Abstract
Duplications of Xq26-27 have been implicated in the etiology of X-linked hypopituitarism associated with mental retardation (MR). Additionally, an expansion of a polyalanine tract (by 11 alanines) within the transcription factor SOX3 (Xq27.1) has been reported in patients with growth hormone deficiency and variable learning difficulties. We report a submicroscopic duplication of Xq27.1, the smallest reported to date (685.6 kb), in two siblings with variable hypopituitarism, callosal abnormalities, anterior pituitary hypoplasia (APH), an ectopic posterior pituitary (EPP), and an absent infundibulum. This duplication contains SOX3 and sequences corresponding to two transcripts of unknown function; only Sox3 is expressed in the infundibulum in mice. Next, we identified a novel seven-alanine expansion within a polyalanine tract in SOX3 in a family with panhypopituitarism in three male siblings with an absent infundibulum, severe APH, and EPP. This mutation led to reduced transcriptional activity, with impaired nuclear localization of the mutant protein. We also identified a novel polymorphism (A43T) in SOX3 in another child with hypopituitarism. In contrast to findings in previous studies, there was no evidence of MR or learning difficulties in our patients. We conclude that both over- and underdosage of SOX3 are associated with similar phenotypes, consisting of infundibular hypoplasia and hypopituitarism but not necessarily MR.
Introduction
The pituitary gland consists of anterior, intermediate, and posterior lobes and is a central regulator of growth, metabolism, and development. Its complex functions are coordinated by signals from the hypothalamus that regulate the release of six different hormones secreted by five different cell types in the anterior pituitary. Each cell type is defined by the hormone that is secreted: corticotropes (adrenocorticotrophic hormone [ACTH]), thyrotropes (thyroid-stimulating hormone [TSH]), gonadotropes (luteinizing hormone [LH] and follicle-stimulating hormone [FSH]), somatotropes (growth hormone [GH]), and lactotropes (prolactin) (Dasen and Rosenfeld 2001). The posterior lobe consists primarily of axon-terminal projections from two populations of hypothalamic neuroendocrine neurons, and it secretes vasopressin and oxytocin.
The origins of the anterior and posterior lobes of the pituitary gland are embryologically distinct. Rathke’s pouch, the primordial anterior pituitary, arises from the oral ectoderm, whereas the posterior lobe derives from the neural ectoderm of the diencephalon. The apposition of Rathke’s pouch and the region of the diencephalon that later develops into the hypothalamus is maintained throughout the early stages of pituitary organogenesis (Takuma et al. 1998) and appears to be critical for normal anterior pituitary development. A number of gene products (Fgf8, Bmp4, and Nkx2.1 [Lazzaro et al. 1991; Ericson et al. 1998; Takuma et al. 1998]) that are expressed in the neural ectoderm but not in Rathke’s pouch are thought to play a significant role in normal anterior pituitary development, as illustrated by the phenotype of mouse mutants that are null or hypomorphic for these alleles. These molecules are thought to activate or repress key regulatory genes encoding transcription factors, such as Hesx1, Lhx3, and Lhx4, within the developing Rathke’s pouch, and these factors are essential for subsequent development of the pituitary (Takuma et al. 1998; Dasen and Rosenfeld 2001).
Failure of normal pituitary development results in congenital hypopituitarism, a condition that is associated with considerable morbidity and occasional mortality if undiagnosed or inadequately treated. Mutations within a number of transcriptional factors have been associated with hypopituitarism in humans, either in isolation or combined with other manifestations, such as optic nerve hypoplasia or a short stiff neck. These genes, mutations of which may be inherited in an autosomal recessive or dominant manner, include POU1F1 (MIM 173110) (Pfaffle et al. 1992; Radovick et al. 1992), PROP1 (MIM 601538) (Wu et al. 1998), HESX1 (MIM 601802) (Dattani et al. 1998), LHX3 (MIM 600577) (Netchine et al. 2000), and LHX4 (MIM 602146) (Machinis et al. 2001).
A number of pedigrees with X-linked hypopituitarism and variable degrees of learning difficulty have recently been described. Duplications at Xq26-27 have been described in many of these pedigrees (Hamel et al. 1996; Lagerstrom-Fermer et al. 1997; Hol et al. 2000; Solomon et al. 2002), which together define a 3.9-Mb critical region between Xq26.1 and Xq27.3 (mental retardation, X-linked, with isolated growth hormone deficiency [MIM 300123]) (Solomon et al. 2004). This duplicated region contains 18 annotated transcripts, including well-characterized genes, ESTs, and predicted exon sequences. One of these, the developmental transcriptional factor SOX3 (MIM 313430), is a good candidate, given that it is expressed in the developing infundibulum in mice, and overexpression in other tissues results in a hypoplastic phenotype in fish (Koster et al. 2000).
SOX3 is a single-exon gene located on the X chromosome in all mammals. It contains an HMG box and is believed to be the gene from which the testis-determining gene SRY evolved. On the basis of sequence homology, SOX3 is closely related to SOX1 and SOX2 (MIM 184429), and the products of all three genes belong to the SOXB1 subfamily and are expressed throughout the developing CNS (Collignon et al. 1996). The three genes encoding members of the SOXB1 family are expressed in neuroepithelial progenitor and stem cells from the earliest stages, and there is considerable overlap in their expression patterns.
Recently, an in-frame duplication within SOX3 that results in an expansion of a polyalanine (PA) tract by 11 alanine residues was described in a pedigree characterized by mental retardation (MR) and short stature due to GH deficiency (Laumonnier et al. 2002). PA expansions have been described in a number of genes associated with diverse human phenotypes (Brown and Brown 2004). These include FOXL2 (MIM 605597) (Crisponi et al. 2001), HOXA13 (MIM 142959) (Goodman et al. 2000), HOXD13 (MIM 142989) (Muragaki et al. 1996), PABPN1 (MIM 602279) (Brais et al. 1998), RUNX2 (MIM 600211) (Otto et al. 2002), ZIC2 (MIM 603073) (Brown et al. 2001), ARX (MIM 300382) (Bienvenu et al. 2002; Stromme et al. 2002), and PHOX2 (MIM 603851) (Nakano et al. 2001). The functional consequences of such expansions are unclear and could be associated with either gain or loss of function. It has been suggested that causative alanine tract expansions within the polyadenine-binding protein nuclear 1 (PABPN1) protein may lead to protein aggregation, with resulting insoluble inclusions and consequent cell death (Brown and Brown 2004), whereas PA expansions within HOXD13 are associated with a shift in localization from the nucleus to the cytoplasm, where the mutant protein forms large amorphous aggregates (Albrecht et al. 2004). Similar cytoplasmic mislocalization was observed in the 11-alanine expansion in SOX3 that was reported by Laumonnier et al. (Laumonnier et al. 2002; Albrecht et al. 2004), although the effects of this expansion on transcription are unknown.
Together, these data suggest that the dosage of SOX3 could be critical for normal hypothalamopituitary development and that both overdosage (by duplication) and underdosage (by PA tract expansion) could cause hypopituitarism. We therefore sought additional examples of over- and underdosage of SOX3 among patients with hypopituitarism who were recruited from national and international centers. We now report a submicroscopic duplication at Xq27.1 in association with variable hypopituitarism in two siblings. This duplication spans 685.6 kb and is the smallest described to date, containing only SOX3 and two other transcripts of unknown function, of which only murine Sox3 is expressed in the infundibulum. These data strongly implicate SOX3 duplication as the cause of the hypopituitary phenotype. We have also identified, in three brothers with panhypopituitarism, a novel PA expansion (from 15 to 22 alanine residues) within SOX3. We show here that the seven-alanine expansion is associated with loss of transcriptional activation, possibly as a result of its impaired cellular localization. We have also identified a novel polymorphism (A43T) in SOX3 in a child with hypopituitarism associated with an unusual abnormality of the corpus callosum. In contrast to findings in previous studies (Hamel et al. 1996; Lagerstrom-Fermer et al. 1997; Hol et al. 2000; Laumonnier et al. 2002; Solomon et al. 2002, 2004), there was no evidence of MR or learning difficulties in any of our patients. Both the duplication and the PA expansion mutations in SOX3 are associated with anterior pituitary hypoplasia (APH), an absent infundibulum, and an ectopic/undescended posterior pituitary. Our data suggest that SOX3 expression is critical for normal development of the hypothalamopituitary axis in humans, that both over- and underdosage of SOX3 give rise to a similar phenotype of infundibular hypoplasia and hypopituitarism, and that neither over- nor underdosage of SOX3 is obligately associated with MR in X-linked hypopituitarism.
Material and Methods
Patient Recruitment
Patients with congenital hypothalamopituitary disorders were recruited into the study from both national and international pediatric and adult endocrinology centers from 1998 through 2004. A total of 76 male probands were screened for mutations within SOX3, and 19 were tested for a duplication by interphase FISH. Approval was obtained from the Institute of Child Health/Great Ormond Street Hospital for Children Joint Research and Ethics Committee. Prior to collection of samples and genomic analysis, informed written consent was obtained from the parents and, where applicable, the patients.
Clinical Evaluation
Clinical details were obtained for all patients recruited into the study. These included birth details, perinatal complications, history of consanguinity, family history, and parental heights, as well as the results of standard dynamic tests of pituitary function and magnetic resonance imaging (MRI). For those patients recruited from centers where hormonal assays were performed using different commercial radioimmunoassay kits, normal values for each center were taken into account for the diagnosis of hormone deficiencies.
Interphase FISH for Detection and Characterization of SOX3 Duplication
Nineteen individuals (14 sporadic cases and 5 familial cases with presumed X-linked inheritance) with variable degrees of hypopituitarism were screened for duplication of the SOX3 locus by interphase FISH, performed using a human genomic BAC clone (bA51C14) containing the SOX3 gene. A control probe was hybridized on a separate slide for each individual, with the use of either another genomic clone from Xq27 or one mapping to Xq22. In many cases, the mother of the affected male was also screened for the presence of possible duplications involving SOX3. FISH was performed on cultured lymphocyte nuclei that were isolated from peripheral blood samples by use of standard protocols. The BAC, PAC, or cosmid clones were labeled with Spectrum Green dUTP by nick translation, performed using a Nick Translation kit (Vysis), and this was used in conjunction with a Spectrum Orange–labeled X centromere probe (Vysis). Interphase FISH was performed as described elsewhere, and hybridization signals in >100 nuclei were scored for each slide (Woodward et al. 2003).
