Abstract
Investigations were carried out to study the production of factors associated with the innate immune response in the systemic and mucosal compartments in adults and children infected with Vibrio cholerae O1 and V. cholerae O139. The levels of nonspecific mediators of the innate defense system, i.e., prostaglandin E2 (PGE2), leukotriene B4 (LTB4), and lactoferrin (Lf), as well as myeloperoxidase (MPO), were elevated at the acute stage of the disease in stools obtained from both O1- and O139-infected adults and children. In the systemic compartment, the levels of Lf were increased after onset of disease, which in children remained elevated up to convalescence compared to the healthy controls. Increased concentrations of C-reactive protein were seen in the sera of adult cholera patients at the acute stage of infection. Elevated levels of the nitric oxide (NO·) metabolites (nitrite and nitrate [NO2− and NO3−]) were detected in plasma but not in urine. The activity of the scavenger of reactive oxygen species, superoxide dismutase, was higher in the plasma of adults immediately after the onset of disease, suggesting that an active scavenging of reactive oxygen species was taking place. The concentration of 8-iso-prostaglandin F2α remained unchanged in the systemic and mucosal compartments in the study subjects. After the recovery of patients from cholera, the concentration of the majority of the metabolites decreased to baseline levels by day 30 after the onset of infection. Immunohistochemical staining showed increased tissue expression of MPO, Lf, and inducible nitric oxide synthase at the acute stage in the duodenal biopsies of adults and rectal biopsies obtained from children with cholera. Very little difference was seen in the levels of the different inflammatory mediators in patients infected with V. cholerae O1 or the encapsulated V. cholerae O139. In summary, these results suggest that elevated concentrations of Lf, MPO, PGE2, LTB4, and NO·, as well as other metabolites, during the acute stage of the disease indicate that the innate defense system, as well as the inflammatory process, is activated in both adults and pediatric patients infected with V. cholerae O1 and O139.
Acute watery diarrhea caused by Vibrio cholerae O1 and V. cholerae O139 induces strong mucosal and systemic antibody responses to cholera toxin (CT) and lipopolysaccharide (LPS), as well as to other cell surface components, resulting in a long-lasting protection from cholera (8, 10, 22, 25-27). However, apart from the specific immune responses, protection from diseases may be afforded by the innate arm of the immune response by the increased secretion of inflammatory mediators with bactericidal or bacteriostatic properties. Although cholera is commonly considered to be a noninflammatory secretory disease, ultrastructural studies of the gut in cholera patients have shown increases and activation of inflammatory cells (17). CT-mediated infections induce a Th2 type of cytokine response reflected by the production of specific immunoglobulin G1 (IgG1), IgG4, and IgE antibodies (16, 22, 23), as well as interleukin-6 (IL-6) production by mast cells (14), further showing an induction of inflammatory responses. Studies conducted in North American volunteers orally challenged with live V. cholerae showed increased levels of lactoferrin (Lf) in stool, an index of an inflammatory response (32). Lf has also been shown to have bactericidal effects on V. cholerae O1 (1).
The oxidative mechanisms involving peroxidases generate reactive products with bactericidal properties and increases in myeloperoxidase (MPO) has been observed in feces in cases of shigellosis (30), as well as in cases of inflammatory bowel disease (34). The involvement of reactive nitric oxide (NO·), another powerful antimicrobial agent, has been shown to increase after infection with V. cholerae O1 (9, 28). Levels of superoxide dismutase (SOD), a scavenger of free radicals, increase in subjects with infectious inflammatory diarrhea, with a concomitant increase in the level of 8-iso-prostaglandin F2α (PGF2α) (30); the latter is a useful index for measuring oxidative injury and is elevated by the free radical-catalyzed lipid peroxidation of arachidonic acid.
Increased intestinal fluid secretion is a protective host response to enteric infections that may be mediated by prostaglandins. PGE2 has been shown to increase in the jejunal aspirates of patients with cholera (33) and, after stimulation with CT, in the intestinal lumen of rabbits (18) and in murine macrophage cell lines treated with enterotoxin (5). PGE2, like CT, also favors a Th2-like response to induce IgE and IgG1 antibodies, which can work in concert with other inflammatory mediators (14).
In the present study, we sought to study the involvement of the different innate mediators in cholera. We have studied the contribution of the bactericidal proteins (Lf and MPO), oxidant-mediated defense factors (NO·), scavengers of the reactive oxygen metabolites (SOD), and eicosanoids (PGE2, leukotriene B4 [LTB4], and PGF2α) in the immunopathogenesis of cholera in patients infected with V. cholerae O1 or by the encapsulated V. cholerae O139 serogroup in both children and adults. The response was studied at the acute phase after onset of illness and at convalescence. The different mediators have been studied in the blood and at the local site in feces as well as mucosal biopsies obtained from patients and healthy volunteers.
MATERIALS AND METHODS
Study groups.
A total of 12 adult male patients with cholera caused by V. cholerae O1 El Tor Ogawa and 13 patients with V. cholerae O139 infection were recruited for the study. Twenty children (2- to 5-year age range) with cholera (19 males and 1 female)—ten infected with V. cholerae O1 El Tor Ogawa and ten infected with V. cholerae O139—were also recruited into the study. The degree of dehydration in the patients (moderate to severe) was assessed by a physician according to the Denver system (35). Ten adult males similar in age and socioeconomic status to the patients but with no history of diarrhea during the previous 3 months were also included as controls. In addition, 10 healthy children (6 males and 4 females) coming for follow-up to the hospital 2 months after onset of shigellosis (30) were treated as controls.
Bacteriological examination of patient stool samples.
Stool samples of patients suffering from acute watery diarrhea were screened by dark-field microscopy and for reactivity with antibodies specific for V. cholerae of the O1 or O139 serogroups (27). Stool samples were then plated on taurocholate-tellurite-gelatin agar and gelatin agar (Difco, Detroit, Mich.) overnight. Suspected vibrio colonies were identified by slide agglutination by using monoclonal antibodies against V. cholerae O1 and O139 serogroups (27). Stools were also cultured to detect other enteric pathogens, e.g., enterotoxigenic Escherichia coli (24) and Salmonella, Shigella, and Campylobacter spp. (36), and were tested by direct microscopy for cyst and vegetative forms of parasites and ova of helminths. The stools of the children and adults included as healthy controls were similarly screened.
Sample collection.
After the microbiological confirmation of stools for V. cholerae, patients were enrolled into the study. Venous blood, feces, and urine were collected from patients after they had been rehydrated. This occurred on the second day of hospitalization and was considered to be ca. 2 days after the onset of diarrhea (day 2) and is indicated as the acute stage in the study. Samples were also collected 5 and 28 days later, during convalescence (that is, 7 and 30 days, respectively, after onset of the disease). Single blood, urine, and fecal samples were collected from the healthy subjects. Blood was collected in heparinized vials or in EDTA-coated sterile vials (Vacutainer System; Becton Dickinson, Rutherford, N.J.) and centrifuged. Samples of plasma and serum separated from blood were aliquoted and stored at −70°C. Stools obtained from patients on the different study days, as well as from healthy controls, were frozen immediately at −70°C. Fecal extracts were prepared by mixing stool (1 g of feces in 4 ml of buffer) with phosphate-buffered saline (PBS) containing EDTA (0.05 M) and the protease inhibitors soybean trypsin inhibitor (100 μg/ml) and phenylmethylsufonyl fluoride (10 mM) (25). Then, 1 ml of fecal extract was equal to 0.25 g of stool. Fecal extracts were frozen in aliquots at −70°C. The total protein content of stool extracts was also determined by using the Bradford protein assay (4). Urine was centrifuged, filtered with a 0.2-μm-pore-size filter, and stored in aliquots at −70°C.
