Abstract
Artificial intelligence (AI) serves as a powerful tool that can revolutionize how personalized, patient-focused care is provided within interventional cardiology. Specifically, AI can augment clinical care across the spectrum for acute coronary syndrome, coronary artery disease, and valvular heart disease, with applications in coronary and structural heart interventions. This has been enabled by the potential of AI to harness various types of health data. We review how AI-driven technologies can advance diagnosis, preprocedural planning, intraprocedural guidance, and prognostication in interventional cardiology. AI automates clinical tasks, increases efficiency, improves reliability and accuracy, and individualizes clinical care, establishing its potential to transform care. Furthermore, AI-enabled, community-based screening programs are yet to be implemented to leverage the full potential of AI to improve patient outcomes. However, to transform clinical practice, AI tools require robust and transparent development processes, consistent performance across various settings and populations, positive impact on clinical and care quality outcomes, and seamless integration into clinical workflows. Once these are established, AI can reshape interventional cardiology, improving precision, efficiency, and patient outcomes.
Keywords: artificial intelligence, interventional cardiology, percutaneous coronary intervention, structural heart intervention
Introduction
Artificial intelligence (AI) is a powerful tool with the potential to transform the delivery of personalized, patient-centered care in interventional cardiology.1, 2, 3 Specifically, AI can augment clinical care across the spectrum for acute coronary syndrome (ACS), coronary artery disease (CAD), and valvular heart disease (VHD). In addition to leveraging diagnostic modalities to identify cardiovascular disease (CVD), AI tools have provided significant benefits for planning and conducting percutaneous coronary interventions (PCI) and structural heart interventions, such as transcatheter aortic and mitral valve replacement. AI-driven innovations are also personalizing prognostication, including postprocedural outcomes and disease progression. Collectively, these advancements promise to redefine the current paradigm in interventional cardiology care delivery.
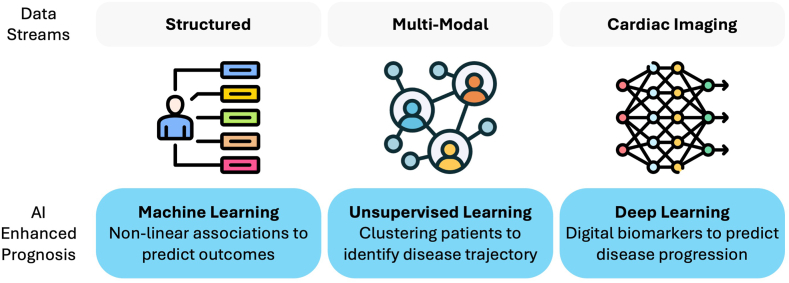
The term AI encapsulates a wide range of computational methods that can infer relevant information from large data sets, often uncovering insights that would be imperceptible to humans. These include machine learning techniques that are effective at capturing complex, nonlinear patterns in structured data but are generally limited to structured data readily measured in health care settings. In contrast, deep learning algorithms—utilizing neural networks—can identify intricate patterns in more complex data streams, such as electrocardiography (ECG) and cardiac imaging. Thus, AI has the potential to harness various health data streams to enhance interventional cardiovascular care.
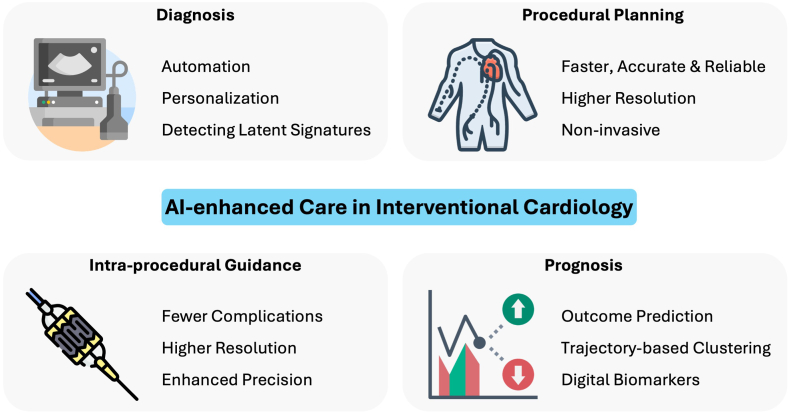
Herein, we survey the potential role of AI in interventional cardiology, discussing advances in the diagnosis of ACS, CAD, and VHD. We also review how AI can transform preprocedural planning, intraprocedural guidance, and prognostication for coronary and structural heart interventions (Central Illustration). Finally, we discuss future areas for innovation and the challenges in developing and integrating AI tools in interventional cardiology care.
Central Illustration.
The role of artificial intelligence in interventional cardiology. AI, artificial intelligence.
Diagnosis
By leveraging existing diagnostic modalities, AI plays a crucial role in improving diagnostic accuracy in health care settings (Figure 1). First, AI-enabled tools can infer latent signatures from a wide range of diagnostic modalities, eg, chest X-ray (CXR), ECG, and transthoracic echocardiography (TTE). Second, AI can facilitate clinical workflow by automating diagnoses through the rapid and accurate interpretation of data. Third, AI can provide a more personalized sequential diagnostic workup.
Figure 1.
AI-enabled precision diagnosis. AI, artificial intelligence; CAG, coronary angiography; CCTA, coronary computed tomography angiography; CXR, chest X-ray; ECG, electrocardiography; IVUS, intravascular ultrasound; OCT, optical coherence tomography; TTE, transthoracic echocardiography.
Acute coronary syndrome
In the emergency room, ACS raises the clinical challenge of accurate triage between patients who require emergent coronary angiography, those who can undergo urgent angiography, and those suitable for noninvasive testing. Currently, this clinician-led decision is informed by a combination of clinical assessments, ECG findings, and levels of high-sensitivity cardiac troponin, which helps classify patients into 1 of 3 categories: ST-elevation myocardial infarction, non-ST-elevation myocardial infarction, or unstable angina. Within this scope, emerging AI tools can be integrated with the existing diagnostic schema for ACS to personalize diagnosis and infer latent signals that are currently underutilized.
