | 1.22 | 0.72 |
Fig. 6.
DI values of HMs by the intake of O. niloticus and C. gariepinus muscles collected from Great Cairo, Nile River.
Target hazard quotient and hazard index
The THQ values explained the single effect of each HM by the fish tissues´ intake. The present results explored an absence of non-carcinogenic risk of all the HMs in the two fish species with THQ values < 1, Fig. 7a. They were in the order of Cd (0.189) > Pb (0.148) > Mn (0.078) > Zn (0.074) > Cu (0.064) for O. niloticus and in the order of Pb (0.620) > Cd (0.210) > Mn (0.096) > Cu (0.089) > Zn (0.059) for C. gariepinus. Following Custodio et al.77, eating either if the two fish species does not present any non-carcinogenic health hazards when consuming the examined HMs separately. The annual mean HI value was estimated to be 0.55 ± 0.15 and 1.07 ± 0.24, for O. niloticus and C. gariepinus, respectively. The HI value was found to be lower than the permitted level of 1 in the muscles of O. niloticus in all seasons, (Fig. 7b). Thus, they referred to no health risk to consumers from O. niloticus consumption in the study area. On the other hand, HI values of C. gariepinus slightly exceeded the threshold value of 1 during summer, autumn, and spring, classified as moderate risk levels for consumers. Pb and Cd were the major contributing metals in HI where Pb contributed 60.71% HI, and Cd with 17.94%, followed by Mn (8.85%), then Cu (7.29%), and Zn (5.18%). The HI results referred to serious problems to human health by the intake of C. gariepinus fishes from the Great Cairo Sector. The findings of the research highlight the urgent need for enhanced environmental management strategies to mitigate heavy metal contamination in the Great Cairo Sector. The estimated HI value in both O. niloticus and C. gariepinus was lower than that reported for the crayfish (1.27–1.92), and (22.9–3.22) collected from El-Kanater Station and El-Rahawy drain, respectively35. In comparison, El-Haddad et al.,68 reported no carcinogenic risk (HI < 1) of consuming O. niloticus or S. galilaeus from the Nile River Islands. Additionally, the results of the health risk assessment justified the importance of monitoring and regulating heavy metal levels in fish species to protect the health of consumers.
Fig. 7.
(a) THQ values and (b) HI of HMs in muscles of O. niloticus and C. gariepinus collected from the Great Cairo Sector, Nile River.
Materials and methods
Study area
The Nile River is a prominent characteristic of Africa’s northeastern basin. It is a significant northward-flowing river in northeastern Africa that arises in Ethiopia and empties into the Mediterranean Sea75. The Nile is the largest river in Africa and has traditionally been regarded as the longest river globally, extending around 6,825 km. The Nile serves as the principal water source for Egypt, Sudan, and South Sudan. The Nile River in Egypt is subjected to various pollutants from both point and non-point sources along its coasts78. Numerous pollutant categories from diverse sources, including sewage, residential, industrial, and agricultural waste effluents, are dumped into the Nile from El-Rahawy drains, exceeding 5 × 108 m³ per day79. In the Delta region, the Nile bifurcates into two primary branches (Rosetta and Damietta) and four subsidiary branches (irrigation canals, referred to as rayahs in colloquial dialect)80. This article pertains to four locations within the Great Cairo Sector of the Nile River. They were chosen based on their proximity to certain polluting activities, (Fig. 8). Table (7) presents the locations and descriptions of sites, together with the types of human activity conducted there.
Fig. 8.
Map showing sampling sites of the Great Cairo, Nile River54.
Sample collection and preservation
Water
Forty-eight surface water samples (0–0.5 cm depth) were obtained from four sites along the Nile River, spanning from Helwan to El-Qanater El-Khayria, between summer 2021 and spring 2022. The sampling sites (S1–S3) were selected due to their closeness to anticipated emission sources, while S4 was considered as a control as it is far from direct pollution sources (Table 7). Prior to water collection, the sampling bottles were washed with distilled water and eventually soaked in 10% HNO3. The samples were collected as triplicates from each site. Water samples were obtained using a 2 L polyvinyl chloride Van Dorn bottle water sampler, stored in an ice box, and then transported to the laboratory. For HMs analysis, the collected samples were acidified with 0.5% HNO3 in well-labeled bottles and were stored at room temperature (4 °C) prior to laboratory evaluation.
Table 7.
Locations and features of the sampling sites.
| Site | Feature | Activities | Latitude | Longitude |
|---|---|---|---|---|
| S1 | Helwan, In Front of Iron and Steel company | I, D | 29°48′0″N | 31°17′45″E |
| S2 | El-Hawamdiya, in front of Starch and Glucose Company | I, D | 29°52′31″N | 31°17′3″E |
| S3 | Shobra, In front of the Electrical power Station | I, D, S | 30° 7′29″N | 31°14′4″ E |
| S4 | El-Qanater El-Khayria, In front of the water purification Station | A | 30°11′1″N | 31° 8′20″E |
Where A: Agricultural, I: Industrial, D: Domestic activities.