Mucosal punch biopsy samples were collected from the adult patients from the duodenum, and the pediatric samples were collected from the rectum at the acute stage and convalescence. Biopsies were collected from the second part of the duodenum by a standard endoscopic procedure by using a local anesthetic and biopsy forceps (Megabite endoscopic forceps; Microvasive; Boston Scientific Corp.). From the adults, rectal biopsy samples were taken 10 to 12 cm from the anus by using a sigmoidscope (Olympus, Tokyo, Japan). Rectal biopsy samples from the children were obtained by using an endoscope. Biopsies were collected only once from the adult healthy subjects (day 0). Rectal biopsy samples collected from the children at follow-up visits to the hospital ca. 60 to 65 days after the onset of diarrhea were treated as controls.
Detection of soluble mediators in samples.
For determination of C-reactive protein (CRP) concentrations in sera, a fluorescence polarization immunoassay procedure was used (Abbott Laboratories, Abbot Park, Ill.) with the Abbott TDx analyzer. PGE2 and LTB4 were measured in plasma and stool samples by using commercial enzyme immunoassay kits (Cayman Chemical Co., Ann Arbor, Mich.). Plasma and stool extracts were purified for the assays, and enzyme immunoassay (EIA) was performed according to the manufacturer's instructions. PGE2 and LTB4 units were expressed in picograms per milliliter of plasma or stool extract. PGF2α was measured in plasma and stool samples by using commercial immunoassay kits (R&D Systems, Minneapolis, Minn.), and units were expressed in picograms per milliliter of stool or plasma. The Lf contents of plasma and stool samples were measured by using a commercial EIA kit (Oxis International, Inc., Portland, Oreg). Lf units were expressed as nanograms per milligram of total protein in stool extracts or as nanograms per milliliter of plasma. The enzyme activity for MPO was determined in the plasma and stool extracts by measuring the H2O2-dependent oxidation of 3,3′,5,5′-tetramethylbenzidine (TMB) spectrophotometrically (Beckman DU 640) at 650 nm. In brief, 1,580 μl of 101 mM sodium phosphate buffer and 200 μl of 16 mM TMB were mixed with 200 μl of plasma or stool extracts, and the background value was set at zero. After the addition of 30 mM H2O2 to the mixture to start the reaction, the absorbance at 655 nm was recorded every 30 s for 2-min period by using the kinetic spectrophotometer. The rate of change of absorbance at 650 nm per min was defined as 1 U of enzyme activity. The specific activity of MPO was expressed as units per milligram of total protein in plasma or stool extracts. The final products of NO· in vivo are nitrate (NO2−) and nitrite (NO3−). The total nitrate and nitrite (designated NO2− and NO3−) contents of plasma and urine were measured photometrically by using a commercial kit (Cayman Chemical) (30). The NO2− or NO3− concentration was expressed in nanomoles per milliliter of plasma. In urine, the NO2− or NO3− content was expressed as a creatinine ratio. To detect the SOD activity, the increase in the rate of autooxidation of 5,6,6a,11b-tetrahydro-3,9,10-trihydroxybenzofluorener was measured by using a commercially available kit (Oxis International). The rate of change of absorbance per minute at 525 nm was defined as 1 U of SOD activity. The specific activity in plasma or stool extracts was expressed as units per milligram of total protein.
Histopathology.
Formalin-fixed, paraffin-embedded tissues were sectioned at 3 μm per section and stained with hematoxylin and eosin. Coded sections from each specimen were examined by the histopathologist, who was unaware of the culture report and clinical profile of the patient. For the evaluation of the biopsy specimens, the histopathological features described earlier were used (17).
Immunohistochemistry.
Paraffin sections 3-μm thick were deparaffinized, rehydrated, and stained with the following antibodies: monoclonal mouse anti-human MPO (1:80; Dako), rabbit anti-inducible nitric oxide synthase (iNOS; 1:250; Serotech, Ltd., Oxford, England), and rabbit anti-human Lf (1:100; Dako). For Lf and iNOS staining, slides were microwaved for 12 min, sections were incubated with 1% trypsin (Sigma) in PBS (pH 7.0) for 5 min at 37°C. To block endogenous peroxidase activity and nonspecific binding sites, sections were incubated with 3% hydrogen peroxide for 1 h, followed by incubation with 20% normal goat serum for 30 min. Sections were incubated overnight with primary antibodies diluted in 1% bovine serum albumin-PBS. After being washed, sections were incubated with biotin-conjugated goat anti-mouse (1:250; Dako) or anti-rabbit (1:500; Dako) antibodies, washed, and overlaid with preformed avidin-biotin peroxidase complex (ABC Complex/HRP; Dako) (1:250). After a final wash, labeling was visualized with 3,3′-diaminobenzidine (DAB).
The reaction was stopped by rinsing the mixtures with water. Sections were counterstained with hematoxylin and mounted with synthetic mountant (Shandon Scientific, Ltd., Cheshire, England). As a control, first-step antibodies were replaced by irrelevant isotype-matched control antibodies.
Quantification of immunoreactivity by computer-assisted analysis of video microscopic images.
Immunohistochemical staining of specific enzymes in duodenal and rectal tissues was examined with a DMLB microscope (Leica Wetzlar, GmbH) equipped with a 3CCD color camera (JVC TK-CF380; Victor Company of Japan, Ltd.). Each image was examined in a Leica Q500IW image analyzer by using the Leica QWin version 2.0 computer program. The standards were set for both positive and negative cells. Positive staining of enzymes in tissue sections was carried out by computer-assisted analysis of video microscopic images as described earlier (29). The positive staining in the tissue section was carried out by computer-assisted analysis of video microscopic images initially read in the true colors red, green, and blue and expressed in gray levels (1 to 256) of the blue part of the red, green, and blue video signals. The acquired image was divided into 512 by 512 pixels, and each pixel was expressed in square micrometers (area) after calibration with the current magnification. The data acquired were imported into Microsoft Excel. Positive immunostaining, as assessed by computer-assisted analysis of video microscopic images, was determined for the studied enzymes in patients and in healthy controls. For each tissue section at least 20 fields (4 × 104 μm2/field) were investigated at a ×400 magnification, and the average values were calculated. The automated video microscopic analysis allowed quantification of the positive immunoreactivity relative to the total cell area of the tissue section, and the results were expressed as the percentage of the ratio of positive pixels to total pixels.
RNA extraction and PCR amplification.