Troponin
Classification of ACS currently relies on either a single elevated troponin value or the delta between serial values over time in the appropriate clinical context. However, troponin levels are known to be influenced by patient demographics such as age and sex.4 Emerging AI-based approaches can enhance the evaluation of chest pain in the emergency department by integrating baseline characteristics to personalize the diagnostic use of troponin levels.5 The myocardial-ischemic-injury-index score uses a gradient-boosting algorithm to integrate patient age and sex with 2 serial troponin measurements to assess the likelihood of myocardial infarction (MI).6 In an external cohort of >20,000 patients, the myocardial-ischemic-injury-index score demonstrated excellent discrimination for identifying MI, with an area under the receiver operating characteristic curve (AUROC) of 0.95. It was also significantly associated with MI-specific and all-cause mortality at 1 year.7 However, the requirement for 2 troponin measurements in all patients to estimate MI probability may limit implementation. Moreover, troponin levels are also influenced by comorbidities such as renal function, and its interpretation is informed by the presence of cardiac symptoms and risk factors.8 The subsequent development of the Collaboration for the Diagnosis and Evaluation of Acute Coronary Syndrome (CoDE-ACS) system integrates these additional features with a single troponin value and has demonstrated superior performance to current guideline-recommended pathways with an AUROC of 0.95 in a cohort of >10,000 patients.9
Electrocardiography
The ECG is a vital tool for ACS diagnosis and management, with current decision making around emergent revascularization relying on the presence or absence of ST-segment elevation. The emerging obstructive myocardial infarction (OMI) paradigm offers a more nuanced framework for identifying acute coronary occlusion in patients who may require urgent revascularization. However, interpreting OMI ECG patterns, such as the presence of de Winter T-waves, Wellens waves, the Aslanger pattern, and the South African pattern, among others, can be challenging for nonexpert readers, potentially leading to missed or delayed diagnoses.10 In this context, the ability of AI algorithms to interpret ECG accurately and reliably enables a refined method to automate the identification of signals that may be challenging for human readers to detect. Recent studies suggest that AI models can infer latent signals to identify patients at risk of OMI earlier in their clinical presentation, providing a more comprehensive evaluation of patients with suspected ACS and potentially prompting timely intervention.11,12
An OMI risk score model was developed using features extracted from prehospital ECG for patients presenting to the emergency department with chest pain to develop a random forest model predicting the likelihood of an acute culprit lesion on coronary angiography.12 The OMI risk model displayed a validation AUROC of 0.87 in an external validation data set of >3000 patients, outperforming the emergency department clinicians who had an AUROC of 0.80.12 Notably, the model could integrate ECG patterns of OMI that cannot be easily appreciated by experts, including subtle T-wave changes (such as prolongation of the Tpeak-Tend interval), and high-dimensional assessment of increased ventricular repolarization dispersion (such as the ratio between the principal components of the ST-T waveforms or the cosine angle between R-to-T).12
Coronary artery disease
The diagnosis of CAD requires an evaluation of whether coronary artery stenosis is present and, if so, quantifying the stenosis severity and the subsequent functional ischemia to assess whether it is actionable. The current diagnostic schema includes distinguishing between those who can be managed with noninvasive testing (imaging or stress tests) and those who need further invasive procedures (coronary angiography). The manual evaluation of coronary investigations is often labor-intensive, requires expertise, and is prone to interobserver variability. The integration of AI could provide automated evaluations as rapidly available, standardized outputs. Furthermore, AI tools also enable personalized assessment of coronary pathology, utilizing hidden signatures present in imaging modalities, providing clinicians with richer information and potentially reducing the need for invasive procedures.
Coronary computed tomography angiography
Coronary computed tomography angiography (CCTA) is a crucial modality for noninvasive anatomical assessment of CAD. The current diagnostic pathway involves manual quantification of calcified plaque burden within the coronary arteries as coronary artery calcium (CAC) scores, with higher CAC scores prompting referral for invasive coronary angiography. With manual CAC scoring being labor-intensive and prone to interobserver variability, AI algorithms can automate the CAC scoring process, providing faster and more consistent CAC assessments than manual methods.13 AI algorithms can use CCTA images to perform inference, providing an extra layer of information that may modulate decision-making about the need for invasive testing. This includes detecting latent signals, such as markers of vascular inflammation, which can be used to identify high-risk plaques that are more prone to rupture, even in cases in which stenosis may not be severe.14 Cross-modality inference is also possible, such as quantification of coronary atherosclerotic burden at comparable accuracy to intracoronary imaging.15 Moreover, fractional flow reserve (FFR) values—typically obtained via pressure wire measurements during angiography—can also be noninvasively inferred from CCTA images, identifying the functional impact of CAD on coronary blood flow.14
Coronary angiography
Coronary angiography is the gold-standard diagnostic modality for CAD. The manual interpretation of coronary angiography requires expertise and is subject to variability. AI algorithms have been developed to automate critical aspects of angiographic assessment. For example, a deep learning model accurately segmented the major coronary arteries from angiographic images (with an F1 score >0.8 for 94% of images) and also quantified stenosis severity.16 AI algorithms can also infer functional information directly from angiographic images, which may reduce the need for further invasive procedures such as pressure wire measurements. Quantitative flow reserve and FFR can be inferred by AI-driven models that analyze the flow dynamics within the coronary arteries and can predict the physiological significance of coronary stenoses, helping to identify which lesions are likely to cause ischemia and require revascularization.17
Intracoronary imaging
Intravascular ultrasound (IVUS) is used for the high-resolution assessment of coronary artery plaques, including identifying the extent of calcification and detecting vulnerable plaques that may be at risk of rupture. AI algorithms can automate the interpretation of IVUS data, reducing the reliance on manual expertise while enhancing the consistency and accuracy of plaque assessments.18 AI-based IVUS analysis can detect and quantify coronary calcifications, segment the coronary artery layers, and identify high-risk plaques. Vulnerable plaques, characterized by thin fibrous caps and large lipid cores, are associated with a higher risk of rupture and subsequent acute coronary events. AI models can specifically identify these high-risk plaques, enhancing the clinical utility of IVUS for more reliable risk stratification.19,20
Intravascular optical coherence tomography (OCT) is another imaging modality characterizing coronary plaques at even greater resolution than IVUS. OCT images depict plaque morphology of fibrous caps and lipid cores, helping to estimate plaque stability and predict the likelihood of causing future coronary events. As with IVUS, the interpretation of OCT images is typically performed manually. AI algorithms have been developed to automate key tasks in OCT evaluation, including lumen segmentation, quantification of plaque morphology, and identification of fibroatheromas.21, 22, 23, 24, 25 AI analysis of OCT images can infer blood flow dynamics to enhance personalized risk stratification.26
Valvular heart disease
The diagnosis of VHD currently requires an assessment of valve pressure gradients and velocities measured by TTE performed by a trained operator, necessitating access to advanced equipment and expertise. AI tools can enhance VHD diagnosis using modalities with greater accessibility—CXR and ECG—and also streamline VHD diagnosis using TTE.