Fish
Two fish species (Oreochromis niloticus and Clarias gariepinus) were collected seasonally using a fisherman’s net. These two species are the most commonly species in the Nile River and the highest consumption by humans. Nearly 30 adult samples from each species were considered and analyzed in triplicates. we collected O. niloticus during summer (mean length 15.9 ± 5.2 cm and mean weight 91.8 ± 5.1 g), autumn (mean length 15.8 ± 1.1 cm and mean weight 91.1 ± 7.1), winter (mean length 17.3 ± 2.2 cm and mean weight 95.6 ± 3.8), and spring (mean length 17.9 ± 5.1 cm and mean weight 97.6 ± 3.7) and C. gariepinus (mean length 25.3 ± 2.7 cm and mean weight 125.5 ± 3.2 g) during summer, (mean length 23.4 ± 4.1 cm and mean weight 122.9 ± 5.3) during autumn, (mean length 26.4 ± 5.4 cm and mean weight 132.6 ± 5.2) during winter and (mean length 24.4 ± 4.3 cm and mean weight 127.6 ± 2.8 g) during spring from the great Cairo sector. The fish samples were securely contained in plastic bags and brought immediately to the laboratory in an icebox at 4 °C, where they were subsequently sliced to extract the muscle tissue.
Analysis
Physicochemical parameters
The water samples’ temperature, pH, electrical conductivity (EC), and dissolved oxygen (DO) levels were promptly evaluated using a Thermo Orion Star (A 329 multiparameter analyzer) during sample collection. The analytical methodologies are addressed in the American Public Health Association81. The biological oxygen demand (BOD) was assessed using the 5-day incubation method outlined in APHA81. The potassium permanganate method was employed to determine chemical oxygen demand (COD)81.
Heavy metals in water
Water samples were subjected to digestion utilizing the Nitric Acid Digestion Method outlined in APHA81. Approximately 5 ml of concentrated HNO3 was introduced to a 250 ml water sample, which was subsequently heated on a hot surface until reduced to 50 ml volume without inducing precipitation. The levels of heavy metals were quantified using Eq. (1):
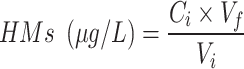 |
1 |
where Ci represents the ICP reported metal concentration in the digested solution (µg/l), Vf denotes the final volume of the digested solution (ml), and Vi indicates the initial water sample volume (ml).
HMs in fish muscles
The heavy metals in fish muscles were digested following the protocol outlined by FAO/SIDA82. A 10 ml aliquot of a freshly produced mixture of Concentrated nitric acid (65%) and perchloric acids (70%) (HNO3: HClO4 = 1:1) was introduced to 5 g of muscle tissue from each species in sealed Teflon containers. The samples were digested for 30 min at 160 °C in a microwave oven (model Milestone, MLS-1200 mega, Germany) until a transparent solution was obtained. The extract was adjusted with double distilled water up to a 50 ml solution in a calibrated measuring flask (50 ml), subsequently filtered, and put into plastic vials for analysis. Blanks were established in each digesting protocol. The amounts of heavy metals in fish muscle tissue were determined using Eq. (2):
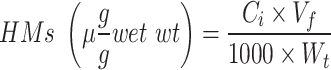 |
2 |
where Ci represents the HMs content in the digested solution (µg/l), Vf denotes the final volume of the digested solution (ml), Wt is the weight of the fish muscle sample (g), and 1000 is the unit conversion factor.
Instrument operation, calibration, and quality control
The total levels of the HMs (Cd, Cu, Pb, Mn, and Zn) in the digested water and fish samples were quantified using Inductively Coupled Plasma Emission Spectrometry (ICP-ES) with Ultra Sonic Nebulizer (USN), model Perkin Elmer optima 7000, USA. Before being utilized, all glassware was cleansed with diluted HCl. The chemicals used were from Germany (E. Merck). A 1000 g/L multi-element validated standard solution served as the baseline stock for apparatus calibration. The standard solutions were prepared with filtered, double-distilled water. The HMs analysis was dependent on the calibration curves (CC), plotted at five different concentrations. Before conducting the sample analyses, runs were carried out for each HM to determine the correlation coefficient (r2) values on the CC. Furthermore, the HMs concentrations were verified utilizing certified Reference materials via the National Institute of Standards and Technology (NIST). The accuracy and precision of the procedure were verified through 3 replicate readings of the standard materials, yielding metal recovery rates between 97.1% and 103.8%, which are within the permitted recovery percent between 80% and 110% defined by Huber83. The wavelengths (nm) for ICP-ES are as follows: 226.499, 324.747, 220.35, 257.604, and 213.855 nm, with corresponding detection limits (µg/L) of 0.1, 0.3, 1.5, 0.04, and 0.2 µg/L, and quantification limits (µg/L) of 0.15, 0.4, 1.8, 0.1 and 0.3 for Cd, Cu, Pb, Mn, and Zn respectively. The operational parameters of the ICP instrument are detailed in Table S3 in the supplementary file.
Metal quality indices (MQI) in water
This study employed many metal indices to evaluate the levels of metal contamination in the Nile River, relevant to drinking water, irrigation, and aquatic life applications. These indices included the Pollution Index (PI), Metal Index (MI), and Heavy Metal Pollution Index (HPI). The PI expresses the single effect of each individual HM by Eq. (3) with different grades reported in Table 8. Moreover, MI explains the combined effect of all investigated HMs. A greater concentration of a metal relative to its maximum permissible limit (MAC) indicates worse water quality (Eq. 4). The HPI elucidates metal pollution by considering the cumulative effects of different heavy metals, utilizing the weighted arithmetic mean method41 as outlined in Eq. (5) and Table (8).
Table 8.