Total RNA was extracted from one piece of duodenal biopsy from adults and one piece of rectal biopsy from children by using the acid guanidinium thiocyanate-phenol-chloroform method described previously (7). cDNAs were synthesized from 2 μg of total cellular RNA/ml and, subsequently, 5 μl of cDNA product was amplified by PCR by using a thermal cycler (Perkin-Elmer, Norwalk, Conn.). PCR amplification of the cDNAs was carried out by using 50 cycles. For PCR amplification, the primers iNOS (236 bp) (31) and SOD (236 bp) (3) were used. Denaturation, annealing, and elongation temperatures and durations for PCR were 94, 60, and 72°C and 1, 1, and 1.5 min, respectively, for iNOS and 94, 60, and 72°C and 1, 1, and 2 min, respectively, for SOD. PCR-amplified products were separated in 1.5% agarose gel.
Statistical analyses.
The Wilcoxon signed rank test and the Mann-Whitney U test were used where applicable for statistical analyses. A P value of ≤0.05 was the criterion for a significant difference. Analyses were carried out by using the statistical software SigmaStat (Jandel Scientific, San Rafael, Calif.). Data were expressed as median values with the 25 and 75 percentiles or as the geometric mean and range (± the standard error of the mean [SEM]).
RESULTS
Clinical history of the study groups.
The clinical features of the patients are shown in Table 1. All 12 of the adult O1 cholera patients and 13 of the O139-infected patients suffered from severe dehydration, and they were, on average, seen at the hospital 8 to 9 h after the onset of illness. The adult cholera patients had a median stool frequency after admission of 24 per day and median vomiting frequency of 6 per day at the initial stage. The 10 children with V. cholerae O1 infection were in the 2- to 8-year age range; 9 arrived with severe dehydration and 1 arrived with moderate dehydration, whereas all of the O139-infected children presented at the hospital with severe dehydration. The children had a 12-h history of diarrhea prior to arrival at the hospital and, on average, had a stool frequency of 21 per day and a vomiting frequency of 5 per day at the initial stage after admission. Examination of stools showed that no bacterial pathogen except V. cholerae was isolated from any of the cholera patients. Stool microscopy revealed the presence of a few ova of Ascaris lumbricoides in one adult cholera patient, both giardia and E. histolytica in one adult O139-infected patient, and A. lumbricoides in one O139-infected adult patient. In the children, A. lumbricoides was isolated from three O1- and three O139-infected patients. Hookworm was detected in one O139-infected child and in two O1-infected children. Giardia was isolated in one O139-infected patient. No bacterial pathogens were isolated from the stool samples of the healthy controls. However, giardia was isolated from one child, and A. lumbricoides was isolated from two children and two adults.
TABLE 1.
Clinical features at the acute phase in patients infected with V. cholerae O1 and O139
| Parameter | Adults
|
Children
|
||||
|---|---|---|---|---|---|---|
| O1 infected | O139 infected | Healthy controls | O1 infected | O139 infected | Healthy controls | |
| Median age in yr (range) | 25 (18-44)d | 29 (19-40) | 27 (22-40) | 5 (2-8) | 6 (2-9) | 4 (3-7) |
| Median duration of illnessa in h (range) | 8 (5-14) | 8 (6-17) | NAe | 12 (5-14) | 11 (3-13) | NA |
| No. (%) with dehydrationb | 12 (100) | 13 (100) | NA | 9 (90) | 9 (100) | NA |
| No. (%) with temp of >37.8°C | 0 | 0 | NA | 0 | 0 | NA |
| Blood leukocyte count (102/mm3)c | 107 (103-112)* | 121 (113-129)* | 92 (90-94) | 111 (106-117)* | 109 (101-118)* | 94 (91-100) |
| Blood lymphocyte count (%) | 32 (30-34) | 31 (29-34) | 36 (34-38) | 39.4 (37-42) | 32 (29-35) | 44 (41-48) |
| Blood PMN count (%)c | 64 (62-66)* | 63 (60-67)* | 56 (54-58) | 53 (49.6-56.5) | 58 (53-63) | 45.2 (43-50) |
| No. (%) with >50 leukocytes/high-powered fieldf | 1 (7.6) | 1 (8) | 0 | 0 | 0 | 0 |
| Creatinine concn in serum in μmol/liter (range)c | 95.7 (87-105)* | 110 (89-136)* | 70 (67-72) | 67 (63.5-71)* | 82 (71-95)* | 49 (47-51) |
| Creatinine concn in urine in μmol/liter (range)c | 3,617 (2,092-11,003)* | 3,410 (2,633-5,897)* | 1,918 (1,407-2,298) | 2,989 (1,742-3,984)* | 2,900 (1,851-3,845)* | 865 (510-1,451) |
That is, duration of illness before hospitalization.
The numbers and percentages of patients with severe dehydration are shown.
Statistical significance (P ≤ 0.05) between the acute stage in O1 and O139 patients and healthy controls is indicated by an asterisk.
Results are expressed as medians with ranges (25th to 75th percentiles) in parentheses.
NA, not applicable in the case of healthy controls.
As determined by microscopic examination of stool samples.
Creatinine levels in serum.
The level of creatinine in sera and urine samples in O1-infected patients, both adults and children, was higher at the acute stage compared to the healthy controls (P = 0.035 to 0.001) (Table 1). In O139-infected patients a similar increase was seen in both children and adults when the values were compared to the healthy controls (P = 0.012 to 0.03).
Blood leukocytes.
The data were analyzed both separately and for the O1 and O139 group of cholera patients together. A higher blood leukocyte level was seen at the acute stage of the disease in children and adults in both groups of cholera patients compared to the healthy controls (Table 1). In the adults, the levels remained elevated up to 7 days after onset of diarrhea but decreased to levels seen in healthy controls by day 30 (data not shown). In children, however, the white blood cell (WBC) count decreased to control levels by day 7 after the onset of illness. No increase was seen in the total lymphocyte counts over the course of the illness in the different study groups. However, the percentages of polymorphs in blood was elevated in the adult cholera patients at the acute stage of the infection compared to the levels at both day 7 (P = 0.011) and day 30 (P < 0.001) after onset of infection or to the levels in the healthy volunteers (P = 0.027). When the data were analyzed separately for O1- and O139-infected adult patients, significantly elevated levels of neutrophil polymorphs were also seen only at the acute stage of infection in both groups of cholera patients compared to the levels seen at follow-up or compared to the healthy group (Table 1). In children, the percentage of polymorphs in blood was somewhat higher at the acute stage, but the difference was not statistically significant from those for the other study periods or to the levels in healthy controls.
A comparison of the percentages of eosinophilic granulocytes in blood showed that the levels were increased from the acute stage (median = 3.2%) to convalescence at day 7 (median = 6.0%; P = 0.022) for the O139-infected patients and between the acute stage (median = 4.11%) and convalescence at day 30 (median = 7.5%; P = 0.049) in the adult O1-infected cholera patients (P = 0.023). The levels in the healthy controls (median = 4.3%) were similar to those in the patients at the acute stage and were only lower than the levels seen in the O139-infected patients at day 30 after onset of illness (P = 0.05). In the children with diarrhea no increase in the blood eosinophil levels were observed either during the course of the disease or in comparison to the levels of the uninfected healthy controls.