Chest X-ray
Recent studies have demonstrated that plain CXR can be effectively used to diagnose aortic stenosis (AS) and mitral regurgitation (MR) when augmented by AI analysis. Deep learning models developed on CXR images could detect AS with an AUROC of 0.83 and MR with an AUROC of 0.80.27,28 Moreover, by integrating information across the entire image, AI models can identify subtle radiographic signs of valvular pathology that may be challenging to detect, even by expert human readers. For example, while clinicians evaluate AS from a CXR by examining for calcification in the aortic region, saliency maps for the deep learning AS model showed hot spots in the aortic valve region and also in the left ventricle and left atrium, suggesting the model considered both aortic valve calcification and signs of elevated pressures in the left cardiac system.27 These models may be useful for opportunistic screening for VHD in CXR repositories in health systems.
Electrocardiography
AI algorithms have been developed to detect VHD from different formats of ECG, including 12-lead and portable single-lead ECG. AI-enhanced interpretation of ECG, as a ubiquitous modality, can substantially improve VHD diagnosis, particularly where the ECG signatures are not recognizable by humans.29, 30, 31, 32, 33 Recently, a 12-lead AI-ECG algorithm, PRESENT-SHD, and a portable-adapted, single-lead AI-ECG model, ADAPT-HEART, have been developed and externally validated using data from 150,000 patients.34,35 These models can detect various structural heart diseases, including moderate or severe AS, with an AUROC of 0.8 from 12-lead ECG images and portable single-lead ECG, respectively.34,35 Further studies have demonstrated that AI-ECG algorithms offer sensitivity and specificity comparable to echocardiography.31,32 Therefore, AI enables leveraging ubiquitous ECG for the large-scale identification of individuals with clinically actionable VHD in various settings.
Transthoracic echocardiography
Diagnostic evaluation for VHD requires a trained cardiac sonographer to capture several high-quality views of the heart and an expert cardiologist to evaluate the results, representing a resource-intensive process. The integration of AI can enhance this workflow by automating the reporting of TTE results, which may help address the problem of interobserver variability and improve the reproducibility of measurements.36,37 AI algorithms that can detect AS and MR and quantify their severity from a single echocardiographic view, even without color Doppler, have been developed and achieved near-perfect accuracy.38, 39, 40 In a multicenter study, an AI algorithm was developed using >17,500 TTE videos to detect severe AS from a parasternal long-axis view without color Doppler—an easy-to-obtain view. The model demonstrated an AUROC of 0.98 and performed consistently across 3 temporally and geographically distinct external validation data sets.39 Together, these AI-enabled capabilities reduce the reliance on the availability of human expertise to interpret TTE scans, potentially accelerating the diagnostic pathway. Lastly, AI models are capable of inferring findings from one imaging modality to another. Cross-modality inference, predicting cardiac magnetic resonance imaging (CMR)-derived parameters or CAC scores from echocardiograms, was demonstrated to achieve good comparability offering a more integrated diagnostic workflow that could streamline clinical decision making and reduce the need for multiple imaging studies.41,42
Preprocedural planning
The preprocedural planning of structural heart interventions, including transcatheter aortic valve replacement (TAVR) and transcatheter edge-to-edge repair (TEER), involves accurate measurements using cardiac imaging. Similarly, successful coronary revascularization requires accurate preprocedural identification of coronary plaque characteristics and coronary vasculature anatomy. However, such assessments are time-consuming and subject to interobserver variability. AI tools have been developed to streamline preprocedural planning, minimize human resources efforts, and ensure accuracy (Figure 2).
Figure 2.
Integrating AI into preprocedural planning. 3D, three-dimensional; AI, artificial intelligence; CCTA, coronary computed tomography angiography; CMR, cardiac magnetic resonance imaging; CT, computed tomography; OCT, optical coherence tomography; TTE, transthoracic echocardiography.