The equations and evaluation criteria of HMs pollution indices used in water, BAF, and human health risk indices of HMs in fish tissues.
| No. | Equation | Variables | Evaluation criteria | References |
|---|---|---|---|---|
| Equation 3 |
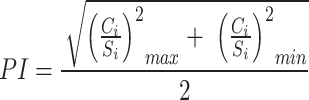
|
Where Ci signifies the HM concentration, Si denotes the HM level outlined in the national water quality criteria. |
PI < 1, no effect 2 > PI > 1, Slightly affected 3 > PI > 2, Moderately affected 5 > PI > 3, Strongly affected PI > 5, Seriously affected |
88 |
| Equation 4 |

|
n is the total number of assessed HMs, MAC is the maximum permitted concentration of the ith metal. | MI > 1, a critical threshold | 89,90 |
| Equation 5 |
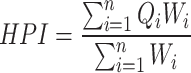


|
Qi is the sub-index of the ith metal, n denotes the quantity of assessed HMs, and Wi is the weight unit of the ith metal (between 0 and 1). |
HPI < 100, no contamination, HPI = 100, Moderate Contamination, HPI > 100, Extreme Contamination and unsuitable for drinking. |
41,91–93 |
| Equation 6 |
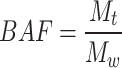
|
Mt is the metal concentration in fish tissues (µg/g wet weight) and Mw is the metal concentration in water (µg/l). |
BAF < 1000, little possibility of accumulation. 5000 > BAF > 1000, an intermediate bioaccumulative metal. BAF > 5000, a highly bioaccumulative metal. |
73 |
| Equation 7 |
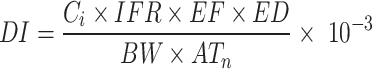
|
Ci is the average concentration of HMs in fish (mg/kg dry weight), IFR is the fish ingestion rate (57 g/person/day), BW is the average adult body weight (70 kg), ED signifies the exposure period (70) years as average lifetime, EF refers to the exposure frequency (365 days/year). ATn is the average exposure time for non-carcinogens (assuming 70 years). | 94 | |
| Equation 8 |

|
RFD is the oral reference dose, 0.001, 0.04, 0.0036, 0.14, and 0.3 for Cd, Cu, Pb, Mn, and Zn, respectively. |
THQs < 1, No harmful effects are anticipated. THQs > 1, a potential threat to health exists. |
87 |
| Equation 9 |

|
N: number of measured HMs. |
HI < 1, no anticipated harmful effects HI > 1, a potential health threat |
87 |
Bioaccumulation factor (BAF)
Bioaccumulation of HM may transpire through interaction with gills and skin and/or through intake of these metals in the digestive system84. The formula (Eq. 6) can be employed to identify the BAF in the consumable muscle of the species under consideration.
Human health risk assessment
Individuals are subjected to HMs in three ways, i.e., inhaling, intake, and interactions through the skin85. The most common route is the ingestion of HMs through fish consumption. The average daily intake (DI) of each HM was estimated based on a diet of 57 g/day of fish tissues87. The target hazard quotient (THQ), a non-cancer estimation of adverse health effects linked to the ingesting of HMs pollutants accumulating in fish tissues, was employed to assess hazard risk84. Each HM´s THQ was established. Furthermore, the hazard index (HI) is a mathematical computation that accounts for the impact of non-carcinogenic risks by using the THQ values of all the HMs under investigation. The equations of DI, THQ, and guideline criteria are in Table 8.
Statistical analysis
The current data were examined for variations in time and space utilizing Minitab 16® Statistical Software (Minitab Inc.), with statistical significance established at p < 0.05 and very significant findings at p < 0.01. Anderson Darling tests were performed to verify the assumptions of parametric tests, and the results indicated that the variables conformed to a normal distribution pattern. A correlogram was generated utilizing R-Studio version 2022.07.1 (R-Studio, Boston, MA, USA). The Pearson correlation coefficient (r) for the measured variables was analyzed using the R program, with code provided in the supplementary file. Triplicate analyses of heavy metals in fish tissues were performed, and the results were presented as mean ± standard deviation. The one-way analysis of variance (ANOVA) and Tukey comparison test were used to ascertain any statistically significant differences in the means of the physicochemical characteristics and heavy metal concentrations at a 5% confidence level across various seasons and locations. Unpaired t-tests were used to compare the HM levels and bioaccumulation between the two fish species.
Conclusion
The proliferation of HMs contamination in the aquatic environment has escalated. Their anthropogenic sources include industrial emissions, domestic, and farming techniques, including the application of fertilizers and pesticides. Global monitoring of drinking water sources is essential. This study evaluated the seasonal and regional distribution of some HMs (Cd, Cu, Pb, Mn, and Zn) in the Great Cairo sector, Nile River, Egypt over the study period (2021–2022). Forty eight surface water samples were collected from the study area and analyzed for physicochemical parameters and HMs content. Furthermore, this study investigated two fish species (O. niloticus and C. gariepinus) to evaluate the HMs distribution, bioaccumulation and heath risk implications. The HMs results in water decreased in the rank; Mn > Zn > Pb > Cu > Cd. The pollution index results indicated that essential Cu and Zn had a negligible pollution effect at all sites under investigation according to WHO drinking water criteria. Moreover, Mn showed a moderate PI effect. On the other hand, the nonessential Pb was seriously affected at all sites while Cd has a slight pollution effect only at S1 while negligible effects at the other sites. The metal index and heavy metal pollution index results outline that HMs exceeded the permissible levels for drinking water and aquatic life utilization and can induce both chronic and acute toxicity and then threaten the human health. Accordingly, obligatory measures must be implemented, and remediation strategies must be advised. The concentrations of HMs in the investigated species ranked in the order: (Zn > Mn > Cu > Pb > Cd), with Cd and Pb exceeding their respective permissible limits. In addition, no significant variations between the BAF measurements of all investigated HMs between the two fish species (unpaired t-test). The Hazard Index results indicated that the HMs in O. niloticus do not present any specific concerns regarding individual health issues and are safe for human consumption. Conversely, the HI in C. gariepinus surpasses the acceptable threshold, and thus an intensive evaluation must be implemented towards this species. This study recommends constant monitoring of industrial effluents for conducting different effective strategies to eliminate metal ions. Furthermore, a multitude of regulations and legislation must be established for the conservation of water resources. In addition, this work can inform policymakers and stakeholders about the potential consequences associated with HM contamination in aquatic systems, facilitating the creation of tailored policies and laws to mitigate these issues.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
Sampling and Methodology, A.K. and H.G.; writing—original draft preparation, A.K.; writing—graph preparation and data analysis, A.K. and H.G.; writing—review and editing, A.K. and H.G.. All the authors reviewed the manuscript.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The authors declare that this study involves animals but ethics approval is not needed as this study does not use live fish, often we use the fish available in the market which are used for eating purposes.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
7/23/2025
This article has been updated to amend the license information.