Acute-phase protein.
The level of CRP was higher in the sera of the adult cholera patients at the acute phase of the infection compared to the levels seen during convalescence or in the healthy controls (P = 0.018) (Table 2). About 84% of the patients showed an increase at day 2 after onset of illness (range, 2 to 11 mg/dl). Although the levels were decreased at day 7 after onset, they remained elevated compared to the healthy controls. In the children, however, the levels of CRP remained at the baseline, as in the healthy controls (Table 2). No difference was seen between the levels in O1- and O139-infected patients, either in children or adults (P = not significant).
TABLE 2.
Levels of the innate mediators of the immune system in blood samplesb
| Age group and mediator | Acute stage (day 2) | Convalescence (day 7) | Convalescence (day 30) | Healthy controls |
|---|---|---|---|---|
| Adults | ||||
| PGE2 (pg/ml) | 1,700 (780-3,890) | 590 (480-4,700) | 690 (390-2,440) | 1,770 (1,060-8,620) |
| PGF2α (pg/ml) | 1.36 (0.83-2.38) | 0.75 (0.43-1.63) | 0.90(0.66-1.40) | 1.27 (0.48-1.55) |
| LTB4 (pg/ml) | 476 (208-1,000)†‡ | 309 (260-493)‡ | 179 (35-850) | 132 (118-265) |
| Lf (ng/ml) | 505 (288-772)‡ | 434 (355-562) | 321 (212-460) | 306 (216-738) |
| SOD (sp act [U/mg of protein]) | 1.20 (1.16-1.25)‡ | 0.063 (0.058-0.067)‡ | 0.048 (0.036-0.064) | 0.024 (0.019-0.03) |
| MPO (sp act [U/mg of protein]) | 0.02 (0.014-0.026) | 0.01 (0.006-0.03) | 0.023 (0.016-0.033) | 0.02 (0.005-0.03) |
| NȮa metabolites (nmol/ml) | 184 (144-323)*†‡ | 58 (47-70) | 37 (32-60) | 38 (32-80) |
| CRP (mg/dl) | 4.1 (2.3-7.5)*†‡ | 2.0 (0.5-3.2) | 0.85 (0.5-2.4) | UDc |
| Children | ||||
| PGE2 (pg/ml) | 1,103 (740-1440) | NDd | 553 (400-1,498) | 784 (557-1,298) |
| PGF2α (pg/ml) | 0.78 (0.31-2.94) | 1.22 (0.16-2.07) | 1.31 (0.22-2.33) | 2.95 (0.76-4.48) |
| LTB4 (pg/ml) | 500 (77-711)†‡ | ND | 80 (47-500) | 79 (47-475) |
| Lf (ng/ml) | 251 (173-360)‡ | 177 (146-246)‡ | 223 (200-325)‡ | 115 (102-141) |
| SOD (sp act [U/mg of protein) | 0.03 (0.024-0.034) | 0.04 (0.02-0.07) | 0.027 (0.023-0.031) | 0.03 (0.028-0.035) |
| MPO (sp act [U/mg of protein]) | 0.036 (0.028-0.045) | 0.026 (0.019-0.035) | 0.023 (0.017-0.032) | 0.02 (0.014-0.023) |
| NȮa metabolites (nmol/ml) | 248 (180-496)*†‡ | 52 (12-164)‡ | 56 (19-69) | 2.5 (1.0-32.0) |
| CRP (mg/dl) | 0.92 (0.7-1.12) | 0.73 (0.57-0.88) | UD | UD |
Nitric oxide (NO) was measured as NO2− and NO3−.
Statistical significance (P ≤ 0.05) between patients at the acute stage and at convalescence at day 7 (*) or day 30 (†) after onset of disease in patients and between patients and healthy controls (‡) is indicated. Results are expressed as medians with ranges (25th to 75th percentiles) given in parentheses
UD, below the detection limit.
ND, not done for day 7 samples.
Lf in stool and plasma.
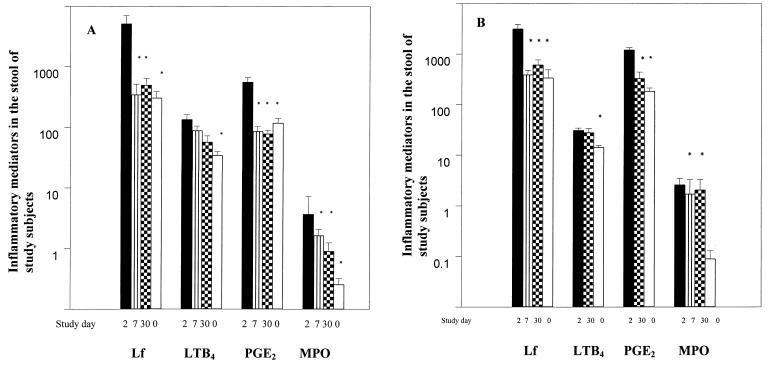
Increased levels of Lf were seen in the stool samples of adult patients with cholera; these levels were elevated at the acute stage compared to those seen at days 7 and 30 after the onset of disease (P < 0.001) or in healthy controls (P < 0.001) (Fig. 1A). A similar pattern was seen in the stools of children with the levels highest at the acute stage of infection compared to those at convalescence at day 7 (P = 0.007) and day 30 (P = 0.002) or to the levels seen in healthy controls (P = 0.002). Again, the levels noted in the children with V. cholerae O1 infection (median, 2,425 ng/mg of protein; range, 607 to 3,814) were similar to those seen in children with V. cholerae O139 infection (median, 3,873 ng/mg; range, 1,397 to 4,914). Lf levels in plasma were higher in adult cholera patients at the acute stage compared to the healthy controls (Table 2). The increased levels at the acute stage were similar in V. cholerae O1 (median, 543 ng/ml; range, 395 to 544)- or V. cholerae O139- (median, 442 ng/ml; range, 218 to 677)- infected patients. In children, Lf levels were higher in plasma over the course of the infection compared to the healthy controls (P = 0.013 to P < 0.001).
FIG. 1.
Levels of innate inflammatory mediators in stool samples of adults (A) or children (B) with cholera. Levels of Lf are expressed as nanograms per milligram of protein, and levels of LTB4 and PGE2 are expressed as picograms per milliliter. For MPO, the specific activity is expressed as units per milligram of protein. Statistical significance (P < 0.05) is shown as a comparison between patients at the acute stage at day 2 and at the convalescence stage at day 7 and/or day 30 after the onset of disease in patients and between patients at the acute stage and healthy controls (day 0) and is indicated by an asterisk. Bars represent geometric means, and lines show the ranges (±SEM).
Nitric oxide metabolites (NO2− and NO3−).