Clones around the SOX3 gene at Xq27.1 (bA197K18, bA35F15, bA364B14, bA189F12, bA359I11, dJ595A18, dJ177G6, and bA338I3) were selected for mapping duplication breakpoints. The clones, which were obtained from the Wellcome Trust Sanger Institute, mapped between positions 137.4 Mb and 138.7 Mb on the X chromosome (Ensembl Genome Browser [NCBI build 34 human genome assembly]).
Genotyping Studies
Polymorphic microsatellite markers mapping near SOX3 (DXS1232, DXS8013, and DXS984) and elsewhere on the X chromosome (DXS6807, DXS9895, DXS6800, DXS1191, DXS8075, DXS6797, DXS8045, DXS1212, and DXS1200) were amplified using fluorescently labeled primers in a standard PCR mix. Cycling conditions were 95°C for 5 min; 30 cycles of 95°C for 45 s, 55°C for 30 s, and 72°C for 45 s; and holding at 72°C for 10 min. Completed reactions were diluted and electrophoresed using an ABI 377 sequencer along with a GeneScan 500 TAMRA size standard (Applied Biosystems) and were analyzed using Genotyper software (Applied Biosystems).
Universal Primer Quantitative Fluorescent Multiplex PCR (UPQFM-PCR)
UPQFM-PCR was performed using primers across the region (see table 1); the method used for UPQFM-PCR was based on that described by Heath et al. (2000). The primary reaction was a standard PCR mix, in a volume of 10 μl, modified only with respect to the concentration of primer (1 pmol, 2 pmol, or 4 pmol of each primer). Up to six pairs of tagged primers were used in each reaction, including two control primer pairs: a primer pair that amplified exon 6 of PLP1, which lies on the X chromosome, and one from the CFTR locus on chromosome 7. Cycling conditions were 95°C hot start for 15 min; 10 cycles of 94°C for 30 s, 56°C for 45 s, and 72°C for 45 s; and holding at 72°C for 10 min. Secondary PCRs were performed in a total volume of 20 μl, which was seeded with 2 μl of the primary reaction as a template; again, a standard PCR mix was used, with 20 pmol of each universal primer. Cycling conditions were identical to the primary reaction, except that 20 cycles were used. From each completed UPQFM-PCR, 1 μl was electrophoresed on an ABI 377 automatic sequencer (as described above). Dosage of the sequence amplified by each primer pair was calculated from the ratio obtained by dividing the ratio of the fluorescent signal from each primer pair to that of each control primer by the same ratio in a group of normal, sex-matched controls.
Table 1.
Primers Used for UPQFM-PCR[Note]
| Primer Name | Primer Sequence |
| SOX3 F1F | GGGCTCGGTAATGATTGG |
| SOX3 F1R | CGGGGTTCTTGAGTTCAGTC |
| SOX3 F2F | GAGTCCCAGGGCCTTTTC |
| SOX3 F2R | GTCGATGAATGGTCGCTTCT |
| SOX3 F3F | AACGCCTTCATGGTATGGTC |
| SOX3 F3R | CGTAGCCCAGCTGCTCCT |
| SOX3 F4F | AGCGCCTGGACACGTACAC |
| SOX3 F4R | GGTGGCAGGTACATGCTGAT |
| SOX3 F5F | CATCGCATCGCACTCTCA |
| SOX3 F5R | ATTCCCAGCCTACAAAGGTG |
| AR1 5′FAM | GCTGTGAAGGTTGCTGTTCCTCAT |
| AR2 | TCCAGAATCTGTTCCAGAGCGTGC |
| DXS1232F | ACCAACAGCCTAATAATGC |
| DXS1232R-HEX | AGAGATGGGAGCAGCA |
| DXS8013F | CCAACCCAACTGTCTATCAA |
| DXS8013R-FAM | GTTTGGTTTTCCATTCCTGA |
| DXS984F-FAM | TTTCTGTCTGCCAAGTGTTT |
| DXS984R | TACTGNGCCCTACTCCATTC |
| cU35F15F5631 | tccgtcttagctgagtggcgtaTCCATGGGGAAGTTCTTGAG |
| cU35F15R5805 | aggcagaatcgactcaccgctaAGCCAGGAGAGTTTGCGTTA |
| 35F15F66750 | tccgtcttagctgagtggcgtaTAACCACCGTCCACTCACAA |
| 35F15R66949 | aggcagaatcgactcaccgctaGAGCCTTGGTTCTGTGGAAG |
| 364B14F16714 | tccgtcttagctgagtggcgtaAGGATCTGGACCAACACAGG |
| 364B14R16959 | aggcagaatcgactcaccgctaTGAAGGCAGGGAACTCCTTA |
| 364B14F25512 | tccgtcttagctgagtggcgtaATGATCCAAGGGTTCCATGA |
| 364B14R25756 | aggcagaatcgactcaccgctaACTGGCAGGAATTGGCTAAC |
| 364B14F48225 | tccgtcttagctgagtggcgtaGATTCCCACCTACTGCTGGA |
| 364b14R48392 | aggcagaatcgactcaccgctaAATGCTGCAGGAGCCTAAGA |
| 364B14F74126 | tccgtcttagctgagtggcgtaTTTCCAGTGGGCTTGGTTAG |
| 364B14R74369 | aggcagaatcgactcaccgctaTAGAGGGCTCTGCGGAATTA |
| 595A18F26757 | tccgtcttagctgagtggcgtaTTTGTTTGCTGCTTCAGTGG |
| 595A18R27039 | aggcagaatcgactcaccgctaTTGGAAGGCGGATACAATTC |
| 595A18F54671 | tccgtcttagctgagtggcgtaAGGAAGGCCCATCAACTTC |
| 595A18R54825 | aggcagaatcgactcaccgctaGAGGCATCATAGGGGCAGTA |
| 595a18F101906 | tccgtcttagctgagtggcgtaGATGCCCCAGATTGTACCAC |
| 595a18R102140 | aggcagaatcgactcaccgctaTATTTGCCGAATTTCAACCA |
| 595a18F114741 | tccgtcttagctgagtggcgtaACTCTATGCGGTGGATGACC |
| 595a18R114935 | aggcagaatcgactcaccgctaGAAAGGTCTGAGCCAGTTGC |
| 291b3F26828 | tccgtcttagctgagtggcgtaTCCTACAAACGCGATTAGCC |
| 291b3R27044 | aggcagaatcgactcaccgctaACCCTGGTCCCGAGAGTAGT |
| 291B3F64796 | tccgtcttagctgagtggcgtaACTTGCCCCACTCTGTATGG |
| 291B3R65011 | aggcagaatcgactcaccgctaTGGGCTCATATGCATGTTGT |
| PLP6F | tccgtcttagctgagtggcgtaTGGAGCATATTACTGCTGTTGC |
| PLP6R | aggcagaatcgactcaccgctaAAGCTTCCCTCCAGCATTTC |
| CFF | tccgtcttagctgagtggcgtaCGAGGCTACAGCTTTGGAAC |
| CFR | aggcagaatcgactcaccgctaCATCACACTTGTGCCATTCC |
| UNIVF | TCCGTCTTAGCTGAGTGGCGTA |
| UNIVR | AGGCAGAATCGACTCACCGCTA |
| 595A18F116286 | TGCCTTGAATCACAGCAGTC |
| 35F15R71430 | TTGCTCTTCACAAACTCGACCATTT |
| 35F15R68658 | AGTGCAGTGGTGCAATGGT |
| RT-1F | AGCTTCCTGTGTGGCAAAGT |
| RT-1R | GGATTCAAGTCGTCCTCCAA |
| RT-3F | AGCAAAAAGGCCGTTTCTAA |
| RT-3R | CCATAGGGACTGCCCGAGTG |
| HPRT-F | CCACGAAAGTGTTGGATATAAGC |
| HPRT-R | GGCGATGTCAATAGGACTCCAGATG |
Note.— Lowercase letters represent the complementary sequence of the universal primers.
PCR to Amplify Duplication Breakpoint Junction
Long-range PCR was performed using oligonucleotide primers with a length generally between 25 and 30 nucleotides; the primers were designed using Primer 3 to be near the breakpoint regions mapped by FISH and UPQFM-PCR. A 25-μl standard reaction mix with the following modifications was used: 280 mM dNTPs, 5 μl Q solution (Qiagen), 0.5 μl (0.2 U) diluted ProofStart enzyme (Qiagen) (2 U diluted in 9.2 μl 1× ProofStart PCR buffer [Qiagen]), and 2.5 U HotStarTaq (Qiagen). Cycling conditions were as follows: a 95°C hot start for 15 min; 40 cycles of 95°C for 15 s, 65°C for 30 s, and 68°C for 10 min; and elongation at 68°C for 10 min.
RT-PCR
Ten micrograms of human brain RNA (6–12 wk gestation [ViroGen]) were treated with 1 U DNase I (Invitrogen), in accordance with the manufacturers' recommendations. One hundred pmol random nonamers and oligo d(T) were added to 10 μg RNA, and reverse transcription was performed using Superscript II reverse transcriptase (Invitrogen), followed by incubation with 4 U RNase H (Invitrogen) at 37°C for 30 min. PCR was then performed under standard conditions with the use of oligonucleotide primers specific to SOX3 (SOX3-3F and SOX3-3R in Xiang et al. [2000]) and to the Ensembl-annotated genes ENST00000343662 (RT-1F and RT-1R) and 000329997 (RT-3F and RT-3R). cDNA was also amplified using primers from the HPRENST00T gene (HPRT-F and HPRT-R) to check for genomic DNA contamination.
RT-PCR and in situ hybridization analysis of the ENSMUSG00000036258 and Sox3 genes was performed as described elsewhere (Solomon et al. 2004). Primers for the following genes were used: ENSMUSG00000036258 (forward: 5′-catcattcgtcgggaagacctc-3′; reverse: 5′-ccattcggttacgcttgtgc-3′) and GAPDH (forward: 5′-cttgctcagtgtccttgctg-3′; reverse: 5′-acccagaagactgtggatgg-3′).