References
- 1.Qingzhen, Y. et al. Artificial water regulation and natural flood processes control heavy metal concentrations and transport in the yellow river, China. Mar. Poll. Bull.209, 117092. 10.2139/ssrn.4522710 (2024). [DOI] [PubMed] [Google Scholar]
- 2.Othman, A. A., Al-Afify, A. D. G., Abdel-Satar, A. M. & Ramadan, M. F. Quality assessment of surface water using the nile chemical pollution index (NCPI) and Microbiological pollution of the Rosetta branch (Nile river, Egypt). Afr. J. Aquat. Sci.46(2), 129–141. 10.2989/16085914.2020.1807898 (2021). [Google Scholar]
- 3.Al–Afify, A. D. G. & Abdel–Satar, A. M. Heavy metal contamination of the river nile environment, Rosetta branch, Egypt. Water Air Soil. Pollut233(302). 10.1007/s11270-022-05759-7 (2022).
- 4.Abdel-Satar, A. M., Ali, M. H. & Goher, M. E. Indices of water quality and metal pollution of nile river, Egypt. Egypt. J. Aquat. Res.43, 21–29. 10.1016/j.ejar.2016.12.006 (2017). [Google Scholar]
- 5.Hussein, M. M., Goher, M. E., Mangood, A. H. & Mousa, I. E. Water quality profile and metal pollution indices of the main stream of the nile river in Egypt. Afr. J. Aquat. Sci.48(2), 138–151 (2023). [Google Scholar]
- 6.Goher, M. E., Ali, M. H. H. & El-Sayed, S. M. Heavy metals contents in Nasser lake and the nile river, Egypt: An overview. Egypt. J. Aquat. Res.45, 301–312. 10.1016/j.ejar.2019.12.002 (2019). [Google Scholar]
- 7.Flefil, N. S., Abdelhameed, M. S., Abdel Monem, A. M. & Abdel Mola, H. R. Relationship between plankton communities and heavy metals in the Rosetta branch, the river Nile, Egypt. EJABF26(6), 635–652 (2022). [Google Scholar]
- 8.Sharma, M. et al. Exploring the impact of heavy metals toxicity in the aquatic ecosystem. Int. J. Energ. Water Res.10.1007/s42108-024-00284 (2024). [Google Scholar]
- 9.Khedr, A. I. Synthesis of novel nanoparticles for photocatalytic degradation of some toxic pollutants in the marine environment. Ph.D thesis, Chemistry Department, Faculty of Science, Ain Shams University (2020).
- 10.Yacoub, A. M., Abd, M. S. A. & El-Satar, A. M. Accumulation of heavy metals in tilapia fish species and related histopathological changes in muscles, gills and liver of Oreochromis niloticus occurring in the area of Qahr El-Bahr, lake Al-Manzalah. Egypt. Oceanol. Hydrobiol. Stud.50(1), 1–15. 10.2478/oandhs-2021-0001 (2021). [Google Scholar]
- 11.Ali, H., Khan, E. & Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: environmental persistence, toxicity, and bioaccumulation. J. Chem.4(1), 14. 10.1155/2019/6730305 (2019).