Coronary interventions
Accurate planning and assessment of coronary revascularization rely on high-resolution visualization of coronary plaques and lumina through OCT using infrared light.43 OCT provides beneficial information on the plaque characteristics, as well as a detailed anatomical map of the coronary vasculature, including the location of vascular bifurcations and side branches, helping to inform preprocedural planning such as stent placement.44, 45, 46 After PCI, stent expansion, apposition, and healing can be inferred from OCT.47, 48, 49 However, OCT interpretation is time-consuming and requires expert readers with potential interobserver variability, significantly curtailing its incremental clinical value.50,51 In OCT images, locating stent struts to measure the protrusion distance and neointimal thickness is essential to assess stent expansion and apposition, respectively, both bearing critical postprocedural implications such as in-stent restenosis, stent fracture, and stent thrombosis.52, 53, 54, 55, 56 AI tools have been developed to accurately detect stent struts from OCT images in as fast as 59 ms per image with a sensitivity and specificity as high as 94%, depending on the case mix.51,57, 58, 59, 60, 61, 62, 63 This was consistent across cases undergoing as well as those with previous stent implantation. However, the performance was modestly lower for struts with thick tissue coverage >0.3 mm.50,51,58,59 AI-automated measurements of the stent and luminal areas had correlation coefficients of 0.96 to 0.99. In addition to substantially reducing the time required for analyzing OCT images, AI reduced interobserver variability by 30%.50,51,58,60
In addition to detecting stent underexpansion and malapposition, OCT provides additional information beyond clinical characteristics to predict these outcomes before stent deployment.64,65 This holds substantial clinical significance as the physician can identify coronary lesions at risk for stent underexpansion and malapposition and might decide to conduct lesion preparation interventions such as atherectomy before stent deployment.64 Conventional machine learning algorithms, including regression, support vector machines, and gradient boosting algorithms, have been used to predict stent underexpansion and malapposition from clinical characteristics as well as OCT features, including plaque calcification length and lipid arc.64,65 These algorithms had a discrimination of 0.85 for predicting stent underexpansion, defined as a stent expansion index ≤80%, and a discrimination of 0.82 for predicting stent malapposition, defined as the lack of contact of at least one stent strut with the vessel wall.64,65
Virtual reality (VR)-based preprocedural planning is emerging as a powerful tool for complex cases involving anomalous coronary anatomy or chronic total occlusion of coronary vessels. A recent case report demonstrated the efficacy of a novel VR-guiding catheter simulation system that uses 3-dimensional reconstructions from ECG-synchronized cardiac computed tomography (CT) imaging to optimize catheter selection and navigation. By simulating catheter type, size, and positioning in a virtual environment, this tool enables precise preprocedural planning while eliminating the need for additional radiation or contrast exposure. Furthermore, in cases involving anomalous origins of the right coronary artery, VR-guided planning has been shown to reduce catheter selection time, improve procedural efficiency, and overcome the challenges posed by complex anatomy.66
Structural heart interventions
TAVR planning and device selection are based on anatomical measurements such as aortic annulus diameter and aortic angle, locating anatomical landmarks, and 3-dimensional visualization of anatomical structures using different cardiac imaging modalities. AI tools enhance preprocedural measurements using echocardiography, CT scan, and CMR, with more accurate measurements from 3-dimensional reconstructions vs 2-dimensional measurements.67,68 AI-enhanced measurements demonstrated significant and strong correlations with manual annotations by senior observers, with a correlation coefficient >0.91, mean error <2 mm, and no clinically relevant bias for anatomical measurements.69, 70, 71, 72, 73, 74, 75, 76 Notably, in a large study from 20 centers in China, the intraclass correlation coefficient values for the AI model were 0.998.69 For device selection, the agreement between AI and the expert was 0.86 to 0.89, similar to the agreement between 2 experts. The expert-level accuracy of AI-automated measurements was achieved without the need for any human inputs, as well as with more reliable measurements in a shorter timeframe.70,77
To visualize anatomical valve structures using CT in a 3-dimensional space, traditional reconstruction methods use filtered back projection, whereas newer iterative reconstruction methods leverage physical modeling with complex math.78 Although iterative reconstruction methods can improve image quality and reduce radiation exposure, they are computationally expensive. To address this challenge, deep learning has been leveraged to provide images with higher contrast-to-noise and signal-to-noise ratios while generating them 6 times faster than iterative reconstruction methods.68,79,80 Therefore, deep learning represents a novel approach that enables high-quality images to visualize anatomical valve structures in 3-dimensional space using fewer computational resources in a shorter duration of time.
The shared pathophysiological pathways between AS and CAD often lead to the co-occurrence of these conditions, with two-thirds of patients undergoing TAVR having comorbid CAD.81 Before aortic valve replacement, candidates should undergo CAD evaluation to receive PCI and TAVR or coronary artery bypass grafting and surgical aortic valve replacement during the same procedure. This preprocedural assessment is conducted using CCTA, which can be facilitated using AI-automated interpretation with an accuracy comparable with expert readers.82 Although CCTA is highly sensitive with a high negative predictive value, enabling the effective exclusion of CAD, it lacks specificity, resulting in a high false positive rate. To further refine the false positive cases, lesion-specific FFR from invasive coronary angiography is needed to detect coronary lesions causing functional ischemia.83 However, the US Food and Drug Administration (FDA) has approved an AI algorithm that noninvasively measures FFR on CCTA with the potential to improve the specificity of CCTA-enabled detection of CAD.84, 85, 86 AI-enhanced FFR assessment in TAVR candidates with coronary lesions on CCTA has shown to have a sensitivity and negative predictive value >95% with a specificity of 52% to 87% and a positive predictive value of 40% to 88%, reducing the burden of pre-TAVR invasive coronary angiography.87, 88, 89, 90
A recent proof-of-concept and validation study introduced a multiuser, multidevice mixed reality application for collaborative cardiac interventional planning.91 This system allows for precise 3-dimensional visualization of patient-specific anatomy using data derived from CT imaging. The mixed reality application facilitated enhanced spatial understanding, improved procedural collaboration, and provided clinicians with tools to virtually manipulate anatomical structures and perform device simulations preoperatively. Notably, clinical validation confirmed that VR-derived measurements were comparable to traditional CT-derived measurements, underscoring the system’s reliability and potential to optimize procedural planning.91
Intraprocedural guidance
In addition to preprocedural planning, AI can advance cardiovascular interventions by providing real-time inputs during the procedure. In particular, enhanced visualization during the procedure can improve the precision and reduce adverse events (Figure 3).
Figure 3.
AI-enhanced intraprocedural guidance for cardiovascular interventions. AI, artificial intelligence; AKI, acute kidney injury; CT, computed tomography; TEE, transesophageal echocardiography. Patrick J. Lynch, medical illustrator, CC BY 2.5, via Wikimedia Commons.