PCR and Automated Sequencing Analysis of DNA
SOX3 is a single-exon gene with a high GC content. Various primer pairs spanning the exon were designed, and different combinations were used for PCR (see table 1) (Xiang et al. 2000). Because of the high GC content of the gene, modified PCR conditions were used; 1 M betaine was included in the PCR mix. An initial denaturation step of 98°C for 15 min was used, followed by the addition of Taq enzyme. A standard PCR protocol was then used: 94°C for 4 min; 35 cycles of 94°C for 1 min, 68°C for 30 s, and 72°C for 30 s; and a final extension of 72°C for 10 min. PCR products were treated with shrimp alkaline phosphatase and exonuclease I (PCR Product Presequencing kit [Amersham Biosciences]) prior to sequencing with the ABI Prism BigDye Terminator kit, version 3.1 (Applied Biosystems). The sequencing protocol for SOX3 was also modified; 1 M betaine was again included in the reaction mix, and an initial denaturation step of 98°C for 15 min was used prior to the addition of the BigDye mix (i.e., BigDye enzyme mix plus dilution buffer) to the PCR products. Sequencing conditions were as follows: 35 cycles of 95°C for 10 s, 62°C for 20 s, and 68°C for 4 min. Sequencing of the duplication breakpoint was performed using a standard sequencing protocol with BigDye version 3.1. The products were directly sequenced using a MegaBace Sequencer (Amersham Biosciences) and were analyzed using Sequencer software (Gene Codes). The sequences that were obtained were compared with the reference sequence (GenBank accession number X71135).
Plasmid Constructs
Since SOX3 is a single-exon gene, it was possible to amplify the entire gene from both unaffected (wild type) and affected (A(7)240∧241 ins) individuals and to clone the wild-type and mutated cDNAs for functional studies. To generate protein for electrophoretic mobility shift assay (EMSA) experiments, SOX3 cDNAs (SOX3 and SOX3(A(7)240∧241 ins)) were subcloned into the expression vector pCMV/SV-Flag, downstream of the T7 promoter. For the transfection and cell localization experiments, Gal4-SOX3 fusion proteins (Gal4-SOX3 and Gal4-SOX3(A(7)240∧241 ins)) were constructed as described elsewhere for HESX1 (Brickman et al. 2000, 2001). A reporter construct containing SOX binding sites within the Hesx1 promoter sequence (Eroshkin et al. 2002), upstream of the firefly luciferase gene, was used in the transcriptional assays. A 570-bp PCR fragment upstream of the initiation ATG was cloned into the pGL3-basic vector (Promega). Primers were 5′-ggcggtaccctgcgttttcattgacaacg-3′ and 5′-ggccctcgagtcttctgggtcgtacagcg-3′. The reporter construct containing reiterated Gal4 binding sites upstream of the SV40 promoter has been described elsewhere (Brickman et al. 2001).
Cell Culture and Transfections
CHO cells were cultured in Dulbecco modified Eagle medium, supplemented with 10% fetal calf serum (FCS) and 2 mM l-glutamine. Transient transfection assays were performed using lipofectamine (Invitrogen), in accordance with the manufacturer’s protocol (with modifications). Transfections were performed on three separate occasions, each in triplicate.
Cell Localization Studies
Cells (n=2×104) were seeded into each chamber of a four-chamber tissue culture plate. The DNA concentration of the transfected Gal4-SOX3 plasmids varied as indicated in the figure legends, but the total amount of DNA transfected per well was normalized to 200 ng by adding the appropriate amount of empty expression vector. Cells were incubated with transfection media (DNA + OptiMem) for ∼15 h and then were refed with normal growth media. Cells were fixed 36 h after transfection in 4% paraformaldehyde. After fixation of the cells, slides were blocked with 10% FCS and then were incubated with an α-Gal4 antibody (Santa Cruz [1:250]) or anti-chick SOX3 antibody (1:500) (a gift from T. Edlund) for 1 h. The slides were washed and incubated with an α-rabbit secondary antibody (Alexa 488 donkey α-rabbit [Molecular Probes]) (1:1,000) for 1 h. Nuclear counterstaining was performed with Vectashield containing 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) (Vector Laboratories). Immunostaining was then visualized on a Zeiss Axioskop 2 Plus microscope, and images were captured using a Zeiss Axiocam camera.
Dual Luciferase Transfection Experiments
In brief, 1×105 cells were seeded into each well of a 12-well plate 24 h prior to transfection with 75 ng of pRL-SV40 Renilla luciferase vector (Promega), to control for transfection efficiency, and 100 ng of the appropriate firefly luciferase reporter. The DNA concentrations of the other transfected plasmids varied as indicated in the figure legends, but the total amount of transfected DNA was normalized to 1.2 μg by addition of the appropriate amount of empty expression vector. Cells were incubated with transfection media (DNA + OptiMem) for ∼15 h and then were refed with normal growth media. Cells were collected 24 h later and assayed for luciferase activity following the Dual Luciferase protocol (Promega).
Purification of Recombinant Proteins and Gel Shift Assays
SOX3 proteins were expressed using the TNT Quick Coupled Transcription/Translation System (Promega). EMSAs were performed as described elsewhere (Brickman et al. 1999, 2000), with the use of a consensus binding sequence for SOX proteins present in the mouse Hesx1 promoter region (Eroshkin et al. 2002) and equivalent amounts of in vitro translated protein. The SOX3 probe was obtained by annealing the primers 5′-agctcaaacaaataaacaattaactc-3′ and 5′-gtcagagttaattgtttatttgtttg-3′ (italics represent the SOX binding sequence). The annealed probe was radioactively labeled using Ready-To-Go DNA Labeling Beads (−dCTP) (Amersham Biosciences) with 32P dCTP (Amersham Biosciences).
Results
Family A: Overdosage of SOX3
Patient 1
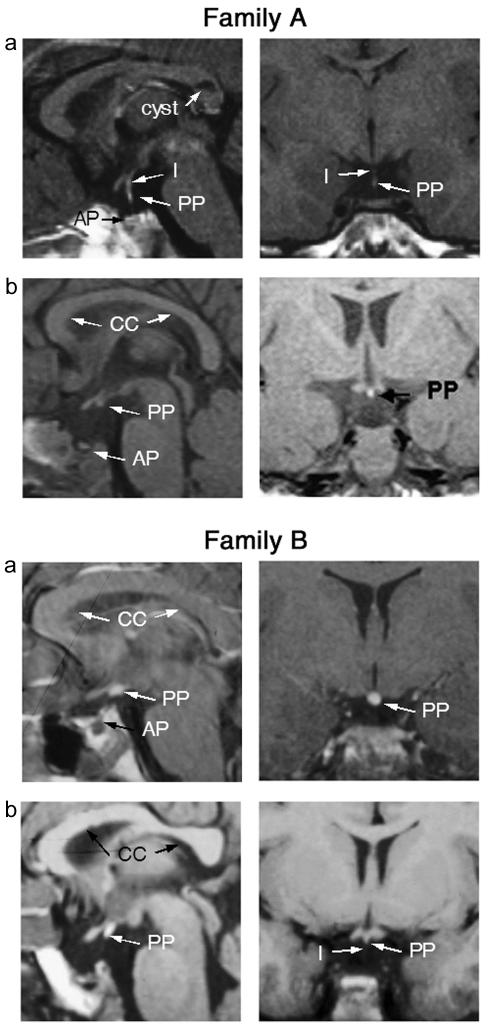
The first patient presented at age 7 years with a height of 107.6 cm (−2.8 SD score [SDS]), weight of 20.5 kg (−0.9 SDS), and history of hypoglycemia and hyponatremia at birth. The midparental height was 179 cm (+0.2 SDS), with a maternal height of 168 cm (+0.7 SDS). A diagnosis of GH insufficiency was made on the basis of combined insulin/arginine provocation testing (table 2), and the patient was treated with recombinant human GH (rhGH). His FT4 concentrations have ranged from 0.5 ng/dl to 1.0 ng/dl, with normal FT3 concentrations, a basal serum TSH concentration of 2.8 μU/ml, and a peak TSH concentration of 13.9 μU/ml in response to TRH. The variable FT4 concentrations to date may indicate the possibility of evolving TSH deficiency. His serum prolactin and cortisol concentrations were normal. He progressed through puberty spontaneously. His current height is 171 cm (−0.7 SDS) at age 17 years. MRI revealed a hypoplastic anterior pituitary, with hypoplasia of the lower half of the infundibulum and a partially descended posterior pituitary at the tip of the infundibulum. A cyst was noted within the splenium of the corpus callosum, and the septum pellucidum was normal (fig. 1 [family A, panel a]). His psychomotor development is normal.
Table 2.
Endocrine Phenotypes of Patients with SOX3 Mutation, Duplication, and Polymorphism[Note]
|
Concentration of |
||||||||||
| Pedigree(Nationality)and Patient | Age at Presentation(years) | Height SDSat Presentation | Weight SDSat Presentation | GH(ng/ml) | FT4(ng/dl) | TSH(μU/ml) | Prolactin(ng/ml) | Peak Cortisol(μg/dl) | Peak LH(IU/liter) | Peak FSH(IU/liter) |
| A (Finnish): | ||||||||||
| 1 | 7.0 | −2.8 | −.9 | 1.0 | 1.0 | 2.8 | 6.2 | 33.7 | 5.0 | 4.5 |
| 2 | .2 | −3.8 | −1.5 | 1.3 | .6 | .01 | 5.5 | 2.5 | .8 | .5 |
| B (Qatari): | ||||||||||
| 1 | 3.0 | −2.5 | NA | 1.7 | .5 | .08 | NA | 2.0 | U | U |
| 2 | 4.5 | −2.5 | NA | .7 | .7 | 3.3 | 7.3 | 3.9 | .5 | .5 |
| 3 | 2.7 | −1.3 | +.7 | .7 | .5 | 2.8 | NA | 3.2 | 1 | <1 |
| C (Ghanaian): | ||||||||||
| 1 | 0 | NA | −.9 | 2.2 | .7 | 4.7 | 34.8 | 1.8 | 1.1 | .6 |
Note.— NA = not available; U = undetectable. Normal ranges: peak GH >6.7 ng/ml, FT4 0.9–1.9 ng/dl, TSH 0.5–6.0 μU/ml, peak cortisol >19.8 μg/dl, and prolactin 5–25 ng/ml.
Figure 1.