- 12.Qu, L. et al. Risk analysis of heavy metal concentration in surface waters across the rural-urban interface of the Wen-Rui Tang river, China. Environ. Pollut.237, 639–649. 10.1016/j.envpol.2018.02.020 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Khammar, S., Behrooz, R. D., Chakraborty, P. & Zeinalipour, N. Risk assessment of heavy metals (Zn, Cu, Pb, and Ni) in the edible tissue of Oncorhynchus Mykiss trout in the rivers of the Southern Caspian sea and the Northern and Southeast Coast of Iran. Reg. Stud. Mar. Sci.79, 103849. 10.1016/j.rsma.2024.103849 (2024). [Google Scholar]
- 14.Singh, G. & Sharma, S. Heavy metal contamination in fish: Sources, mechanisms, and consequences. Aquat. Sci.86, 107. 10.1007/s00027-024-01121-7 (2024). [Google Scholar]
- 15.Mali, M. et al. Mobility of trace elements in a coastal contaminated site under groundwater salinization dynamics. Sci. Rep.14, 24859. 10.1038/s41598-024-75974-1 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Priyadarshi, M., Das, P. & Hussain, A. Assessment of heavy metal diffusion rates from river Yamuna and its effect on the surrounding geology: An experimental and theoretical study. J. Indian Chem. Soc.101, 101333. 10.1016/j.jics.2024.101333 (2024). [Google Scholar]
- 17.Wang, M. et al. A triple increase in global river basins with water scarcity due to future pollution. Nat. Commun.15, 880. 10.1038/s41467-024-44947-3 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Degwy, A. A., Negm, N. A., El-Tabl, A. S. & Goher, M. E. Assessment of heavy metal pollution in water and its effect on nile tilapia (Oreochromis niloticus) in mediterranean lakes: A case study at Mariout lake. Appl. Wat Sci.13, 50. 10.1007/s13201-022-01858-2 (2023). [Google Scholar]
- 19.Salaah, S. M. & El-Gaar, D. M. Physiological and histological alterations in fishes induced by pollution in lake Nasser and the potential human risk assessment. Egypt. J. Aquat. Biol. Fish.24(4), 373–390. 10.21608/ejabf.2020.100264 (2020). [Google Scholar]
- 20.Witkowska, D., Słowik, J. & Chilicka, K. Heavy metals, and human health: Possible exposure pathways and the competition for protein binding sites. Molecules26(19), 6060. 10.3390/molecules26196060 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdel-Khalek, A. A., Elhaddad, E., Mamdouh, S. & Marie, M. A. S. Assessment of metal pollution around Sabal drainage in river nile and its impacts on bioaccumulation level, metals correlation and human risk hazard using Oreochromis niloticus as a bioindicator. Turkish J. Fish. Aquat. Sci.16(2), 227–239 (2016). [Google Scholar]
- 22.Jolaosho, T. L. et al. Bioaccumulation dynamics, noncarcinogenic and carcinogenic risks of heavy metals in commercially valuable shellfish and finfish species from the world largest floating slum. Makoko Nigeria Mar. Pollut Bul. 207, 116807. 10.1016/j.marpolbul.2024.116807 (2024). [DOI] [PubMed] [Google Scholar]
- 23.Amptmeijer, D. & Bieser, J. Bioaccumulation as a key driver of hg bioaccumulation in high trophic level fish. EGusphere10.5194/egusphere-2025-312 (2025). [Google Scholar]
- 24.Jolaosho, T. L., Mustapha, A. A. & Hundeyin, S. T. Hydrogeochemical evolution and heavy metal characterization of groundwater from Southwestern, Nigeria: An integrated assessment using Spatial, indexical, irrigation, chemometric, and health risk models. Heliyon10 (19), e38364. 10.1016/j.heliyon.2024.e38364 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khedr, A. et al. Water quality assessment of the Northern part of Suez Gulf (Red Sea, Egypt), using principal component analysis. EGABF23, 527–538. 10.21608/ejabf.2019.58410 (2019). [Google Scholar]
- 26.Shalaby, S. E. M. et al. Levels of pesticide residues in water, sediment, and fish samples collected from nile river in Cairo, Egypt. Environ. Forensic.19, 228–238. 10.1080/15275922.2018.1519735 (2018). [Google Scholar]
- 27.Khedr, A. I. Impacts of seawater inlets and effluent outlets from the Nuweiba desalination plant on the marine coastal area. A MSc. Thesis, Faculty of Science, Benha University (2016).
- 28.Saad, A. E. H., Emam, W., Mola, H. & Omar, H. Effect of pollution on macrobenthic invertebrates in some localities along the river nile at great Cairo, Egypt. Egypt. J. Aquat. Biol. Fish.19(2), 1–11. 10.21608/ejabf.2015.2252 (2015). [Google Scholar]
- 29.El Sayed, S. M., Hegab, M. H., Mola, H. R. A., Ahmed, N. M. & Goher, M. E. An integrated water quality assessment of Damietta and Rosetta branches (Nile river, Egypt) using chemical and biological indices. Environ. Monit. Assess.192, 228. 10.1007/s10661-020-8195-4 (2020). [DOI] [PubMed] [Google Scholar]
- 30.USEPA (United States Environmental Protection Agency). National Recommended Water Quality Criteria—Aquatic Life Criteria Table (2021). https://www.epa.gov/wqc/national-recommendedwaterquality-criteria-aquatic-life-criteria-table [accessed 29 November 2021].
- 31.Hussein, A. M., Mahmoud, R. K., Sillanpää, M. & Abdelwahed, M. S. M. Impacts alum DWTPs sludge discharge and changes in flowregime of the nile river on the quality of surface water and cultivated soils in Fayoum watershed, Egypt. Sci. Total Environ.766, 144333 (2021). [DOI] [PubMed] [Google Scholar]
- 32.Ali, E. M., Shabaan-Dessouki, S. A., Soliman, A. I. & El Shenawy, A. S. Characterization of chemical water quality in the nile river. Egypt. Int. J. Pure Appl. Biosci.2(3), 35–53 (2014). [Google Scholar]
- 33.Ghannam, H. E. Risk assessment of pollution with heavy metals in water and fish from river Nile, Egypt. Appl. Wat Sci.11(7), 125. 10.1007/s13201-021-01449-7 (2021). [Google Scholar]
- 34.Masindi, V., Mkhonza, P. T., Tekere, M. & Chapter Source s of heavy metals pollution, in book: Remediation of heavy metals edition: (eds Inamuddin et al.) Publisher: Springer Nature Environmental Chemistry for a Sustainable World. 70, 419–454 10.1007/978-3-030-80334-6_17. (2021). [Google Scholar]
- 35.Abd El-Aziz, M. A. Assessment influence of some pollutants on extract components of Procambarus Clarkii in the Nile River water and impact of these extracts on some organisms. a MSc thesis, Faculty of Science (Boys), Department of Zoology and Entomology, Al-Azhar University.