Coronary interventions
Intraprocedural guidance during PCI has been enabled by innovative technologies, such as dynamic coronary roadmap (DCR), with improved procedural outcomes. DCR dynamically overlays the coronary arteries on fluoroscopic PCI images in real time while compensating for cardiac and respiratory-induced motions. Therefore, DCR allows for enhanced real-time visualizations during PCI and potentially diminishes fluoroscopy time and contrast volume.92 Multiple studies, including randomized clinical trials and retrospective analyses, have demonstrated that DCR use during PCI significantly lowers contrast medium volume and fluoroscopy time without compromising clinical success rates, regardless of the operator’s experience level.93, 94, 95 These beneficial effects have also been observed in patients with chronic kidney disease at risk for contrast-induced acute kidney injury (AKI).96 In a propensity score-matched retrospective study on patients with chronic kidney disease receiving PCI, those with DCR-enhanced PCI received less contrast (92 mL vs 116 mL) and incurred fewer contrast-induced AKI (0.9% vs 6.2%) compared with patients undergoing PCI without DCR, while the success rate was comparable (99.1% vs 98.2%).96 Additionally, integrating preprocedural CT scans with live fluoroscopy has proven feasible and offers valuable insights into chronic total occlusion PCI, enhancing visualization without increasing procedural risks.97
Structural heart interventions
Intraprocedural transesophageal echocardiography (TEE) plays a key role in real-time guidance of structural heart interventions such as TAVR and TEER.98 Although TEE uses safe ultrasound waves and feasibly provides critical hemodynamic and anatomical information during the procedure, the quality of TEE images is inferior compared with CT, providing suboptimal procedural guidance.99 However, using intraprocedural CT for real-time guidance is limited due to radiation exposure. To improve the real-time guidance using TTE without imposing the radiation exposure of CT, mathematical methods have been suggested to fuse 2-dimensional TEE images with preprocedural cardiac CT images to produce a 3-dimensional image using temporal and spatial registration.100, 101, 102, 103, 104 This method can serve as a real-time guidance tool, which potentially leads to more successful interventions and improves procedural outcomes.100 Although AI has not been leveraged in this domain, it has the potential to improve the efficiency and accuracy of such processes.80 Notably, the implications of such guidance tools in terms of short- and long-term patient outcomes should be defined before wide clinical adoption.
Prognosis
AI has enabled the prediction of short- and long-term outcomes for cardiovascular interventions, which allows for personalized periprocedural risk stratification to tailor procedural conduction or to plan a postprocedural follow-up (Figure 4). Additionally, the predictions can be improved by including both postprocedural and preprocedural data. The prognostic and predictive value of diagnostic modalities such as cardiac imaging are being increasingly recognized and incorporated into risk prediction models.
Figure 4.
AI tools to improve prognostication in interventional cardiology. AI, artificial intelligence.
Coronary interventions
In-hospital mortality following PCI can be predicted from structured clinical variables using machine learning models with an AUROC of 0.90 to 0.96.105, 106, 107, 108, 109, 110, 111, 112 Furthermore, short-term mortality can also be predicted at modestly lower performances with an AUROC of 0.86 to 0.93 for 30-day mortality112, 113, 114, 115 and 0.82 to 0.87 for 1-year mortality.116, 117, 118 In addition to mortality, machine learning models predicted adverse in-hospital outcomes, such as bleeding (AUROC: 0.70-0.89),108, 109, 110, 111,119,120 AKI (AUROC: 0.75-0.89),108, 109, 110, 111,121,122 and stroke (AUROC: 0.70-0.75).108, 109, 110 Machine learning models provided a modest improvement in predicting in-hospital mortality, AKI, and bleeding over the NCDR-CathPCI risk scores (AUROC: 0.96 vs 0.95 for mortality, 0.84 vs. 0.82 for AKI, and 0.79 vs. 0.76 for bleeding) in a registry from Japan.111 Moreover, postdischarge outcomes, including 30-day heart failure hospitalization (AUROC: 0.90),112 and 1-year target vessel revascularization (AUROC: 0.72) have been predicted.117,118 These models may allow for personalized risk stratification before PCI and potentially lead to enhanced outcomes.
Structural heart interventions
Machine learning models showed good discrimination in predicting in-hospital (AUROC: 0.92), 30-day (AUROC: 0.75), and 1-year mortality (AUROC: 0.72-0.8) after TAVR using structured clinical variables.123, 124, 125, 126 Considering TAVR outcomes other than mortality, machine learning models predicted the need for post-TAVR permanent pacemaker implantation (AUROC: 0.81),127, 128, 129 and 6-month infective endocarditis (AUROC: 0.75) using structured data.130 Furthermore, deep learning algorithms have been used to predict paravalvular leak (AUROC: 0.86),131 2-year mortality (AUROC: 0.70),132 30-day cerebrovascular events (AUROC: 0.79),133 and new-onset atrial fibrillation (AUROC: 0.74) after TAVR using cardiac CT images in addition to structured data,134 showing the incremental value of cardiac images over readily available clinical variables.