Family A, MRI scans in patients with SOX3 duplication. Coronal and sagittal MRI scans of patient 1 (panel a) and patient 2 (panel b) from family A, showing APH (“AP”), partial hypoplasia of the infundibulum (“I”) in patient 1 (absent in patient 2), and an undescended/ectopic posterior pituitary (“PP”) (partial in patient 1). Note the cyst in the corpus callosum identified in patient 1. Family B, MRI scans in patients with PA expansion in SOX3. Coronal and sagittal MRI scans of patient 2 (panel a) and patient 3 (panel b) from family B, showing APH (“AP”), hypoplasia of the infundibulum (“I”), and an undescended/ectopic posterior pituitary (“PP”).
Patient 2
The half brother of patient 1 presented at age 2 mo (height 54.2 cm [−3.8 SDS]; weight 5.1 kg [−1.5 SDS]) with a history of neonatal hypoglycemia and was found to have severe cortisol, TSH, GH, and gonadotrophin deficiency (table 2). He had hypoplastic genitalia, with both testes palpable high in the inguinal canal and a micropenis. He was treated with thyroxine, rhGH, and hydrocortisone. He is currently 2.5 years old, with a height of 94 cm (+0.6 SDS) and weight of 14.5 kg (+0.4 SDS). MRI revealed hypoplasia of the anterior pituitary, absence of the infundibulum, and an ectopic/undescended posterior pituitary. The corpus callosum was normal (fig. 1 [family A, panel b]). He has normal psychomotor development but has been noted to be hyperactive.
Family B: Underdosage of SOX3
Patient 1
Patient 1 is currently 17 years old. He was born to first-degree consanguineous Qatari parents, and he first presented with short stature (height 83 cm [−2.5 SDS]) at age 3 years. His heterozygous mother is of normal height. General examination revealed two small testes lying in the scrotum. He had elevated fasting triglycerides, and clonidine and L-dopa stimulation revealed low peak GH concentrations (peak GH 1.7 ng/ml and 1.9 ng/ml, respectively) (table 2). The patient showed an excellent response to treatment with rhGH, with a height velocity increasing from 4 cm/year pre-GH to 11 cm/year with rhGH treatment. A CT scan was reported as being normal. A trial period of suspended rhGH treatment was associated with a marked reduction in the patient's height velocity to 1 cm/year. rhGH treatment was therefore recommenced. Although the initial total thyroxine concentration was normal, repeat investigations at age 6.2 years confirmed secondary TSH deficiency (free T4 0.5 ng/dl; TSH 0.08 μU/ml). At age 15 years, he was noted to be prepubertal, with small testes and undetectable concentrations of LH and FSH. Treatment with a depot preparation of testosterone (Sustanon) was therefore commenced. The patient also complained of weakness and was found to have a stimulated serum cortisol concentration of 2.0 μg/dl, confirming ACTH deficiency. Hydrocortisone treatment was therefore commenced. He is currently being treated with Sustanon, rhGH, thyroxine, and hydrocortisone. His current height is 162.5 cm (−1.9 SDS), and his weight is 66.5 kg (+0.2 SDS). His neurodevelopment is normal.
Patient 2
Patient 2 is currently 15 years old. He presented at age 4.5 years with a height SDS of −2.5 and a poor growth velocity of 4 cm/year. He had a micropenis, with bilaterally undescended testes. Investigations (including a clonidine provocation test) at age 6 years revealed GH deficiency (peak GH 0.7 ng/ml; IGF-1 undetectable), cortisol deficiency (peak cortisol on provocation 3.9 μg/dl), hypothyroidism (FT4 0.7 ng/dl; basal TSH 3.3 μU/ml; peak TSH in response to TRH provocation 24.8 μU/ml [120 min]), and a normal serum prolactin concentration (7.3 ng/ml) (table 2). A 3-mo course of Sustanon led to an increase in the size of the phallus. MRI identified a severely attenuated anterior pituitary gland in the sella turcica, a hypoplastic infundibulum, and an undescended/ectopic posterior pituitary at the level of the third ventricle. The rest of the intracranial structures, including the optic pathways, were normal (fig. 1 [family B, panel a]). Treatment with rhGH, hydrocortisone, and thyroxine was commenced. Puberty was induced with Sustanon at age 13.5 years, when the basal and peak serum gonadotrophins following LH-releasing hormone stimulation were <0.5 IU/liter. He has normal neurodevelopment to date, with good school progress. His current height is 159 cm (−1.2 SDS), and his weight is 61.4 kg (+0.6 SDS).
Patient 3
Patient 3 is currently 13 years old. He was first seen at age 2.7 years, with short stature (height 88.5 cm [−1.3 SDS]; weight 15.2 kg [+0.7 SDS]), micropenis, and undescended testes. Investigations revealed a free T4 concentration of 0.5 ng/dl (normal 0.6–1.9), TSH concentration of 2.8 μU/ml, LH concentration of 1.0 IU/liter, FSH concentration of <1.0 IU/liter, and a low IGF-1 concentration (table 2). Treatment with thyroxine was commenced. Subsequent clonidine provocation testing revealed a peak GH concentration of 0.7 ng/dl, with a stimulated cortisol concentration of 3.2 μg/dl. rhGH and hydrocortisone replacement was commenced. Although the testes descended into the scrotum in response to 3 wk of human chorionic gonadotrophin, the testosterone response was poor (from <0.1 ng/ml to 0.6 ng/ml). His height SDS and weight SDS are currently −0.1 and +1.5, respectively. Pubertal induction was required, and the patient is currently also receiving Sustanon. MRI revealed a small anterior pituitary gland in a small pituitary fossa. The infundibulum was difficult to visualize, since it was severely attenuated, with an ectopic/undescended posterior pituitary (fig. 1 [family B, panel b]).
Another male sibling presented at age 9 years with deficiencies of GH (peak GH 2.8 ng/ml on clonidine provocation) and, possibly, gonadotrophin (basal and peak serum LH and FSH <0.5 IU/liter). He has normal serum thyroxine (free T4 1.0 ng/dl), prolactin (8.7 ng/ml), and basal cortisol (12.1 μg/dl) concentrations. Currently, he is 12.5 years old, with a height of 138 cm (−1.8 SDS) and weight of 59.8 kg (+1.9 SDS). MRI revealed APH, an absent infundibulum, and an undescended/ectopic posterior pituitary gland. DNA from this child is unavailable.
Overdosage of SOX3
Identification of a novel SOX3 duplication
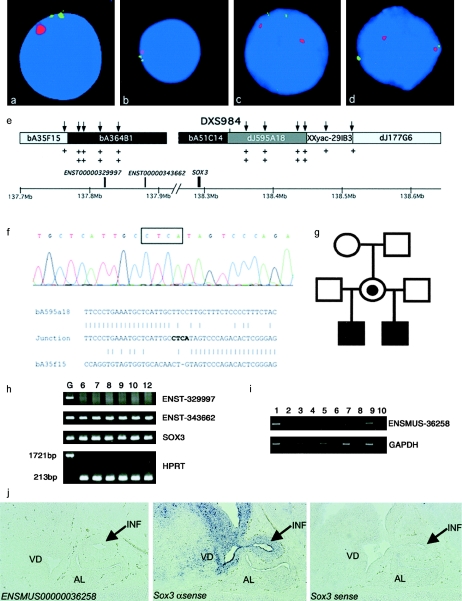
A duplication including SOX3 was found in one family from Finland, with two half brothers affected with differing degrees of hypopituitarism (fig. 2a, 2b, 2e, and 2g). Their mother is a carrier of the duplication (fig. 2c) and is phenotypically unaffected, with a height of 168 cm; the maternal grandmother does not carry the duplication (fig. 2d).
Figure 2.
Duplication of SOX3 in family A. a–d, Representative interphase nuclei from four members of the family (older sibling [a], younger half brother [b], mother [c], and maternal grandmother [d]), with bA51C14 shown in green and the X centromere shown in red. e, Data used to map the extent of the duplication. Clones from the SOX3 genomic region are shown along with position (in Mb) on the X chromosome (NCBI build 34 assembly of the human genome). The clones duplicated by interphase FISH are blackened, those not duplicated are shaded light gray, and the clone containing the breakpoint is shaded dark gray; unknown duplication status is indicated by the unblackened area. The positions of genes within the duplicated region are designated by blackened boxes above the scale bar. The positions of UPQFM-PCR primer pairs are designated by vertical arrows above the contig. Two plus signs underneath a primer pair indicate that dosage quotients for this primer pair were consistent with a duplication; one plus sign shows that this region had a normal copy number. The position of the informative polymorphic marker, DXS984, is also shown. f, Electropherogram showing sequence from the duplication breakpoint in family A. The four nucleotides not present in either the proximal or the distal normal sequence are boxed above the electropherogram. Underneath, a ClustalW alignment of the normal distal (bA595A18), normal proximal (bA35F15), and junction sequence is shown, demonstrating that there is no homology at the proximal and distal ends of the duplication. g, Pedigree of family A. h, RT-PCR for the three genes within the duplication, performed using human brain mRNA at 6, 7, 8, 9, 10, and 12 wk of gestation. Genomic DNA was included as a positive control. Transcripts were present for SOX3 and a novel gene (Ensembl ENST00000343662). The larger 1,721-bp product was not amplified from the brain RNA, showing that there was no genomic DNA contamination. i, RT-PCR analysis for ENSMUSG00000036258 (murine homologue of ENSG00000343662), showing expression in the mouse embryo at 14.5 days postcoitum (dpc). Expression was not detected in the hypothalamic/pituitary region, the pituitary progenitor cell line aT1-1, or the adult pituitary. No amplification was evident in the −RT controls, indicating that the cDNA was not contaminated with genomic DNA. Lane 1, 14.5–dpc embryo +RT; lane 2, 14.5-dpc embryo −RT; lane 3, 14.5-dpc hypothalamus/pituitary +RT; lane 4, 14.5-dpc hypothalamus/pituitary −RT; lane 5, aT1-1 +RT; lane 6, aT1-1 −RT; lane 7, adult pituitary +RT; lane 8, adult pituitary −RT; lane 9, genomic DNA as template; lane 10, water as a blank. j, In situ analysis of the ENSMUSG00000036258 and Sox3 genes in the developing murine hypothalamus, infundibulum, and pituitary. No expression of ENSMUSG00000036258 was detected (left panel). Sox3 is expressed in the infundibulum (arrow) and ventral diencephalon but not in the presumptive anterior pituitary (middle panel). No staining was observed using the Sox3 sense control probe (right panel). INF = infundibulum; VD = ventral diencephalon; AL = anterior lobe.