There were increased levels of NO2− and NO3− in plasma in adult patients at the acute stage (P < 0.001) which decreased at convalescence at day 7 (P < 0.001) or day 30 (P < 0.001) after the onset of disease (Table 2). In urine, however, the levels of NO2− and NO3− in patients at different stages of the disease were similar and not different from those of the healthy controls (range, 0.13 to 0.50 nmol/mg of creatinine). In children, the levels of NO2− and NO3− in plasma were higher at the acute stage compared to the level at convalescence at day 7 (P = 0.001) or day 30 (P < 0.001) or that determined in healthy controls (P < 0.001). However, the levels in urine were not significantly altered during the different phases of the disease or from values determined in healthy children. When the nitrate levels in plasma and urine were analyzed separately for V. cholerae O1 and O139 patients, the levels were comparable. At the acute stage, NO2− and NO3− levels in plasma were, on average, 236 and 127 nmol/ml for O1- and O139-infected adult patients, respectively. In children, the levels were 241 nmol/ml in O1 patients and 348 nmol/ml for O139 patients. The levels in urine were comparable in the two groups of patients and were not elevated during the course of infection.
Levels of eicosanoids after infection.
Significantly higher concentrations of PGE2 in stools were observed at the acute stage in adult patients compared to the levels measured at the later stages during convalescence (P = 0.003) or to the levels seen in healthy controls (P = 0.037) (Fig. 1A). This trend was also seen in the children (P = 0.002 to P < 0.001) (Fig. 1B), although the level of PGE2 was significantly higher than in the adults at the acute stage (P = 0.004) or at day 30 after the onset of illness (P = 0.005). There were no differences in the levels of PGE2 in the stools of cholera patients infected with V. cholerae O1 or O139. At variance with the observation in stools, PGE2 levels in plasma in both adults and children remained unchanged over the study period and were not different from those seen in healthy controls (Table 2). The concentrations of PGF2α in stool were lower in the adult cholera patients at the acute stage of infection (1.9 pg/ml), but this was not significantly different from the levels seen at later stages of the disease or in the controls. In plasma, PGF2α levels were also not different from those seen at follow-up or in the controls. A similar pattern was seen in the children both in plasma (Table 2) and in stool. No differences were seen between O1 and O139 patients in the levels of PGF2α in the stool samples or in the systemic circulation.
LTB4 levels were higher in the stools of adults and children with cholera infection at the acute stage of the disease compared to the healthy groups (P = 0.047 to 0.001) (Fig. 1). In the children, the levels were still somewhat elevated after 30 days of onset of the disease compared to the healthy controls (P = 0.049). In plasma samples in the adults, the levels were higher at the acute stage and at day 7 and decreased to control levels later on. In children, an increase in the level of LTB4 was also observed in plasma at the acute stage of infection. Again, no difference was seen in LTB4 levels between the O1- and the O139-infected cholera patients.
Mediators of the oxidative and antioxidative pathway.
MPO activity was elevated in feces at the acute stage of infection in both children and adults (P = 0.05 to 0.022) (Fig. 1). In the stool of both adults and children with cholera, SOD activity was not elevated over the disease process (range, 1.5 to 0.5 U/mg of protein) or compared to the healthy controls. In sera, only in the adult patients were the levels of the enzyme found to be increased after the onset of the disease (P = 0.004) (Table 2). SOD levels were similar in both V. cholerae O1- and O139-infected patients.
Detection of mRNA for iNOS, and SOD at the mucosal surface.
mRNA for iNOS and SOD were expressed in the duodenal tissues obtained from adult patients throughout the disease process, as well as in healthy controls. In the children, rectal biopsies showed a similar trend and mRNA could be detected at the acute stage, as well as at convalescence and in the healthy controls.
Histopathological analysis of tissue sections and immunohistochemical staining.
The duodenal biopsy sections from the adults and rectal biopsies from the children were histologically evaluated. It was observed that the small intestinal mucosa in patients with cholera seems histologically intact. However, at the acute stage of the disease there was marked congestion of blood vessels and infiltration of neutrophil polymorphs (PMN) in the lamina propria and at the surface of the crypt epithelium. At ca. days 7 and 30 postinfection there was a decrease in the numbers of PMN in the epithelium and lamina propria. Since only rectal biopsies could be obtained from the children with cholera, these were studied. As in the duodenal biopsy samples from the adults, it was observed that there was congestion of blood vessels and edema at the acute stage, with an increase in the numbers of PMN in the lamina propria and surface epithelium. However, the PMN levels decreased during convalescence at day 30 after onset of infection.
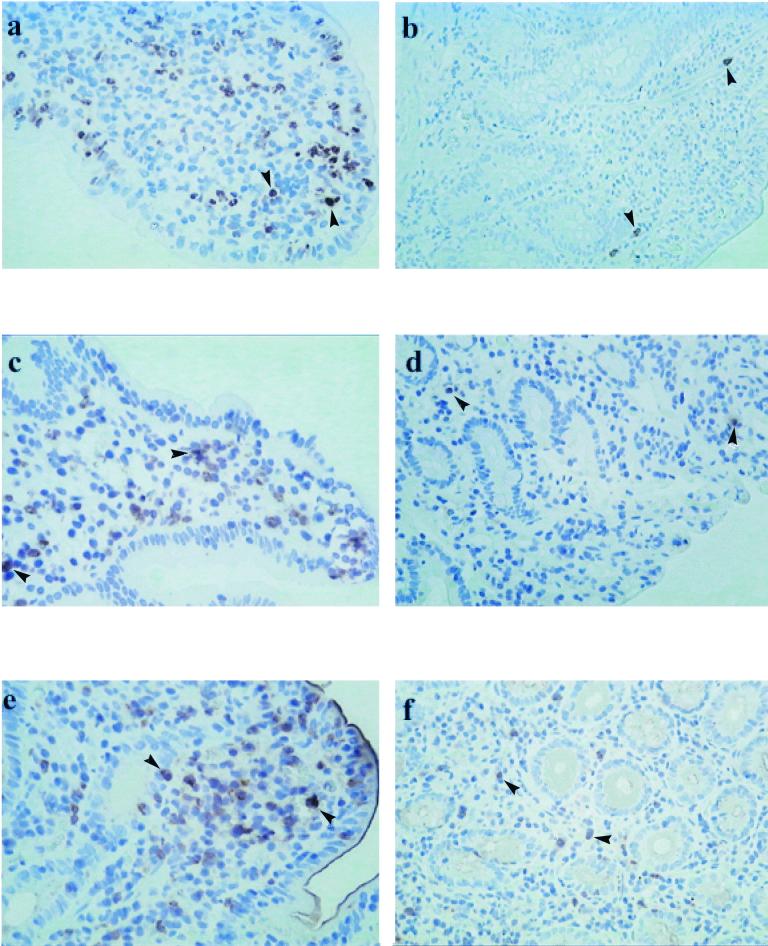
Analyses of paraffin-embedded duodenal sections obtained from adult patients were immunostained to detect MPO (Fig. 2a, Table 3) showed that MPO levels were increased at the acute stage of infection and, in comparison, decreased at convalescence at day 7 (P < 0.001) (Table 3) and day 30 (P < 0.001) and was also higher compared to healthy controls (P < 0.001) (Fig. 2b). Similarly, the Lf level was also increased at the acute stage (Fig. 2c); this level was significantly higher than the levels observed at convalescence (P = 0.002) or in healthy controls (P = 0.001) (Fig. 2d). Both MPO and Lf could be detected in the PMN in sections.