Similar efforts have been made for TEER, albeit to a lesser extent than TAVR. Using structured data, machine learning models predicted in-hospital (AUROC: 0.83)135,136 and 1-year mortality (AUROC: 0.79)137 as well as 30-day readmission (AUROC: 0.72) after TEER.138 Of note, the MITRALITY score consistently predicted 1-year mortality following TEER (AUROC: 0.78) in an external validation cohort and also demonstrated improved performance over existing risk scores.139
Advanced prognostication
Unsupervised and semisupervised AI represents a novel approach to predicting procedural outcomes by identifying phenotypic differences across patient clusters using their preprocedural data. This approach has been adopted for TAVR,140, 141, 142 TEER,143 and PCI,144 using machine learning models. The observed differences in survival outcomes between patient clusters based on echocardiogram and clinical variables enable risk stratification and prediction of the disease trajectory. However, identifying human-invisible signatures of worse outcomes from more complex data streams, such as cardiac imaging, can lead to more precise prognostication strategies.145 Recently, a multimodal video-based AI biomarker, DASSi (Digital AS Severity index), for AS progression has been developed and externally validated. Derived from TTE or CMR videos, higher DASSi was associated with faster AS progression, as determined by increased peak aortic valve velocity and a higher risk of aortic valve replacement.40
Future directions
Cardiovascular disease screening
AI is set to revolutionize CVD screening by enhancing modalities available at the point of care, such as phonocardiography, ECG, and point-of-care ultrasound. Early detection of CVD improves clinical outcomes through prompt treatment, but traditional screening methods are resource-intensive and require skilled professionals for interpretation. AI-driven interpretations can overcome these limitations by automating data analysis, making screening programs more accessible and scalable, especially in resource-constrained settings. For instance, AI-driven interpretations of phonocardiography can classify heart murmurs with near-perfect accuracy and detect VHD effectively.146, 147, 148, 149, 150, 151, 152, 153 Similarly, AI algorithms applied to ECG data—including images of 12-lead ECG and portable single-lead ECG—enabled scalable screening for VHD and other structural heart diseases in the community.33, 34, 35,154,155 Furthermore, integrating AI with point-of-care ultrasound automated the detection of structural heart conditions, such as severe AS, even when images are acquired by minimally trained operators.39,156, 157, 158 These developments demonstrate the feasibility of screening approaches for early detection of VHD, such as severe AS, whose timely treatment with transcatheter interventions, especially in asymptomatic stages, improves patient outcomes.159, 160, 161 However, such approaches are yet to be widely implemented for CVD screening.
From bytes to bedside
Bridging the gap from bytes to bedside to bring transformative AI technology to patients involves overcoming several challenges. The development and integration of AI tools into clinical workflows require careful consideration of key aspects, as outlined in reporting checklists.162, 163, 164, 165, 166 Notable examples include TRIPOD-AI, STARD-AI, and MI-CLAIM, which focus on ensuring the robust development of AI tools.162, 163, 164 Additionally, checklists such as DECIDE-AI and CONSORT-AI provide guidance for the implementation of AI tools in real-world settings.165,166 These reporting checklists serve as an auxiliary tool to help clinicians assess the robustness and feasibility of AI technologies. In this section, we review essential concepts in AI tool development and implementation, including external validity, fairness, additional benefits over the standard of care, and effective integration into clinical workflows.162, 163, 164, 165, 166, 167, 168, 169 We also highlight the infrastructure investments required for AI implementation.
The development of AI algorithms marks the first step, which should be followed by evaluating its external validity across disparate settings to ensure the algorithm can generalize well. This is essential because high-performing models may not retain their original performance in external data sources due to overfitting to the development data.170,171 Nevertheless, the external validity of AI models has not always been reported, particularly for some proprietary algorithms for which the model development process remains obscure. Model development using a diverse population representing a broad range of sociodemographic and clinical subgroups can improve the model’s generalizability.1,172 While data privacy concerns can cause hurdles in validating models using external data, advancements in the field of information technology, such as the sharing of a docker container, enable end-to-end model deployment in external data without the need for sharing the underlying model or original data set.173
The fairness of AI algorithms—exhibiting consistent performance across age, sex, racial, and ethnic subgroups—should be established before their clinical adoption. This ensures the deployment of AI algorithms will not lead to or exacerbate health disparities. This is essential because some AI algorithms may perform worse in older adults or minoritized populations.174 Although model development in large and diverse populations improves the model’s fairness, specific measures, such as age-/sex-matching during training, should be adopted.175 Model training using age-/sex-matched cases and controls enforces learning specific biological features of the target, independent of age and sex. However, the biological effects of age and sex can then be incorporated into an ensemble model. This approach has been shown to generalize well across various settings for AI-ECG models.34,35
AI algorithms should be adopted in clinical practice only if they demonstrate clear advantages over the standard of care. This requires a head-to-head comparison of AI tools with the current clinical care in terms of clinical benefit, clinical workload, and cost. It should be noted that the advantage of AI over existing tools needs to be established in external data sources as well as in the development population, because the advantage of the AI algorithm might arise from its overfitting to the development population rather than from a true added value. Moreover, automation bias should be considered and minimized, as clinicians may trust assistive AI tools more than their clinical judgment, which can lead to worse clinical performance compared with standard care in the absence of the AI tool.176
The successful integration of AI algorithms into clinical workflow hinges on their interoperability across health systems. The more tech-agnostic the algorithms are, the more effectively they can be incorporated into clinical workflow.1 For example, ECG images can be used more readily than ECG signals with smaller infrastructural investments. Therefore, given the comparable performance of image- vs signal-based AI-ECG models, image models have an advantage in transforming care.177,178 Furthermore, AI tools should improve care without increasing the already exhausting clinical workload. To this end, online platforms that allow for the deployment of AI algorithms with real-time clinically meaningful outputs are needed.179
Generating streamlined, real-time AI outputs to enhance patient care often requires significant infrastructural investments, including sufficient computational power and secure data storage solutions.1, 2, 3 While these investments require extensive planning and financial resources upfront, they support the scalability of AI-driven initiatives in the long term.180,181 Furthermore, the emergence of cloud computing services has enabled organizations to access high-performance computational resources on demand, reducing upfront costs and eliminating the need for extensive on-site infrastructure.180
Contemporary AI applications in interventional cardiology
Recently, AI has transitioned from theoretical promise to practical application in interventional cardiology, enhancing diagnostic accuracy and procedural efficiency. Among the AI-enabled medical devices in the cardiovascular domain that have met the FDA premarket requirements,182 the FFRangio System and FEops HEARTguide have established use cases in interventional cardiology. CathWorks FFRangio is a software tool that analyzes angiography images to calculate FFRangio, a mathematically derived value based on simulated blood flow from 3-dimensional coronary models, supporting the functional evaluation of CAD.183,184 Similarly, FEops HEARTguide is a simulation software designed to assist in procedural planning for transcatheter left atrial appendage occlusion device implantation. It provides patient-specific predictions of implant frame deformation, helping clinicians determine the optimal device size and placement.185
Another significant AI application in interventional cardiology is the CorPath GRX System, developed by Corindus Vascular Robotics. This robotic platform is designed to improve precision during PCI. In 2018, the FDA granted 510(k) clearance for the system's “Rotate on Retract” (RoR) feature, marking the first automated robotic movement in the technIQ Series. The RoR software enhances procedural efficiency by automatically rotating the guide wire upon joystick retraction, allowing operators to navigate to targeted lesions more effectively. Preclinical data demonstrated a significant reduction in wiring time among experienced physicians using the RoR feature compared with conventional methods.186 In 2020, the platform also received FDA 510(k) clearance for a new feature that provides enhanced accuracy of movements for advanced PCI.187
Conclusion
In summary, we demonstrated how AI-driven technologies can advance diagnosis, preprocedural planning, intraprocedural guidance, and prognostication in interventional cardiology (Central Illustration). AI can automate clinical operations, increase efficiency while ensuring accuracy, improve reliability, and individualize clinical care, establishing its potential to revolutionize interventional cardiology care. However, AI-enabled, community-based screening programs are yet to be implemented to harness the full potential of AI to improve patient outcomes. For AI tools to move the needle in clinical practice, robust and transparent development processes, consistent performance across various settings and populations, improvements in clinical and care quality outcomes benchmarked against the current standard of care, and seamless integration into clinical workflows are required. With these efforts, AI can reshape interventional cardiology, improving precision, efficiency, and patient outcomes.