Characterization of duplication
The size of the duplication was mapped using genomic clones mapping close to SOX3 (fig. 2e). This mapped the extent of the duplication proximal to SOX3 to between two adjacent genomic clones, ∼500 kb distant from SOX3, and the distal end of the duplication mapped within a clone adjacent to bA51C14. Refinement of the location of the two duplication breakpoints was performed by UPQFM-PCR, with the use of unique tagged primer pairs close to the breakpoint regions, as mapped by interphase FISH (fig. 2e). We have deduced the size of the duplication from the position of the breakpoints, under the assumption that there are no further deletions/duplications associated with the rearrangement. Long-range PCR amplification across the breakpoint junction was performed using oligonucleotide primers designed within the breakpoint regions, because dual-color interphase FISH, performed using two probes within the duplication, indicated a tandem head-to-tail rearrangement (data not shown). One primer pair combination (35F15R71430 and 595a18F114741), with each oligonucleotide mapping to one end of the duplicated region, amplified a 4–5 kb product from genomic DNA from the members of the family known to carry the duplication, confirming that the duplication rearrangement was in tandem. No amplification was observed with this combination of primers in DNA from normal individuals or the grandmother, who did not carry the duplication.
The use of primers 595A18F116286 and 35F15R68658 to sequence the breakpoint in both directions from genomic DNA from one of the affected males revealed no homology between the two sequences at either end of the duplication. Four nucleotides (CTCA) were inserted at the breakpoint, consistent with a duplication mechanism involving nonhomologous end joining (Lieber et al. 2003) (fig. 2f). The breakpoint at the proximal end of the duplication was located within an AluJb repeat from genomic clone bA35F15, and the distal breakpoint was within a unique sequence in genomic clone bA595A18.
The tandem duplication in this family is the smallest described to date (685.6 kb in length). Microsatellite markers on the X chromosome were genotyped to determine the origin of the duplication in the family. Three microsatellites mapping within the duplicated region were typed, but only one, DXS984 (fig. 2e), was informative. Both affected males shared the same allele at this locus, which was inherited from the maternal grandfather (data not shown). No skewing of X inactivation was observed (using a method that is based on differential methylation of HpaII restriction sites near a polymorphic CAG repeat in the androgen receptor gene) in DNA extracted from peripheral blood obtained from the mother, who carried the duplication (data not shown) (Allen et al. 1992).
Expression studies suggest that SOX3 duplication is associated with an infundibular phenotype
The expression of the three genes mapping within the duplicated interval (SOX3 and two Ensembl-predicted genes, ENST00000343662 and ENSG00000329997) was investigated by RT-PCR. SOX3 and ENST00000343662 (a novel coding sequence predicted to contain a proline-rich domain) could be amplified from total human fetal brain RNA (6–12 wk gestation [fig. 2h]). However, with the use of E14.5 murine infundibulum, Sox3—but no ENSG00000343662-related product (ENSMUSG00000036258)—could be amplified (fig. 2i). The third predicted human transcript, ENST00000329997, was not amplified from human fetal brain (fig. 2h). This transcript contains a putative RNA-binding domain with homology to the heterogeneous nuclear ribonucleoprotein family, but it has no murine orthologue, and its biological role remains unclear. Given the above data, and given that murine Sox3 is expressed within the infundibulum whereas ENSMUSG00000036258 is not (fig. 2j), we suggest that the phenotype in the patients with the 685.6-kb duplication is most likely due to overdosage of SOX3.
Underdosage of SOX3
Identification of a novel PA expansion in SOX3
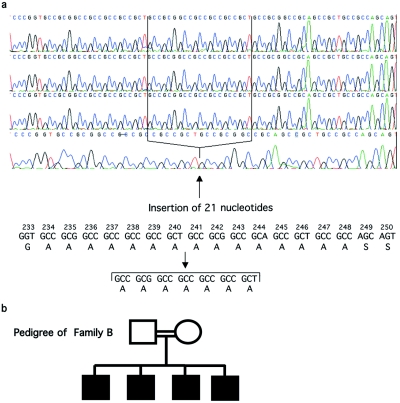
We next investigated the role of possible loss of SOX3 function by screening for mutations in SOX3 in a cohort of 76 patients with variable hypopituitarism. Thirteen cases were assigned as familial (more than one case in each family), whereas 63 cases were assigned as sporadic. The coding region, as well as 145 bp upstream and 121 bp downstream, was sequenced. An expansion by seven alanines of the first PA tract was identified in a consanguineous family (family B) with four affected males (A(7)240∧241 ins), with the insertion occurring between nucleotides 720 and 721 (the A of the ATG is designated “1”) (fig. 3a and 3b). The mother of these four children was heterozygous for this mutation and did not exhibit significantly skewed X inactivation in DNA extracted from peripheral blood, as observed in family A (data not shown).
Figure 3.
a, Protein and genomic sequence showing a PA expansion within SOX3. PA expansion (from 15 to 22 alanine residues) in three brothers (top three electropherograms) born to a consanguineous union (family B), compared with the normal wild-type sequence (bottom electropherogram). b, Family tree of a pedigree with a mutation (A(7)240∧241 ins) within SOX3.
SOX3 Polymorphism
We identified a novel missense change (c.127 G→A [p.A43T]) in a child originally from Ghana, with sporadic combined pituitary hormone deficiency (CPHD) (tables 2 and 3). Although this change was not identified in 92 white controls (46 male and 46 female) or in 72 other patients with hypopituitarism, it was identified in a heterozygous state in 3 of 19 normal controls from an Afro-Caribbean background. We therefore conclude that this change is in fact a common Afro-Caribbean polymorphism. A polymorphism was also identified within the first PA tract, as has been described elsewhere (732 A→C) (Raverot et al. 2004). We identified no further changes and found no other expansions in the first PA tract in an additional 72 male patients with hypopituitarism. Previous data have shown no mutations within SOX3 in 56 patients with idiopathic oligo-/azoospermia and infertility (Raverot et al. 2004); 15 patients with X-linked MR and two Australian families with hypopituitarism (Laumonnier et al. 2002); 8 patients with 46,XX sex reversal and 25 patients with 46,XY gonadal dysgenesis (Lim et al. 2000); and 10 patients with X-linked Rett syndrome (Xiang et al. 2000). Additionally, Laumonnier et al. (2002) sequenced the PA tract in question in 600 normal chromosomes and found no changes.
Table 3.
Summary of Neuroradiological Findings in Patients with SOX3 Mutations, Duplications, and Polymorphisms[Note]
|
Neuroradiological Finding in |
|||||
| Pedigree(Nationality)and Patient | Anterior Pituitary | Posterior Pituitary | Infundibulum | Corpus Callosum | Septum Pellucidum |
| A (Finnish): | |||||
| 1 | Hypoplastic | Partially descended | Partial hypoplasia | Cyst | Normal |
| 2 | Hypoplastic | Undescended | Absent | Normal | Normal |
| B (Qatari): | |||||
| 1 | NA | NA | NA | NA | NA |
| 2 | Hypoplastic | Undescended | Hypoplastic | Normal | Normal |
| 3 | Hypoplastic | Undescended | Hypoplastic | Normal | Normal |
| C (Ghanaian): | |||||
| 1 | Hypoplastic | Undescended | Absent | Hypoplastic | Normal |
Note.— NA = not available.
The Novel PA Expansion within SOX3(A(7)240∧241 ins) Is Associated with Loss of Function
Reporter assays
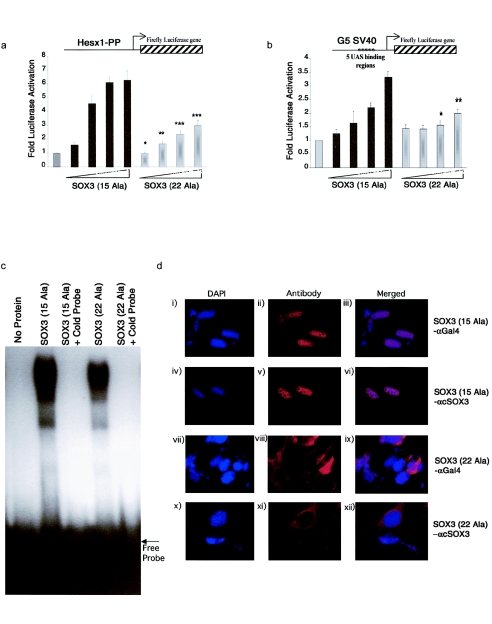
To characterize the properties of wild-type and mutant SOX3 proteins, we used the firefly luciferase gene downstream of the proximal promoter of Hesx1 to make a reporter construct, since this gene contains purported binding sites for SOX proteins. In figure 4a, increasing amounts of wild-type SOX3 activated the Hesx1 reporter up to 6-fold in a dose-dependent manner. With the mutated SOX3 construct, the maximal activation achieved was 3-fold, suggesting that expansion of the PA tract reduced the efficacy of SOX3 in this assay.