FIG. 2.
Immunohistochemistry of duodenal mucosa for inflammatory mediators. The expression of MPO (a), Lf (c), and iNOS (e) in duodenal biopsies from adult patients with cholera at the acute stage of infection is depicted. Sections from healthy adult controls stained for MPO (b), Lf (d), and iNOS (f) are also shown. Arrow shows immunoperoxidase-labeled cells. Magnification, ×320.
TABLE 3.
Immunohistochemical staining for components at the local site in study subjects
| Mediator | Study day | % Mediator (range)a in:
|
|
|---|---|---|---|
| Adults | Children | ||
| MPO | 2 | 1.45 (1.08-8.87)*†‡ | 1.21 (1.13-2.23)‡ |
| 7 | 0.01 (0.01-0.05) | 0.21 (0.05-0.35)‡ | |
| 30 | 0.01 (0.005-0.01) | 0.06 (0.01-0.28) | |
| HCb | 0.01 (0.002-0.02) | 0.01 (0.01-0.07) | |
| Lf | 2 | 1.16 (0.53-1.71)*†‡ | 1.18 (0.85-1.79)‡¶ |
| 7 | 0.02 (0.01-0.23)‡ | 0.51 (0.22-1.63)‡ | |
| 30 | 0.05 (0.01-0.12) | 0.30 (0.18-0.95)‡ | |
| HC | 0.003 (0.001-0.01) | 0.01 (0.01-0.42) | |
| iNOS | 2 | 3.77 (3.13-5.61)†‡ | 3.1 (2.3-5.3)†‡ |
| 30 | 0.97 (0.28-1.64) | 2.0 (1.31-3.26)‡ | |
| HC | 1.23 (1.11-1.76) | 0.16 (0.03-1.51) | |
The average studied areas for each section were 9.3 × 105 μm2 for the duodenal biopsies and 10.2 × 105 μm2 for the rectal biopsies. Quantification of immunoreaction-positive areas relative to the total tissue section was determined by a computerized image-analyzing technique, and the results are expressed as the percentage of the ratio of positive area to the total area. Paraffin-embedded duodenal sections were studied in adults and in rectal sections in children in the study subjects. The Mann-Whitney U test was used to compare the statistical significance (P ≤ 0.05) in comparison between patients at the acute stage and at convalescence at day 7 (*) or day 30 (†) or between day 7 and day 30 (¶) after the onset of disease in patients or between patients at different stages of the disease and healthy controls (‡). Data are given as medians with ranges (25th and 75th percentiles) in parentheses.
HC, healthy controls.
Immunostaining for iNOS showed upregulation at the acute stage (Fig. 2e) and in comparison to that seen at convalescence or in the controls (Fig. 2f). In children from whom only rectal biopsies could be collected, statistically significant increases in the levels of MPO, Lf, and iNOS were seen at the acute stage (Table 3). The levels of these mediators were significantly higher at the acute stage compared to the levels seen at convalescence (P = 0.05 to 0.006) or in healthy controls (P = 0.008).
Comparison of adults and children.
Since both adults and children with cholera were studied, we compared their responses. In the blood, the elevation of PMN, eosinophil, and CRP levels were only observed in the adult cholera patients. The levels of SOD in plasma were higher in adults than in children (P = 0.003). The mean level of PGE2 in the stool was higher in children than in adults. For the other factors, such as nitric oxide, LTB4, and MPO in the stool and sera, the patterns and levels were similar in both adults and children over the course of the disease. However, the expression of MPO, Lf, and iNOS persisted longer at the mucosal surface in children compared to adults.
DISCUSSION
We studied the contribution of different mediators of the innate immune response in pediatric and adult cholera patients. The majority of the patients studied presented to the hospital with severe dehydration and severe disease.
A number of factors believed to play a role in the association of V. cholerae with the host gastrointestinal tract can either result in successful elimination of the bacteria or result in colonization and production of acute watery diarrhea. Cholera was long considered a classical paradigm of a noninflammatory toxigenic diarrhea until ultrastructural studies showed that, in reality, inflammatory cells such as the PMN, eosinophils, and mast cells were increased in V. cholerae O1 infections at the acute stage of the disease in the gut (17). In the present study we show that in specimens obtained from patients infected with V. cholerae O1 and O139, there were increases in a number of inflammatory mediators that have bactericidal, bacteriostatic, and immunoregulatory properties. Although these increases were predominantly seen in the stool and in gut biopsies of patients during the acute phase of infection, increases were also seen in the systemic circulation. It was interesting to observe that there were elevations in the level of the acute-phase protein, CRP, in the adult patients at the acute stage of infection. The acute-phase protein is normally present in very low concentrations in the sera of healthy subjects and increases rapidly in response to an inflammatory stimulus (13). It has been shown to increase more in bloody dysentery than in secretory diarrhea (12). In the present study we demonstrate significantly increased levels of CRP in adult cholera patients, levels that were higher than those seen in healthy individuals. This suggests that V. cholerae, although a noninvasive pathogen, does can stimulate the induction of the acute-phase protein. Why this was not observed in the children with cholera is difficult to determine since the levels of this protein are known to increase in infectious diseases in both children and adults. At the acute phase of infection, some increase in the number of leukocytes, including total WBC and PMN, was seen in the blood. Increased WBC counts have been observed earlier in cholera patients with severe disease (17). Increases in the levels of the bactericidal protein Lf and MPO were also seen in the patients with cholera in the present study. Both of these proteins are known to be present in neutrophil polymorphs. The bactericidal activities of Lf against V. cholerae have been shown earlier in in vitro studies (1). Detection of the protein in stool samples has been used as a useful index of inflammation and as a marker for the presence of leukocytes. In a study in North American volunteers immunized with the live cholera vaccine candidate, CVD 110, increases in the levels of the protein were seen in stool when an Lf agglutination assay was used (32). By using a more sensitive enzyme-linked immunosorbent assay, we show that there are increases in both O1- and O139-infected patients not only in stool and plasma samples but also in the mucosal compartment in the biopsies. The increased localization of Lf, which is mainly produced by the secondary granules of PMN, demonstrates that these cells are activated in cholera. It is, however, important to note that this protein not only is involved in bacterial clearance but also has immunotropic functions and promotes the maturation of B and T cells, the antigen-presenting function of B cells, and the induction of the release of cytokines, which may in turn have an effect on the generation of specific immune responses (20). In addition to Lf, MPO, which is also involved in bactericidal activity mediated via the oxidative pathway, is produced by the PMN. Its upregulation at the local site in both tissue and in stool suggests that it may play a role in bactericidal activities by generating reactive products that have potent antibacterial properties (20). MPO may also protect the mucosal surfaces by preventing the accumulation of toxic products of oxygen reduction.