Acknowledgments
Declaration of competing interest
Rohan Khera is an Associate Editor of JAMA. He receives research support, through Yale, from Bristol-Myers Squibb, Novo Nordisk, BridgeBio, and the Blavatnik Family Foundation. He is a coinventor of US Pending Patent Applications WO2023230345A1, US20220336048A1, 63/346,610, 63/484,426, 63/508,315, 63/580,137, 63/606,203, 63/619,241, 63/562,335, and 18/813,882. He is a cofounder of Evidence2Health, a precision health platform to improve evidence-based cardiovascular care, and an academic cofounder of Ensight-AI, Inc, an AI-ECG analytics company. Arya Aminorroaya, Dhruva Biswas, and Aline Pedroso reported no financial interests.
Funding
This work was supported by the National Institutes of Health (grant numbers R01AG089981, R01HL167858, and K23HL153775 [R.K.]), and the Doris Duke Charitable Foundation (grant number 2022060 [R.K.]). The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Ethics statement and patient consent
This review article adheres to all relevant ethical guidelines. As it is based on a synthesis of previously published literature, it does not involve human participants, patient data, or new clinical interventions requiring ethical approval or patient consent.
References
- 1.Oikonomou E.K., Khera R. Artificial intelligence-enhanced patient evaluation: bridging art and science. Eur Heart J. 2024;45(35):3204–3218. doi: 10.1093/eurheartj/ehae415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thangaraj P.M., Benson S.H., Oikonomou E.K., Asselbergs F.W., Khera R. Cardiovascular care with digital twin technology in the era of generative artificial intelligence. Eur Heart J. 2024;45(45):4808–4821. doi: 10.1093/eurheartj/ehae619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khera R., Oikonomou E.K., Nadkarni G.N., et al. Transforming cardiovascular care with artificial intelligence: from discovery to practice: JACC state-of-the-art review. J Am Coll Cardiol. 2024;84(1):97–114. doi: 10.1016/j.jacc.2024.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee K.K., Ferry A.V., Anand A., et al. Sex-specific thresholds of high-sensitivity troponin in patients with suspected acute coronary syndrome. J Am Coll Cardiol. 2019;74(16):2032–2043. doi: 10.1016/j.jacc.2019.07.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thangaraj P.M., Khera R. Accelerating chest pain evaluation with machine learning. Eur Heart J Acute Cardiovasc Care. 2023;12(11):753–754. doi: 10.1093/ehjacc/zuad117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Than M.P., Pickering J.W., Sandoval Y., et al. Machine learning to predict the likelihood of acute myocardial infarction. Circulation. 2019;140(11):899–909. doi: 10.1161/CIRCULATIONAHA.119.041980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doudesis D., Lee K.K., Yang J., et al. Validation of the myocardial-ischaemic-injury-index machine learning algorithm to guide the diagnosis of myocardial infarction in a heterogenous population: a prespecified exploratory analysis. Lancet Digit Health. 2022;4(5):e300–e308. doi: 10.1016/S2589-7500(22)00025-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller-Hodges E., Anand A., Shah A.S.V., et al. High-sensitivity cardiac troponin and the risk stratification of patients with renal impairment presenting with suspected acute coronary syndrome. Circulation. 2018;137(5):425–435. doi: 10.1161/CIRCULATIONAHA.117.030320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doudesis D., Lee K.K., Boeddinghaus J., et al. Machine learning for diagnosis of myocardial infarction using cardiac troponin concentrations. Nat Med. 2023;29(5):1201–1210. doi: 10.1038/s41591-023-02325-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McLaren J., de Alencar J.N., Aslanger E.K., Meyers H.P., Smith S.W. From ST-segment elevation MI to occlusion MI: the new paradigm shift in acute myocardial infarction. JACC: Adv. 2024;3(11) doi: 10.1016/j.jacadv.2024.101314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herman R., Meyers H.P., Smith S.W., et al. International evaluation of an artificial intelligence-powered electrocardiogram model detecting acute coronary occlusion myocardial infarction. Eur Heart J Digit Health. 2024;5(2):123–133. doi: 10.1093/ehjdh/ztad074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Zaiti S.S., Martin-Gill C., Zègre-Hemsey J.K., et al. Machine learning for ECG diagnosis and risk stratification of occlusion myocardial infarction. Nat Med. 2023;29(7):1804–1813. doi: 10.1038/s41591-023-02396-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Q., Xie G., Tang C.X., et al. Development and validation of CCTA-based radiomics signature for predicting coronary plaques with rapid progression. Circ Cardiovasc Imaging. 2023;16(9) doi: 10.1161/CIRCIMAGING.123.015340. [DOI] [PubMed] [Google Scholar]