- 36.Elnazer, A. A. et al. Temporal and Spatial evaluation of the river nile water quality between Qena and Sohag cities, Egypt. Bull. Natl. Res. Cent.42, 3. 10.1186/s42269-018-0005-6 (2018). [Google Scholar]
- 37.Tayel, S. I., Mahmoud, S. A., Ahmed, N. A. M., Abdel & Rahman A.A.S. Pathological impacts of environmental toxins on Oreochromis niloticus fish inhabiting the water of Damietta branch of the river Nile, Egypt. EJABF22 (5), 309–321 (2018). [Google Scholar]
- 38.Omar, W. A., Mikhail, W. Z. A., Abdo, H. M., Abou El Defan, T. A. & Poraas, M. M. Ecological risk assessment of metal pollution along greater Cairo sector of the river nile, Egypt, using nile tilapia, Oreochromis niloticus, as bioindicator. J. Toxicol.167319, 11. 10.1155/2015/167319 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goher, M. E., Hassan, A. M., Abdel-Moniem, I. A., Fahmy, A. H. & El-sayed, S. M. Evaluation of surface water quality and heavy metal indices of Ismailia Canal, Nile River, Egypt. Egypt. J. Aquat. Res.40, 225–233 (2014). 10.1016/j.ejar.2014.09.001
- 40.Abdel-Satar, A. M., Abdo, M. H., Othman, A. A. & Al-Afify, A. D. G. Assessment of water quality in the Shores of the nile river Islands, Egypt: Chemical and Microbiological analysis. Ecol. Front.10.1016/j.ecofro.2024.02.012 (2024). [Google Scholar]
- 41.Hassouna, M. E., Goher, M. E., El-Sayed, S. M. & Hassan, R. A. Integrated approach to quality indices and health risk assessment of water in the Bahr Yusuf canal, Fayoum, Egypt. Oceanol. Hydrobiol. Stud.48(4), 337–354. 10.2478/ohs-2019-0031 (2019). [Google Scholar]
- 42.Goher, M. E., Abdo, M. H., Mangood, A. H. & Hussein, M. M. water quality and potential health risk assessment for consumption of Oreochromis niloticus from El-Bahr El-Pharaony drain, Egypt. Fresenius Environ. Bull.24(11), 3590–3602 (2015). [Google Scholar]
- 43.EWQS-Egyptian Standards for Drinking Water and Domestic Uses (in Arabic), Egyptian Ministry of Health and Population. Resolution No. 458 of 2007 defining limits for criteria and requirements necessary for drinking water and domestic use. Official Gazette No. 24 (supplementary), 22 12 pp (in Arabic). October (2007). https://faolex.fao.org/docs/pdf/egy83626.pdf
- 44.WHO-World Health Organization. Guidelines for Drinking Water Quality 4th edn (WHO, 2011). https://www.paho.org/en/documents/guidelines-drinking-waterquality-4o-ed-2011
- 45.FAO, Food Agriculture Organization of the United Nations (FAO), Ayers, R. S., Westcot, D. W. & Food Water Quality for Agriculture, Irrigation and Drainage, Rome, Paper No. 29. Rev. 1, M-6 (1994).
- 46.Hashem, H. M., Tayel, I. S., Sabra, A. E., Yacoub, M. A. & Heiba, A. A. Impact of the water quality of El-Rahawy drain on some genetic and histopathological aspects of Oreochromis NiloticusEgypt. J. Aquat. Biol. Fish.24(2), 19–38 (2020). [Google Scholar]
- 47.Dey, M. et al. Assessment of contamination level, pollution risk and source apportionment of heavy metals in the Halda river water, Bangladesh. Heliyon7, e08625 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paronda, G. R. A., David, C. P. C. & Apodaca, D. C. River flow pattern and heavy metals concentrations in Pasig river, Philippines as affected by varying seasons and astronomical tides. IOP Conf. Series: Earth Environ. Sci.344, 012049. 10.1088/1755-1315/344/1/012049 (2019). [Google Scholar]
- 49.Jiao, C. Z., Li, H., Song, M. & Wang, L. Ecological risk assessment of heavy metals in water and sediment of the Pearl River estuary. IOP Conference Series: Materials Science and Engineering394, 052055 (2018). 10.1088/1757-899X/394/5/052055 052055.
- 50.Kılıç, E. & Can, M. Determination of Spatiotemporal variations in heavy metal concentration through Orontes river. TURJAF5, 1086. 10.24925/turjaf.v5i9.1086-1093.1298 (2017). [Google Scholar]
- 51.Abd El-Aal, R. F., El-Sayed, S. M., Attia, M. S., Donia, N. S. & Goher, M. E. Pollution indices and distribution pattern of heavy metals in Qarun lake water, Egypt. Egypt. J. Aquat. Biol. Fish.24(1), 593–607. 10.21608/EJABF.2020.75893 (2020). [Google Scholar]
- 52.Bahnasawy, M., Khidr, A. & Dheina, N. Assessment of heavy metal concentrations in water, plankton and fish of lake Manzala, Egypt. Turk. J. Zool.35(2), 271–280 (2011). [Google Scholar]
- 53.Nwabueze, A. & Oghenevwairhe, E. Heavy metal concentrations in the West African clam, Egeria radiata (Lammark, 1 804) from Mciver market, Warri, Nigeria. Int. J. Sci. Nat.3(2), 309–315 (2012). [Google Scholar]
- 54.Ibrahim, A. A. & Omar, H. M. Seasonal variation of heavy metals accumulation in muscles of the African catfish Clarias Gariepinus and in river nile water and sediments at Assiut Governorate, Egypt. J. Biol. Earth Sci.3(2), 236–248 (2013). [Google Scholar]
- 55.Vasistha, P. & Ganguly, R. Assessment of spatio-temporal variations in lake water body using indexing method. Environ. Sci. Pollut Res.27(33), 41856–41875. 10.1007/s11356-020-10109-3 (2020). [DOI] [PubMed] [Google Scholar]
- 56.El-Sheekh, M. M. Impact of water quality on ecosystems of the Nile River. In: Negm AM (ed); The Nile river. Springer International Publishing, Cham, 357–385 (2017).