Figure 4.
a, Impaired activation of Gal4-SOX3(A(7)240∧241 ins) (labeled “SOX3 (22 ala)”) at SOX binding sites. Increasing concentrations of Gal4-SOX3 (labeled “SOX3 (15 ala)”) and Gal4-SOX3(A(7)240∧241 ins) (10 ng, 100 ng, 500 ng, and 1,000 ng) were cotransfected with the HESX1 promoter (Hesx1-PP) luciferase reporter. Gal4-SOX3 led to a dose-dependent activation of the HESX1 promoter (a 6-fold activation at the highest concentration tested), whereas Gal4-SOX3(A(7)240∧241 ins) was associated with impaired activation (a 3-fold activation at the highest concentration tested). b, Impaired activation of Gal4-SOX3(A(7)240∧241 ins) (labeled “SOX3 (22 ala)”) at the SV40 promoter. Increasing concentrations of Gal4-SOX3 (labeled “SOX3 (15 ala)”) and Gal4-SOX3(A(7)240∧241 ins) (10 ng, 100 ng, 500 ng, and 1,000 ng) were cotransfected with the SV40 promoter reporter construct. Gal4-SOX3 led to a dose-dependent activation of the SV40 promoter (a 3.3-fold activation at the highest concentration tested), whereas Gal4-SOX3(A(7)240∧241 ins) was associated with impaired activation (a 2-fold activation at the highest concentration tested). a and b, One asterisk (*) indicates P<.05, two asterisks (**) indicate P<.001, and three asterisks (***) indicate P<.0001. The results represent the means of three independent experiments, each performed in triplicate. c, Gal4-SOX3(A(7)240∧241 ins) (labeled “SOX3 (22 ala)”) binds to SOX binding sites in a manner that is similar to that of wild-type SOX3 (labeled “SOX3 (15 ala)”). Identical amounts of in vitro translated wild-type SOX3 or SOX3(A(7)240∧241 ins) (10 μl) were added to the consensus SOX DNA-binding site (lanes 2 and 4). Duplicate experiments—but with the addition of 15 pmol of cold probe, showing specific loss of DNA binding—are shown in lanes 3 and 5. Lane 1 shows the effect of free probe in the absence of protein. Western blot analysis showed equivalent expression levels of the constructs (data not shown). d, Impaired nuclear localization in Gal4-SOX3(A(7)240∧241) (labeled “SOX3 (22 ala)”). CHO cells were transfected with 50 ng of the respective plasmid construct, as indicated. Nuclei in the cells were counterstained with DAPI. Panels i–vi, wild-type SOX3 (labeled “SOX3 (15 ala)”), using either α-GAL4 (panels i–iii) or α-cSOX3 (panels iv–vi) antibodies; panels vii–xii, Gal4-SOX3(A(7)240∧241) (labeled “SOX3 (22 ala)”), using either α-GAL4 (panels vii–ix) or α-cSOX3 (panels x–xii) antibodies.
This result could be due to the inability of the protein to bind to DNA, or it could be due to an alteration in some other function of the protein (e.g., nuclear localization). To test this, Gal4-SOX3 constructs were cotransfected with a reporter construct containing reiterated Gal4 binding sites (upstream activation sequence [UAS]) upstream of an SV40 promoter driving a luciferase reporter. As shown in figure 4b, the loss of activation of the firefly luciferase gene observed with the mutant SOX3 construct did not appear to reflect direct DNA binding—a finding that supports data from EMSAs that use a consensus binding site for SOX3 (fig. 4c). On the other hand, the p.A43T missense sequence variant identified in family C activated transcription and bound to DNA in a manner that was similar to wild-type SOX3 (data not shown), supporting the hypothesis that this change reflects a polymorphic variant.
Cell localization
Given the hypothesis that nucleocytoplasmic shuttling plays a key role in the function of SOX proteins (Smith and Koopman 2004), we studied the cellular localization of wild-type and mutant SOX3 proteins. First, fusion proteins, with enhanced green fluorescent protein (EGFP) added N- or C-terminal to wild-type or mutant SOX3, were expressed in Chinese hamster ovary (CHO) cells. These showed fluorescence evenly distributed throughout the cell, regardless of the position of the EGFP, with both constructs over a wide range of plasmid concentrations (data not shown).
However, since addition of EGFP could itself cause mislocalization of SOX3 proteins, the experiments were repeated using the wild-type and mutant SOX3/GAL4 fusion constructs, and the transfected, fixed cells were immunostained with antibodies to SOX3 or to the Gal4 DNA-binding domain (fig. 4d). These results showed a distinct difference in cellular localization between the wild-type and mutant SOX3 proteins. Wild-type SOX3/Gal4 was predominantly localized to the nucleus of the cell, as would be expected of a transcription factor, although some protein is also present in the cytoplasm. The mutant SOX3/Gal4 protein was largely excluded from the nucleus, although some residual staining was found within the nucleus.
Discussion
Congenital pituitary hormone deficiencies occur in ∼1 in 4,000 births. Of these, isolated GH deficiency is the commonest phenotype, followed by CPHD and septo-optic dysplasia. To date, a number of genetic abnormalities have been described in association with these disorders (Dattani and Robinson 2000; Cohen and Radovick 2002). X-linked hypopituitarism is a rare and variable condition. Recent studies have implicated duplications at Xq26-27 in the etiology of this form of hypopituitarism. Solomon et al. (2004) recently narrowed the critical region to a 3.9-Mb interval at Xq27 that contains 18 annotated transcripts. We have now refined the critical interval to a 685.6-kb region in a pedigree in which two half brothers manifest evidence of hypopituitarism. Within this region are three transcripts: SOX3 and two other transcripts. ENST0000032997 is not expressed in human fetal brain and does not have a murine homologue. ENSG00000343662 is expressed in the human fetal brain, but the murine orthologue (ENSMUSG00000036258) is not expressed in the infundibulum. On the other hand, murine Sox3 is expressed in the infundibulum (Solomon et al. 2004). Hence, our data support the notion that SOX3 duplication is implicated in the etiology of X-linked hypopituitarism. The first child (patient 1, family A) manifested GH deficiency, with borderline low FT4 concentrations, whereas the second sibling (patient 2, family A) manifested a more severe CPHD phenotype. In keeping with the more severe phenotype in patient 2, the infundibulum was absent in this sibling, whereas that of his half brother, patient 1, was present but was hypoplastic in the distal portion. However, the corpus callosum in patient 1 was abnormal, whereas that in the second sibling was normal, suggesting that SOX3 is variably implicated in the development of the midline forebrain structures. This variability in phenotype between the two half brothers may reflect differences in genetic background from their different fathers. Their mother, who is a carrier of the duplication, did not manifest an abnormal phenotype. The allele on which the duplication is present was inherited from the maternal grandfather, suggesting either that the duplication was inherited from him, in which case he would have been expected to have an abnormal phenotype, or that it arose de novo in the mother. There was no evidence of skewed X inactivation in DNA extracted from peripheral blood in the mother, but, given that SOX3 is not expressed in lymphocytes, these data are not surprising and do not exclude the possibility of locally skewed X inactivation (e.g., within the brain and infundibulum).
We have also identified, in three brothers from a consanguineous pedigree, a novel PA expansion within SOX3 that increases the size of a PA tract from 15 to 22 alanine residues. The phenotype was similar in all three siblings with profound and complete panhypopituitarism in association with APH, an absent or hypoplastic infundibulum, and an ectopic/undescended posterior pituitary. A fourth sibling had GH and possible gonadotrophin deficiencies, but with similar neuroradiological findings. Although DNA was unavailable for genetic testing, it is highly likely that this fourth child also harbored the PA expansion in SOX3. The similarity in phenotype may reflect the relative genetic homogeneity of the consanguineous union of their parents.
We have shown that SOX3 can act as an activator of transcription and that the PA expansion was functionally associated with impairment—but not complete loss—of transcriptional activation in this assay. The PA expansion did not appear to affect DNA binding but did lead to impairment of nuclear localization. Thus, the residual transcriptional activity and, hence, partial loss of function might potentially be explained by the presence of some residual SOX3 protein within the nucleus. Our data suggesting that the PA expansion is associated with loss, rather than gain, of function are consistent with those of Albrecht et al. (2004), who have recently shown that PA expansions within Hoxd13 that are associated with synpolydactyly result in a shift in the localization of Hoxd13 from the nucleus to the cytoplasm, where it forms large amorphous aggregates. They suggested that this cytoplasmic aggregation is influenced by the length of the repeat, the level of expression, and the efficacy of degradation by the proteosome. Similar findings have recently been obtained for the previously published PA expansion within SOX3, which resulted in the expansion of the same alanine tract from 15 to 26 alanine residues (Albrecht et al. 2004). The longer PA expansion in this pedigree (Laumonnier et al. 2002) appears to be associated with a more severe phenotype, in that the affected members of the family manifested MR and mild craniofacial abnormalities, in addition to GH deficiency. In contrast, in the present study, the pituitary phenotype is more severe in patients with the shorter PA tract, and none of our patients manifested evidence of MR or learning difficulties. Hence, the relationship between the length of the PA tract and the phenotype remains far from clear, and the brain and infundibulum could be differentially affected, reflecting differences in the relative contributions of the three SOXB1 genes in the two tissues.
The most significant conclusion we draw from these studies is that the gene dosage of SOX3 appears to be critical for the normal development of the diencephalon and the infundibulum in humans, since both overdosage and underdosage of SOX3 are associated with somewhat similar phenotypes. The phenotype is predominantly that of infundibular hypoplasia with variable effects on the corpus callosum. As SOX3 is not expressed in Rathke’s pouch or in the developing anterior pituitary (Collignon et al. 1996; Solomon et al. 2004), the resulting APH is probably secondary, confirming the importance of inductive mechanisms from the hypothalamus and infundibulum on normal anterior pituitary development. An ectopic/undescended posterior pituitary may well directly reflect the effects of infundibular hypoplasia. A similar phenotype has previously been observed in connection with mutations within HESX1 and LHX4, both of which are associated with APH and an ectopic/undescended posterior pituitary (Machinis et al. 2001; Thomas et al. 2001).
These data are consistent with observations in mice, in which Sox3 is expressed throughout the CNS but particularly in the ventral diencephalon and infundibulum (Rizzoti et al. 2004; Solomon et al. 2004). Mice with Sox3 deletions are affected with variable reductions in growth rate and in pituitary concentrations of GH, TSH, LH, and FSH, in mice that survived to adulthood. Also, as observed in some of the human patients with the longer PA expansion, 50% of the mutant mice displayed craniofacial defects (Rizzoti et al. 2004). Sox3 was shown to be required at different levels for the development and function of the hypothalamopituitary axis; indeed, Sox3 is first necessary in the infundibulum, for correct morphogenesis of Rathke’s pouch, and it is also necessary, later, in the hypothalamus, for normal hypothalamopituitary function (Rizzoti et al. 2004). More precisely, in heterozygous female embryos in which one copy of Sox3 had been deleted and replaced with green fluorescent protein, which was expressed under the control of the Sox3 promoter, Sox3 null cells, in which the wild-type copy has been X inactivated, were shown to be clustered in the infundibulum/hypothalamic region. Within this cluster, cell proliferation was reduced, showing a specific requirement for Sox3 in this region. Moreover, the infundibulum was less evaginated, and expression of secreted factors within this territory was expanded, probably explaining the bifurcations that were observed in the pouch and that persist in the adult gland as extra clefts. However, since heterozygous female mice displayed dysmorphic glands but were not affected with hypopituitarism, it is very likely that the hypothalamus, where Sox3 expression is maintained, is also affected in the Sox3 null animals. The null mutants also showed dysgenesis of the corpus callosum and failure of the dorsal hippocampal commissure to cross the midline. A similarly variable phenotype was reported in connection with an independent Sox3-targeted mutation in mice (Weiss et al. 2003).