We also observed an increase in the levels of nitric oxide metabolites in plasma and an elevation of iNOS activity in the mucosa. Nitric oxide metabolites are necessary for protection of the gut by killing bacteria. It has been speculated that increased production of NO· may result in enhanced peristalsis in the gut in cholera due to its effect on intestinal smooth muscle and neurons and have a prosecretory role (11). It has also been shown that CT-induced NO· production originates from the intestinal epithelium and is mediated by iNOS (9). CT may stimulate iNOS gene expression in the intestine. Our results show that iNOS expression is increased in the duodenal and rectal sections obtained from cholera patients. In addition to the presecretory role of nitric oxide, it is possible that the metabolite, by formation of toxic peroxynitrite, may also be involved in bactericidal activities in the gut and thereby decrease fluid secretion and help in recovery from the disease. We found that although the level of the NO· was elevated in the serum at the acute stage of infection it decreased to levels seen in the healthy individuals by ca. day 7 after the onset of illness. The iNOS activity in the tissue sections also decreased to control levels at convalescence.
The upregulation of MPO, as well as the sustained levels of SOD, in the local secretion suggests that the two enzymes strike a balance to maintain the oxidation reduction potential in the disease process. The levels of SOD in plasma also remained at normal or somewhat higher than normal levels in children and adults with cholera during the acute stage, suggesting that active scavenging of the reactive products by MPO was taking place. This may be the reason why there seems to be recovery in cholera so soon after such an extremely acute dehydrating infection, whereas in shigellosis the SOD levels decrease in both children and adults, predisposing the tissues to reactive oxygen species and resulting in damage and lowered defense (30).
The eicosanoid PGE2 has been shown to be upregulated when epithelial cells are infected by invasive bacteria (7, 30). Our results show that in patients with cholera there is an increase in the level of PGE2 in stool at the acute stage of infection. Evidence suggests that CT induces an increase of PGE2 in jejunal aspirates of humans after natural cholera infection (33), as well as in experimental animals (18, 19). PGE2 has been shown to have an anti-inflammatory, as well as an immunoregulatory, role. In addition, it is believed to prime T cells for the production of anti-inflammatory cytokines (6). The various functions of PGE2 may help in the induction of a protective response for the host and in the recovery from disease. In contrast to PGE2, the levels of LTB4, an eicosanoid which has a proinflammatory role, were also increased in cholera patients. LTB4 has a role to play in neutrophil and macrophage chemotaxis, as well as in modulating and increasing vascular permeability. The level of LTB4 was increased both in stool and serum samples and decreased to normal levels by convalescence. Earlier studies have shown that in the rat jejunum, CT stimulates the production of LTB4, PGE2, and LTC4 (2). These earlier findings and those of the present study are consistent with the observation that arachidonate metabolites are involved in diarrhea induced by CT. These lipid mediators may be produced by the cells at the mucosal surface and by those in the circulation. Ultrastructural studies of the rectal sections have demonstrated increased accumulation of lipid bodies in the mucosal mast cells at the acute stage of infection (21). Lipid bodies are the site of arachidonic acid metabolism in the cell, and the increase of lipid bodies in acute watery diarrhea suggests that there is active synthesis of these inflammatory mediators in these cells which, upon release into the mucosal environment, could contribute to the pathogenesis of the disease.
The children and adults with cholera that were studied here were seen at the hospital very soon after the onset of illness, and ca. 90% suffered from severe dehydration and severe disease. The protective or harmful effects of the mediators studied here in recovery from the disease can only be speculated upon. Inflammation and innate components are believed to be a prerequisite for the host to mount an appropriate adaptive immune response to an infection (15). It is possible that in cholera the different mediators of the innate system are activated, leading to better protection. However, the inflammation observed is of a low-grade nature and may be needed to clear bacteria or be helpful in mounting an appropriate adaptive immune response. In conclusion, the present study provides evidence that there is an increase in the levels of different mediators and inflammatory components in disease caused by V. cholerae O1 and O139 in both children and adults.
Acknowledgments
This study was conducted at the ICDDR,B Centre for Health and Population Support was provided through grants from the the Swedish Agency for Research Cooperation with Developing Countries (Sida-SAREC) (1998-05440). The ICDDR,B acknowledges with gratitude the commitment of SIDA-SAREC to the Centre's research efforts.
REFERENCES
- 1.Arnold, R. R., M. Brewer, and J. J. Gauthier. 1980. Bactericidal activity of human lactoferrin: sensitivity of a variety of microorganisms. Infect. Immun. 28:893-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Autore, G., F. Capasso, G. Di Carlo, and N. Mascolo. 1987. Effect of cholera toxin on the production of eicosanoids by rat jejunum. Br. J. Pharmacol. 92:149-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boven, L. A., L. Gomes, C. Hery, F. Gray, J. Verhoef, P. Portegies, M. Tardieu, and H. S. Nottet. 1999. Increased peroxynitrite activity in AIDS dementia complex: implications for the neuropathogenesis of HIV-1 infection. J. Immunol. 162:4319-4327. [PubMed] [Google Scholar]
- 4.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 5.Burch, R. M., C. Jelsema, and J. Axelrod. 1988. Cholera toxin and pertussis toxin stimulate prostaglandin E2 synthesis in a murine macrophage cell line. J. Pharmacol. Exp. Ther. 244:765-773. [PubMed] [Google Scholar]
- 6.Demeure, C. E., L. P. Yang, C. Desjardins, P. Raynauld, and G. Delespesse. 1997. Prostaglandin E2 primes naive T cells for the production of anti-inflammatory cytokines. Eur. J. Immunol. 27:3526-3531. [DOI] [PubMed] [Google Scholar]
- 7.Eckmann, L., W. F. Stenson, T. C. Savidge, D. C. Lowe, K. E. Barrett, J. Fierer, J. R. Smith, and M. F. Kagnoff. 1997. Role of intestinal epithelial cells in the host secretory response to infection by invasive bacteria. Bacterial entry induces epithelial prostaglandin H synthase-2 expression and prostaglandin E2 and F2α production. J. Clin. Investig. 100:296-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glass, R. I., S. Becker, M. I. Huq, B. J. Stoll, M. U. Khan, M. H. Merson, J. V. Lee, and R. E. Black. 1982. Endemic cholera in rural Bangladesh, 1966-1980. Am. J. Epidemiol. 116:959-970. [DOI] [PubMed] [Google Scholar]