- 57.Al–Afify, A. D. G., Othman, A. & Ramadan, M. F. Characterization of chemical and Microbiological quality of nile river surface water at Cairo (Egypt). Rend. Lincei Scienze Fis. E Naturali29, 725–736. 10.1007/s12210-018-0721-8 (2018). [Google Scholar]
- 58.Kispang, N. K., Kibet, J. K. & Adongo, J. O. A review of the current status of the water quality in the nile water basin. Bull. Natl. Res. Cent.48, 30. 10.1186/s42269-024-01186-2 (2024). [Google Scholar]
- 59.Rashed, M. N. Monitoring of environmental heavy metals in fish from Nasser lake. Environ. Int.27, 27–33. 10.1016/S0160-4120(01)00050- (2001). [DOI] [PubMed] [Google Scholar]
- 60.ANZECC/ARMCANZ. Australian and New Zealand guidelines for fresh and marine water quality. Australian and New Zealand Environmental Conservation Council & Agriculture and Resource Management Council of Australian and New Zealand, Canberra, 1–123. (2000).
- 61.Union, E. U. E. Commission regulation as regards heavy metals. Directive22, 466 (2001). [Google Scholar]
- 62.MAFF-Ministry of. Agriculture, fisheries, and food. Monitoring and surveillance of non-radioactive contaminants in the aquatic environment and activities regulating the disposal of wastes at Sea, 1997. In: Aquatic Environment Monitoring Report No. 52. Center for Environment, Fisheries and Aquaculture Science, Lowestoft, UK (2000). [Google Scholar]
- 63.EC-European Community. Commission regulation. Official J. Eur. Union, 78, (2005). L16/43-L16/45.
- 64.FAO/WHO-Food and Agriculture Organization/World Health Organization. Evaluation of certain food additives and the contaminants mercury, lead and cadmium, WHO technical report series No. 505. In: Aquatic environment monitoring report No. 52, Center for Environment, Fisheries and Aquaculture Science, Lowestoft, UK. (1989).
- 65.Ghannam, H. E., Haddad, E., Talab, A. S. & E.S.E. & Bioaccumulation of heavy metals in tilapia fish organs. J. Bio Env Sci.7(2), 88–99 (2015). [Google Scholar]
- 66.FAO-Food and Agriculture Organization. Compilation of legal limits for hazardous substances in fish and fishery products. FAO Fish. Circular464, 5–100 (1983). [Google Scholar]
- 67.WHO-World Health Organization. Environment Health criteria No. 85. Heavy metals environmental aspects, Geneva, Switzerland. (1989).
- 68.Elhaddad, E., Salaah, S. M., Salama, H. M. M., El–Sherif, D. M. & Gaber, H. S. Risk assessment and hazardous effects of metal contamination in O. niloticus and S. galilaeus from four islands of the River Nile. Bull. Environ. Contam. Toxicol.109, 839–851(2022). 10.1007/s00128-022-03589-1 [DOI] [PMC free article] [PubMed]
- 69.Dural, M., Göksu, M. Z. & Őzak, A. A. Investigation of heavy metal levels in economically important fish species captured from the Tuzla lagoon. Food Chem.102(1), 415–421. 10.1016/j.foodchem.2006.03.001 (2007). [Google Scholar]
- 70.Kumar, M. et al. A review on heavy metal-induced toxicity in fishes: Bioaccumulation, antioxidant defense system, histopathological manifestations, and transcriptional profiling of genes. J. Trace Elem. Med. Biol.83, 127377. 10.1016/j.jtemb.2023.127377 (2024). [DOI] [PubMed] [Google Scholar]
- 71.Beyersmann, D. & Hartwig, A. Carcinogenic metal compounds: Recent insight into molecular and cellular mechanisms. Arch. Toxicol.82, 493–512. 10.1007/s00204-008-0313-y (2008). [DOI] [PubMed] [Google Scholar]
- 72.Rani, L., Srivastav, A. L., Kaushal, J., Grewal, A. S. & Madhav, S. Chapter 3 - Heavy metal contamination in the river ecosystem, Editor(s): Madhav, S., Kanhaiya, S., Srivastav, A., Singh, V. & Singh, P. Ecological significance of river ecosystems, Elsevier, 37–50, ISBN 9780323850452 (2022). 10.1016/B978-0-323-85045-2.00016-9
- 73.Olayinka-Olagunju, J. O., Dosumu, A. A. & Olatunji-Ojo, A. M. Bioaccumulation of heavy metals in pelagic and benthic Fshes of Ogbese river, Ondo State, South-Western Nigeria. Water Air Soil. Pollut.232(2), 44. 10.1007/s11270-021-04987-7 (2021). [Google Scholar]
- 74.Marzouk, M. Fish and environmental pollution. J. Vet. Med.42, 51–52 (1994). [Google Scholar]
- 75.Talab, A. S., Goher, M. E., Ghannam, H. E. & Abdo, M. H. Chemical compositions and heavy metal contents of Oreochromis niloticus from the main irrigated canals (Rayahs) of nile delta. Egypt. J. Aquat. Res.42, 23–31. 10.1016/j.ejar.2016.01.003 (2016). [Google Scholar]
- 76.Zeitoun, M. M. & Mehana, E. E. Impact of water pollution with heavy metals on fish health: Overview and updates. Global Vet.12(2), 219–231. 10.5829/idosi.gv.2014.12.02.82219 (2014). [Google Scholar]