The similarity between the null mutant mice and the pedigrees with the PA expansion and duplication described in the present study encourage us in the notion that the PA expansion reflects a loss of function. Given the similarity in phenotype, we cannot rule out the possibility that the duplication could also represent a loss of functional SOX3. It will be interesting to know whether overexpression of Sox3 in mice generates a phenotype that is similar to that of Sox3 null mice.
None of the patients in the present study manifested craniofacial defects or any evidence of MR, in contrast to findings in previous reports (Laumonnier et al. 2002). Given that the PA expansion observed in our patients may represent only a partial loss of function and that the duplication reported in the present study is the smallest reported to date, there may indeed be some correlation between the genotype and phenotype, but further studies are required to explore this possibility. Similar dosage dependence has also been suggested in patients with mutations and duplications encompassing PAX6 (MIM 607108), SOX9 (MIM 608160), WNT4 (MIM 603490), and DAX1 (MIM 300473) (Huang et al. 1999; Jordan et al. 2001; Hanson 2003; Meeks et al. 2003; Vincent et al. 2003). Indeed, normal development of the pituitary gland itself appears to be critically dependent on the timing of expression and dosage of Hesx1 and Prop1 in the mouse (Dasen et al. 2001). The underlying mechanism whereby both under- and overdosage of SOX3 can lead to an abnormal phenotype remains unknown. Although our data are consistent with those of previous studies that have suggested that Sox3 is a transcriptional activator (Uchikawa et al. 1999), recent data have suggested that, in Xenopus laevis, Sox3 can repress expression of Xnr5, a nodal-related gene that is expressed early in embryonic development (Zhang et al. 2003). This phenomenon—whereby a transcription factor can act as both an activator and a repressor, in a context-dependent manner—has been described elsewhere (Scully and Rosenfeld 2002). In this context, target genes could be critically sensitive to dosage of SOX3. It is interesting that our preliminary data suggest that the prevalence of SOX3 duplications (1/18) and mutations (1/74) in unselected patients with hypopituitarism is comparable to the prevalence of mutations in other transcription factors, such as HESX1, but is lower than the prevalence of mutations in the pituitary-specific genes POU1F1 and PROP1 (J.T. and M.T.D., unpublished data), although the prevalence increases if the cohort is carefully selected for familial CPHD.
In conclusion, we have shown that the dosage of SOX3 is critical for the normal development of the infundibulum and, hence, the anterior pituitary in humans. We report the smallest duplication interval containing SOX3, which suggests that this candidate is the gene most likely to be responsible for the hypopituitarism observed in individuals with duplications of Xq26-27. We also describe patients with a seven-alanine expansion in a PA tract within SOX3 that is associated with impaired transcriptional activity possibly consequent to incorrect cellular localization of the protein. Two of the patients in the present study had abnormalities of the corpus callosum in addition to hypopituitarism, but, in contrast to previous studies, none of our patients showed evidence of learning difficulties or MR. This marked phenotypic variation should be borne in mind when considering potential X-linked GH deficiency and/or MR.
Acknowledgments
We thank all the pediatricians and patients who participated in the study. This study was supported by grants from the Medical Research Council U.K. (to M.T.D., K.S.W., J.T., A.M., and M.C.), the Wellcome Trust (to K.J.W.), Novo Nordisk (to A.M.), and the Child Growth Foundation (to A.M.). Research at the Institute of Child Health and the Great Ormond Street Hospital for Children NHS Trust benefits from research-and-development funding received from the NHS Executive.
Electronic-Database Information
The accession number and URLs for data presented herein are as follows:
- Ensembl Genome Browser, http://www.ensembl.org (for ENST00000343662, ENSG00000329997, and ENSMUSG 00000036258)
- GenBank, http://www.ncbi.nlm.nih.gov/Genbank/ (for reference sequence [accession number X71135])
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for POU1F1, PROP1, HESX1, LHX3, LHX4, mental retardation, X-linked, with isolated growth hormone deficiency, SOX3, SOX2, FOXL2, HOXA13, HOXD13, PABPN1, RUNX2, ZIC2, ARX, PHOX2, PAX6, SOX9, WNT4, and DAX1)
References
- Albrecht AN, Kornak U, Boddrich A, Suring K, Robinson PN, Stiege AC, Lurz R, Stricker S, Wanker EE, Mundlos S (2004) A molecular pathogenesis for transcription factor associated poly-alanine tract expansions. Hum Mol Genet 13:2351–2359 [DOI] [PubMed] [Google Scholar]
- Allen RC, Zoghbi HY, Moseley AB, Rosenblatt HM, Belmont JW (1992) Methylation of HpaII and HhaI sites near the polymorphic CAG repeat in the human androgen-receptor gene correlates with X chromosome inactivation. Am J Hum Genet 51:1229–1239 [PMC free article] [PubMed] [Google Scholar]
- Bienvenu T, Poirier K, Friocourt G, Bahi N, Beaumont D, Fauchereau F, Ben Jeema L, Zemni R, Vinet MC, Francis F, Couvert P, Gomot M, Moraine C, Van Bokhoven H, Kalscheuer V, Frints S, Gecz J, Ohzaki K, Chaabouni H, Fryns JP, Desportes V, Beldjord C, Chelly J (2002) ARX, a novel Prd-class-homeobox gene highly expressed in the telencephalon, is mutated in X-linked mental retardation. Hum Mol Genet 11:981–991 [DOI] [PubMed] [Google Scholar]
- Brais B, Bouchard JP, Xie YG, Rochefort DL, Chretien N, Tome FM, Lafreniere RG, Rommens JM, Uyama E, Nohira O, Blumen S, Korczyn AD, Heutink P, Mathieu J, Duranceau A, Codere F, Fardeau M, Rouleau GA, Korcyn AD (1998) Short GCG expansions in the PABP2 gene cause oculopharyngeal muscular dystrophy. Nat Genet 18:164–167 [DOI] [PubMed] [Google Scholar]
- Brickman JM, Adam M, Ptashne M (1999) Interactions between an HMG-1 protein and members of the Rel family. Proc Natl Acad Sci USA 96:10679–10683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman JM, Clements M, Tyrell R, McNay D, Woods K, Warner J, Stewart A, Beddington RS, Dattani M (2001) Molecular effects of novel mutations in Hesx1/HESX1 associated with human pituitary disorders. Development 128:5189–5199 [DOI] [PubMed] [Google Scholar]
- Brickman JM, Jones CM, Clements M, Smith JC, Beddington RS (2000) Hex is a transcriptional repressor that contributes to anterior identity and suppresses Spemann organiser function. Development 127:2303–2315 [DOI] [PubMed] [Google Scholar]
- Brown LY, Brown SA (2004) Alanine tracts: the expanding story of human illness and trinucleotide repeats. Trends Genet 20:51–58 [DOI] [PubMed] [Google Scholar]
- Brown LY, Odent S, David V, Blayau M, Dubourg C, Apacik C, Delgado MA, Hall BD, Reynolds JF, Sommer A, Wieczorek D, Brown SA, Muenke M (2001) Holoprosencephaly due to mutations in ZIC2: alanine tract expansion mutations may be caused by parental somatic recombination. Hum Mol Genet 10:791–796 [DOI] [PubMed] [Google Scholar]
- Cohen LE, Radovick S (2002) Molecular basis of combined pituitary hormone deficiencies. Endocr Rev 23:431–442 [DOI] [PubMed] [Google Scholar]
- Collignon J, Sockanathan S, Hacker A, Cohen-Tannoudji M, Norris D, Rastan S, Stevanovic M, Goodfellow PN, Lovell-Badge R (1996) A comparison of the properties of Sox-3 with Sry and two related genes, Sox-1 and Sox-2. Development 122:509–520 [DOI] [PubMed] [Google Scholar]
- Crisponi L, Deiana M, Loi A, Chiappe F, Uda M, Amati P, Bisceglia L, Zelante L, Nagaraja R, Porcu S, Ristaldi MS, Marzella R, Rocchi M, Nicolino M, Lienhardt-Roussie A, Nivelon A, Verloes A, Schlessinger D, Gasparini P, Bonneau D, Cao A, Pilia G (2001) The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthus inversus syndrome. Nat Genet 27:159–166 [DOI] [PubMed] [Google Scholar]
- Dasen JS, Barbera JP, Herman TS, Connell SO, Olson L, Ju B, Tollkuhn J, Baek SH, Rose DW, Rosenfeld MG (2001) Temporal regulation of a paired-like homeodomain repressor/TLE corepressor complex and a related activator is required for pituitary organogenesis. Genes Dev 15:3193–3207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasen JS, Rosenfeld MG (2001) Signaling and transcriptional mechanisms in pituitary development. Annu Rev Neurosci 24:327–355 [DOI] [PubMed] [Google Scholar]
- Dattani MT, Martinez Barbera JP, Thomas PQ, Brickman JM, Gupta R, Martensson IL, Toresson H, Fox M, Wales JK, Hindmarsh PC, Krauss S, Beddington RS, Robinson IC (1998) Mutations in the homeobox gene HESX1/Hesx1 associated with septo-optic dysplasia in human and mouse. Nat Genet 19:125–133 [DOI] [PubMed] [Google Scholar]
- Dattani MT, Robinson IC (2000) The molecular basis for developmental disorders of the pituitary gland in man. Clin Genet 57:337–346 [DOI] [PubMed] [Google Scholar]
- Ericson J, Norlin S, Jessell TM, Edlund T (1998) Integrated FGF and BMP signaling controls the progression of progenitor cell differentiation and the emergence of pattern in the embryonic anterior pituitary. Development 125:1005–1015 [DOI] [PubMed] [Google Scholar]