- 9.Janoff, E. N., H. Hayakawa, D. N. Taylor, C. E. Fasching, J. R. Kenner, E. Jaimes, and L. Raij. 1997. Nitric oxide production during Vibrio cholerae infection. Am. J. Physiol. 273:G1160-G1167. [DOI] [PubMed] [Google Scholar]
- 10.Jertborn, M., A. M. Svennerholm, and J. Holmgren. 1986. Saliva, breast milk, and serum antibody responses as indirect measures of intestinal immunity after oral cholera vaccination or natural disease. J. Clin. Microbiol. 24:203-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang, M. M., A. Kirchgessner, M. D. Gershon, and A. Surprenant. 1993. Cholera toxin-sensitive neurons in guinea pig submucosal plexus. Am. J. Physiol. 264:G86-G94. [DOI] [PubMed] [Google Scholar]
- 12.Khan, W. A., M. A. Salam, and M. L. Bennish. 1995. C reactive protein and prealbumin as markers of disease activity in shigellosis. Gut 37:402-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kushner, I., H. Gewurz, and M. D. Benson. 1981. C-reactive protein and the acute-phase response. J. Lab. Clin. Med. 97:739-749. [PubMed] [Google Scholar]
- 14.Leal-Berumen, I., D. P. Snider, C. Barajas-Lopez, and J. S. Marshall. 1996. Cholera toxin increases IL-6 synthesis and decreases TNFα production by rat peritoneal mast cells. J. Immunol. 156:316-321. [PubMed] [Google Scholar]
- 15.Marcinkiewicz, J. 1997. Neutrophil chloramines: missing links between innate and acquired immunity. Immunol. Today 18:577-580. [DOI] [PubMed] [Google Scholar]
- 16.Marinaro, M., H. F. Staats, T. Hiroi, R. J. Jackson, M. Coste, P. N. Boyaka, N. Okahashi, M. Yamamoto, H. Kiyono, H. Bluethmann, et al. 1995. Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) cells and IL-4. J. Immunol. 155:4621-4629. [PubMed] [Google Scholar]
- 17.Mathan, M. M., G. Chandy, and V. I. Mathan. 1995. Ultrastructural changes in the upper small intestinal mucosa in patients with cholera. Gastroenterology 109:422-430. [DOI] [PubMed] [Google Scholar]
- 18.Peterson, J. W., Y. Lu, S. Duncan, J. Cantu, and A. K. Chopra. 1994. Interactions of intestinal mediators in the mode of action of cholera toxin. J. Med. Microbiol. 41:3-9. [DOI] [PubMed] [Google Scholar]
- 19.Peterson, J. W., and L. G. Ochoa. 1989. Role of prostaglandins and cAMP in the secretory effects of cholera toxin. Science 245:857-859. [DOI] [PubMed] [Google Scholar]
- 20.Pruitt, K. M., B. Rahemtulla, F. Rahemtulla, and M. W. Russell. 1999. Innate humoral factors, p. 65-88. In P. L. Ogra, J. Mestecky, M. E. Lamm, W. Strober, J. Bienenstock, and J. R. McGhee (ed.), Handbook of mucosal immunology. Academic Press, Inc., San Diego, Calif.
- 21.Pulimood, A. B., M. M. Mathan, and V. I. Mathan. 1998. Quantitative and ultrastructural analysis of rectal mucosal mast cells in acute infectious diarrhea. Dig. Dis. Sci. 43:2111-2116. [DOI] [PubMed] [Google Scholar]
- 22.Qadri, F., F. Ahmed, M. M. Karim, C. Wenneras, Y. A. Begum, M. Abdus Salam, M. J. Albert, and J. R. McGhee. 1999. Lipopolysaccharide- and cholera toxin-specific subclass distribution of B-cell responses in cholera. Clin. Diagn. Lab. Immunol. 6:812-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qadri, F., M. Asaduzzaman, C. Wenneras, G. Mohi, M. J. Albert, M. Abdus Salam, R. B. Sack, M. Jertborn, J. R. McGhee, D. A. Sack, and J. Holmgren. 2000. Enterotoxin-specific immunoglobulin E responses in humans after infection or vaccination with diarrhea-causing enteropathogens. Infect. Immun. 68:6077-6081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qadri, F., S. K. Das, A. S. Faruque, G. J. Fuchs, M. J. Albert, R. B. Sack, and A. M. Svennerholm. 2000. Prevalence of toxin types and colonization factors in enterotoxigenic Escherichia coli isolated during a 2-year period from diarrheal patients in Bangladesh. J. Clin. Microbiol. 38:27-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qadri, F., G. Jonson, Y. A. Begum, C. Wenneras, M. J. Albert, M. A. Salam, and A. M. Svennerholm. 1997. Immune response to the mannose-sensitive hemagglutinin in patients with cholera due to Vibrio cholerae O1 and O139. Clin. Diagn. Lab. Immunol. 4:429-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qadri, F., G. Mohi, J. Hossain, T. Azim, A. M. Khan, M. A. Salam, R. B. Sack, M. J. Albert, and A. M. Svennerholm. 1995. Comparison of the vibriocidal antibody response in cholera due to Vibrio cholerae O139 Bengal with the response in cholera due to Vibrio cholerae O1. Clin. Diagn. Lab. Immunol. 2:685-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qadri, F., C. Wenneras, M. J. Albert, J. Hossain, K. Mannoor, Y. A. Begum, G. Mohi, M. A. Salam, R. B. Sack, and A. M. Svennerholm. 1997. Comparison of immune responses in patients infected with Vibrio cholerae O139 and O1. Infect. Immun. 65:3571-3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rabbani, G. H., S. Islam, A. K. Chowdhury, A. K. Mitra, M. J. Miller, and G. Fuchs. 2001. Increased nitrite and nitrate concentrations in sera and urine of patients with cholera or shigellosis. Am. J. Gastroenterol. 96:467-472. [DOI] [PubMed] [Google Scholar]
- 29.Raqib, R., A. A. Lindberg, L. Bjork, P. K. Bardhan, B. Wretlind, U. Andersson, and J. Andersson. 1995. Down-regulation of gamma interferon, tumor necrosis factor type I, interleukin 1 (IL-1) type I, IL-3, IL-4, and transforming growth factor beta type I receptors at the local site during the acute phase of Shigella infection. Infect. Immun. 63:3079-3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raqib, R., S. M. Mia, F. Qadri, T. I. Alam, N. H. Alam, A. K. Chowdhury, M. M. Mathan, and J. Andersson. 2000. Innate immune responses in children and adults with shigellosis. Infect. Immun. 68:3620-3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selleri, C., T. Sato, A. M. Raiola, B. Rotoli, N. S. Young, and J. P. Maciejewski. 1997. Induction of nitric oxide synthase is involved in the mechanism of Fas-mediated apoptosis in haemopoietic cells. Br. J. Haematol. 99:481-489. [DOI] [PubMed] [Google Scholar]
- 32.Silva, T. M., M. A. Schleupner, C. O. Tacket, T. S. Steiner, J. B. Kaper, R. Edelman, and R. Guerrant. 1996. New evidence for an inflammatory component in diarrhea caused by selected new, live attenuated cholera vaccines and by El Tor and Q139 Vibrio cholerae. Infect. Immun. 64:2362-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Speelman, P., G. H. Rabbani, K. Bukhave, and J. Rask-Madsen. 1985. Increased jejunal prostaglandin E2 concentrations in patients with acute cholera. Gut 26:188-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugi, K., O. Saitoh, I. Hirata, and K. Katsu. 1996. Fecal lactoferrin as a marker for disease activity in inflammatory bowel disease: comparison with other neutrophil-derived proteins. Am. J. Gastroenterol. 91:927-934. [PubMed] [Google Scholar]
- 35.World Health Organization. 1990. Diarrhoeal diseases control programme. Global activities, 1988-1989. Wkly. Epidemiol. Rec. 65:289-292. [PubMed] [Google Scholar]
- 36.World Health Organization. 1987. Programme for control of diarrhoeal diseases 83.3(Rev. 1):9-20.