- 77.Custodio, M., Peñaloza, R., Ochoa, S. & Cuadrado, W. Human risk associated with the ingestion of artichokes grown in soils irrigated with water contaminated by potentially toxic elements, Junin Peru. Saudi J. Biol. Sci.28(10), 5952–5962. 10.1016/j.sjbs.2021.06.054 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abdo, M. H., Ahmed, H. B., Helal, M. H., Fekry, M. M. & Abdelhamid, A. E. Water quality index and environmental assessment of Rosetta branch aquatic system, nile river, Egypt. Egypt. J. Chem.65, 321–331. 10.21608/ejchem.2021.92605.4405 (2022). [Google Scholar]
- 79.Abou El-Anwar, E., Salman, S., Asmoay, A. & Elnazer, A. Geochemical, mineralogical and pollution assessment of river nile sediments at Assiut Governorate, Egypt. J. Afr. Earth Sci.180, 104227. 10.1016/j.jafrearsci.2021.104227 (2021). [Google Scholar]
- 80.Bendary, R. E. & Ibrahim, S. M. Diversity and density of microbenthic invertebrates associated with macrophytes in the El-Rayah El-Nasery and El-Rayah El-Behery, nile river, Egypt. Egypt. J. Aquat. Biol. Fish.25, 511–526. 10.21608/EJABF.2021.203279 (2021). [Google Scholar]
- 81.APHA-American Public Health Association. Standard Methods for the Examination of Water and Wastewater 21th edn, Vol. 1015 (pp, AWWA, WCPF, 2012).
- 82.FAO/SIDA. Manual of methods in aquatic environment research [Part 9. Analyses of metals and organochorines in fish]. FAO Fish. Tech. Paper212, 33 (1983). [Google Scholar]
- 83.Huber, L. Validation and Qualification in Analytical Laboratories 2nd edn p 288 (CRC, 2007).
- 84.Monroy, M., Maceda-Veiga, A. & de Sostoa, A. Metal concentration in water, sediment, and four fish species from lake Titicaca reveals a large-scale environmental concern. Sci. Tot Environ.487, 233–244. 10.1016/j.scitotenv.2014.03.134 (2014). [DOI] [PubMed] [Google Scholar]
- 85.Al-Swadi, H. A. et al. Sources, toxicity potential, and human health risk assessment of heavy metals-laden soil and dust of urban and suburban areas as affected by industrial and mining activities. Sci. Rep.12, 8972. 10.1038/s41598-022-12345-8 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Khedr, A. I. et al. Assessment of PAH pollution in mediterranean lakes and health implications for fish and consumers, case study: Manzala lake, Egypt. Water Cycle5, 199–214. 10.1016/j.watcyc.2024.05.003 (2024). [Google Scholar]
- 87.USEPA- United States Environmental Protection Agency. Regional Screening Levels (RSLs)-Generic Tables (Summary Table, Nov. 2018).
- 88.Caeiro, S. et al. Assessing heavy metal contamination in Sado estuary sediment: An index analysis approach. Ecol. Ind.5, 151–169. 10.1016/j.ecolind.2005.02.001 (2005). [Google Scholar]
- 89.Tamasi, G. & Cini, R. Heavy metals in drinking waters from Mount Amiata (Tuscany, Italy). Possible risks from arsenic for public health in the Province of Siena. Sci. Total Environ.327(1–3), 41–51. 10.1016/j.scitotenv.2003.10.011 (2004). [DOI] [PubMed] [Google Scholar]
- 90.Bakan, G., Özkoç, H. B., Tülek, S. & Cüce, H. Integrated environmental quality assessment of Kızılırmak river and its coastal environment. Turk. J. Fish. Aquat. Sci.10, 453–462. 10.4194/trjfas.2010.0403 (2010). [Google Scholar]
- 91.Milivojević, J., Krstić, D., Šmit, B. & Djekić, V. Assessment of heavy metal contamination and calculation of its pollution index for Uglješnica river, Serbia. Bull. Environ. Contam. Toxicol.97(5), 737–742. 10.1007/s00128-016-1918-092 (2016). [DOI] [PubMed] [Google Scholar]
- 92.Ndimele, P. E. et al. Source apportionment, ecological and health risk assessment of potentially toxic elements in water, sediment and Blackchin tilapia {sarotherodon melanotheron (rüppell 1852)} from Lagos and Ologe lagoons, Lagos State, Nigeria. J. Trace Elem. Minerals. 100173. 10.1016/j.jtemin.2024.100173 (2024).
- 93.Jolaosho, T. L. et al. Occurrence, distribution, source apportionment, ecological and health risk assessment of heavy metals in water, sediment, fish and Prawn from Ojo river in Lagos. Nigeria Environ. Monit. Assess.196(2), 109. 10.1016/j.jtemin.2024.100173 (2024). [DOI] [PubMed] [Google Scholar]
- 94.Song, B. et al. Assessing the health risk of heavy metals in vegetables to the general population in Beijing, China. J. Environ. Sci.21(08), 1702–1709. 10.1016/s1001- (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article [and its supplementary information files].