- Eroshkin F, Kazanskaya O, Martynova N, Zaraisky A (2002) Characterization of cis-regulatory elements of the homeobox gene Xanf-1. Gene 285:279–286 [DOI] [PubMed] [Google Scholar]
- Goodman FR, Bacchelli C, Brady AF, Brueton LA, Fryns JP, Mortlock DP, Innis JW, Holmes LB, Donnenfeld AE, Feingold M, Beemer FA, Hennekam RC, Scambler PJ (2000) Novel HOXA13 mutations and the phenotypic spectrum of hand-foot-genital syndrome. Am J Hum Genet 67:197–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel BC, Smits AP, Otten BJ, van den Helm B, Ropers HH, Mariman EC (1996) Familial X-linked mental retardation and isolated growth hormone deficiency: clinical and molecular findings. Am J Med Genet 64:35–41 [DOI] [PubMed] [Google Scholar]
- Hanson IM (2003) PAX6 and congenital eye malformations. Pediatr Res 54:791–796 [DOI] [PubMed] [Google Scholar]
- Heath KE, Day IN, Humphries SE (2000) Universal primer quantitative fluorescent multiplex (UPQFM) PCR: a method to detect major and minor rearrangements of the low density lipoprotein receptor gene. J Med Genet 37:272–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hol FA, Schepens MT, van Beersum SE, Redolfi E, Affer M, Vezzoni P, Hamel BC, Karnes PS, Mariman EC, Zucchi I (2000) Identification and characterization of an Xq26-q27 duplication in a family with spina bifida and panhypopituitarism suggests the involvement of two distinct genes. Genomics 69:174–181 [DOI] [PubMed] [Google Scholar]
- Huang B, Wang S, Ning Y, Lamb AN, Bartley J (1999) Autosomal XX sex reversal caused by duplication of SOX9. Am J Med Genet 87:349–353 [DOI] [PubMed] [Google Scholar]
- Jordan BK, Mohammed M, Ching ST, Delot E, Chen XN, Dewing P, Swain A, Rao PN, Elejalde BR, Vilain E (2001) Up-regulation of WNT-4 signaling and dosage-sensitive sex reversal in humans. Am J Hum Genet 68:1102–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster RW, Kuhnlein RP, Wittbrodt J (2000) Ectopic Sox3 activity elicits sensory placode formation. Mech Dev 95:175–187 [DOI] [PubMed] [Google Scholar]
- Lagerstrom-Fermer M, Sundvall M, Johnsen E, Warne GL, Forrest SM, Zajac JD, Rickards A, Ravine D, Landegren U, Pettersson U (1997) X-linked recessive panhypopituitarism associated with a regional duplication in Xq25-q26. Am J Hum Genet 60:910–916 [PMC free article] [PubMed] [Google Scholar]
- Laumonnier F, Ronce N, Hamel BC, Thomas P, Lespinasse J, Raynaud M, Paringaux C, Van Bokhoven H, Kalscheuer V, Fryns JP, Chelly J, Moraine C, Briault S (2002) Transcription factor SOX3 is involved in X-linked mental retardation with growth hormone deficiency. Am J Hum Genet 71:1450–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzaro D, Price M, de Felice M, Di Lauro R (1991) The transcription factor TTF-1 is expressed at the onset of thyroid and lung morphogenesis and in restricted regions of the foetal brain. Development 113:1093–1104 [DOI] [PubMed] [Google Scholar]
- Lieber MR, Ma Y, Pannicke U, Schwarz K (2003) Mechanism and regulation of human non-homologous DNA end-joining. Nat Rev Mol Cell Biol 4:712–720 [DOI] [PubMed] [Google Scholar]
- Lim HN, Berkovitz GD, Hughes IA, Hawkins JR (2000) Mutation analysis of subjects with 46,XX sex reversal and 46,XY gonadal dysgenesis does not support the involvement of SOX3 in testis determination. Hum Genet 107:650–652 [DOI] [PubMed] [Google Scholar]
- Machinis K, Pantel J, Netchine I, Leger J, Camand OJ, Sobrier ML, Dastot-Le Moal F, Duquesnoy P, Abitbol M, Czernichow P, Amselem S (2001) Syndromic short stature in patients with a germline mutation in the LIM homeobox LHX4. Am J Hum Genet 69:961–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeks JJ, Weiss J, Jameson JL (2003) Dax1 is required for testis determination. Nat Genet 34:32–33 [DOI] [PubMed] [Google Scholar]
- Muragaki Y, Mundlos S, Upton J, Olsen BR (1996) Altered growth and branching patterns in synpolydactyly caused by mutations in HOXD13. Science 272:548–551 [DOI] [PubMed] [Google Scholar]
- Nakano M, Yamada K, Fain J, Sener EC, Selleck CJ, Awad AH, Zwaan J, Mullaney PB, Bosley TM, Engle EC (2001) Homozygous mutations in ARIX (PHOX2A) result in congenital fibrosis of the extraocular muscles type 2. Nat Genet 29:315–320 [DOI] [PubMed] [Google Scholar]
- Netchine I, Sobrier ML, Krude H, Schnabel D, Maghnie M, Marcos E, Duriez B, Cacheux V, Moers A, Goossens M, Gruters A, Amselem S (2000) Mutations in LHX3 result in a new syndrome revealed by combined pituitary hormone deficiency. Nat Genet 25:182–186 [DOI] [PubMed] [Google Scholar]
- Otto F, Kanegane H, Mundlos S (2002) Mutations in the RUNX2 gene in patients with cleidocranial dysplasia. Hum Mutat 19:209–216 [DOI] [PubMed] [Google Scholar]
- Pfaffle RW, DiMattia GE, Parks JS, Brown MR, Wit JM, Jansen M, Van der Nat H, Van den Brande JL, Rosenfeld MG, Ingraham HA (1992) Mutation of the POU-specific domain of Pit-1 and hypopituitarism without pituitary hypoplasia. Science 257:1118–1121 [DOI] [PubMed] [Google Scholar]
- Radovick S, Nations M, Du Y, Berg LA, Weintraub BD, Wondisford FE (1992) A mutation in the POU-homeodomain of Pit-1 responsible for combined pituitary hormone deficiency. Science 257:1115–1118 [DOI] [PubMed] [Google Scholar]
- Raverot G, Lejeune H, Kotlar T, Pugeat M, Jameson JL (2004) X-linked sex-determining region Y box 3 (SOX3) gene mutations are uncommon in men with idiopathic oligoazoospermic infertility. J Clin Endocrinol Metab 89:4146–4148 [DOI] [PubMed] [Google Scholar]
- Rizzoti K, Brunelli S, Carmignac D, Thomas PQ, Robinson IC, Lovell-Badge R (2004) SOX3 is required during the formation of the hypothalamo-pituitary axis. Nat Genet 36:247–255 [DOI] [PubMed] [Google Scholar]
- Scully KM, Rosenfeld MG (2002) Pituitary development: regulatory codes in mammalian organogenesis. Science 295:2231–2235 [DOI] [PubMed] [Google Scholar]
- Smith JM, Koopman PA (2004) The ins and outs of transcriptional control: nucleocytoplasmic shuttling in development and disease. Trends Genet 20:4–8 [DOI] [PubMed] [Google Scholar]
- Solomon NM, Nouri S, Warne GL, Lagerstrom-Fermer M, Forrest SM, Thomas PQ (2002) Increased gene dosage at Xq26-q27 is associated with X-linked hypopituitarism. Genomics 79:553–559 [DOI] [PubMed] [Google Scholar]
- Solomon NM, Ross SA, Morgan T, Belsky JL, Hol FA, Karnes PS, Hopwood NJ, Myers SE, Tan AS, Warne GL, Forrest SM, Thomas PQ (2004) Array comparative genomic hybridisation analysis of boys with X linked hypopituitarism identifies a 3.9 Mb duplicated critical region at Xq27 containing SOX3. J Med Genet 41:669–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromme P, Mangelsdorf ME, Shaw MA, Lower KM, Lewis SM, Bruyere H, Lutcherath V, Gedeon AK, Wallace RH, Scheffer IE, Turner G, Partington M, Frints SG, Fryns JP, Sutherland GR, Mulley JC, Gecz J (2002) Mutations in the human ortholog of Aristaless cause X-linked mental retardation and epilepsy. Nat Genet 30:441–445 [DOI] [PubMed] [Google Scholar]
- Takuma N, Sheng HZ, Furuta Y, Ward JM, Sharma K, Hogan LM, Pfaff SL, Westphal H, Kimura S, Mahon KA (1998) Formation of Rathke’s pouch requires dual induction from the diencephalon. Development 125:4835–4840 [DOI] [PubMed] [Google Scholar]
- Thomas PQ, Dattani MT, Brickman JM, McNay D, Warne G, Zacharin M, Cameron F, Hurst J, Woods K, Dunger D, Stanhope R, Forrest S, Robinson IC, Beddington RS (2001) Heterozygous HESX1 mutations associated with isolated congenital pituitary hypoplasia and septo-optic dysplasia. Hum Mol Genet 10:39–45 [DOI] [PubMed] [Google Scholar]
- Uchikawa M, Kamachi Y, Kondoh H (1999) Two distinct subgroups of group B Sox genes for transcriptional activators and repressors: their expression during embryonic organogenesis of the chicken. Mech Dev 84:103–120 [DOI] [PubMed] [Google Scholar]
- Vincent MC, Pujo AL, Olivier D, Calvas P (2003) Screening for PAX6 gene mutations is consistent with haploinsufficiency as the main mechanism leading to various ocular defects. Eur J Hum Genet 11:163–169 [DOI] [PubMed] [Google Scholar]
- Weiss J, Meeks JJ, Hurley L, Raverot G, Frassetto A, Jameson JL (2003) Sox3 is required for gonadal function, but not sex determination, in males and females. Mol Cell Biol 23:8084–8091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward K, Cundall M, Palmer R, Surtees R, Winter RM, Malcolm S (2003) Complex chromosomal rearrangement and associated counseling issues in a family with Pelizaeus-Merzbacher disease. Am J Med Genet A 118:15–24 [DOI] [PubMed] [Google Scholar]
- Wu W, Cogan JD, Pfaffle RW, Dasen JS, Frisch H, O’Connell SM, Flynn SE, Brown MR, Mullis PE, Parks JS, Phillips JA, Rosenfeld MG (1998) Mutations in PROP1 cause familial combined pituitary hormone deficiency. Nat Genet 18:147–149 [DOI] [PubMed] [Google Scholar]
- Xiang F, Buervenich S, Nicolao P, Bailey ME, Zhang Z, Anvret M (2000) Mutation screening in Rett syndrome patients. J Med Genet 37:250–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Basta T, Jensen ED, Klymkowsky MW (2003) The β-catenin/VegT-regulated early zygotic gene Xnr5 is a direct target of SOX3 regulation. Development 130:5609–5624 [DOI] [PubMed] [Google Scholar]