- 14.Oikonomou E.K., Marwan M., Desai M.Y., et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. Lancet. 2018;392(10151):929–939. doi: 10.1016/S0140-6736(18)31114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narula J., Stuckey T.D., Nakazawa G., et al. Prospective deep learning-based quantitative assessment of coronary plaque by computed tomography angiography compared with intravascular ultrasound: the REVEALPLAQUE study. Eur Heart J Cardiovasc Imaging. 2024;25(9):1287–1295. doi: 10.1093/ehjci/jeae115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang S., Kweon J., Roh J.H., et al. Deep learning segmentation of major vessels in X-ray coronary angiography. Sci Rep. 2019;9 doi: 10.1038/s41598-019-53254-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Westra J., Andersen B.K., Campo G., et al. Diagnostic performance of in-procedure angiography-derived quantitative flow reserve compared to pressure-derived fractional flow reserve: the FAVOR II Europe-Japan study. J Am Heart Assoc. 2018;7(14) doi: 10.1161/JAHA.118.009603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu S., Neleman T., Hartman E.M.J., et al. Automated quantitative assessment of coronary calcification using intravascular ultrasound. Ultrasound Med Biol. 2020;46(10):2801–2809. doi: 10.1016/j.ultrasmedbio.2020.04.032. [DOI] [PubMed] [Google Scholar]
- 19.Bae Y., Kang S.J., Kim G., et al. Prediction of coronary thin-cap fibroatheroma by intravascular ultrasound-based machine learning. Atherosclerosis. 2019;288:168–174. doi: 10.1016/j.atherosclerosis.2019.04.228. [DOI] [PubMed] [Google Scholar]
- 20.Jun T.J., Kang S.J., Lee J.G., et al. Automated detection of vulnerable plaque in intravascular ultrasound images. Med Biol Eng Comput. 2019;57(4):863–876. doi: 10.1007/s11517-018-1925-x. [DOI] [PubMed] [Google Scholar]
- 21.Chu M., Jia H., Gutiérrez-Chico J.L., et al. Artificial intelligence and optical coherence tomography for the automatic characterisation of human atherosclerotic plaques. EuroIntervention. 2021;17(1):41–50. doi: 10.4244/EIJ-D-20-01355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akbar A., Khwaja T.S., Javaid A., Kim J.S., Ha J. Automated accurate lumen segmentation using L-mode interpolation for three-dimensional intravascular optical coherence tomography. Biomed Opt Express. 2019;10(10):5325–5336. doi: 10.1364/BOE.10.005325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gessert N., Lutz M., Heyder M., et al. Automatic plaque detection in IVOCT pullbacks using convolutional neural networks. IEEE Trans Med Imaging. 2019;38(2):426–434. doi: 10.1109/TMI.2018.2865659. [DOI] [PubMed] [Google Scholar]
- 24.Lee J., Pereira G.T.R., Gharaibeh Y., et al. Automated analysis of fibrous cap in intravascular optical coherence tomography images of coronary arteries. Sci Rep. 2022;12 doi: 10.1038/s41598-022-24884-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu R., Zhang Y., Zheng Y., Liu Y., Zhao Y., Yi L. Automated detection of vulnerable plaque for intravascular optical coherence tomography images. Cardiovasc Eng Technol. 2019;10(4):590–603. doi: 10.1007/s13239-019-00425-2. [DOI] [PubMed] [Google Scholar]
- 26.Hong H., Jia H., Zeng M., et al. Risk stratification in acute coronary syndrome by comprehensive morphofunctional assessment with optical coherence tomography. JACC: Asia. 2022;2(4):460–472. doi: 10.1016/j.jacasi.2022.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ueda D., Yamamoto A., Ehara S., et al. Artificial intelligence-based detection of aortic stenosis from chest radiographs. Eur Heart J Digit Health. 2022;3(1):20–28. doi: 10.1093/ehjdh/ztab102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ueda D., Ehara S., Yamamoto A., et al. Development and validation of artificial intelligence-based method for diagnosis of mitral regurgitation from chest radiographs. Radiol Artif Intell. 2022;4(2) doi: 10.1148/ryai.210221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ulloa-Cerna A.E., Jing L., Pfeifer J.M., et al. rECHOmmend: an ECG-based machine learning approach for identifying patients at increased risk of undiagnosed structural heart disease detectable by echocardiography. Circulation. 2022;146(1):36–47. doi: 10.1161/CIRCULATIONAHA.121.057869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elias P., Poterucha T.J., Rajaram V., et al. Deep learning electrocardiographic analysis for detection of left-sided valvular heart disease. J Am Coll Cardiol. 2022;80(6):613–626. doi: 10.1016/j.jacc.2022.05.029. [DOI] [PubMed] [Google Scholar]
- 31.Kwon J.M., Lee S.Y., Jeon K.H., et al. Deep learning-based algorithm for detecting aortic stenosis using electrocardiography. J Am Heart Assoc. 2020;9(7) doi: 10.1161/JAHA.119.014717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen-Shelly M., Attia Z.I., Friedman P.A., et al. Electrocardiogram screening for aortic valve stenosis using artificial intelligence. Eur Heart J. 2021;42(30):2885–2896. doi: 10.1093/eurheartj/ehab153. [DOI] [PubMed] [Google Scholar]
- 33.Aminorroaya A., Dhingra L.S., Sangha V., et al. Deep learning-enabled detection of aortic stenosis from noisy single lead electrocardiograms. Preprint. Posted online October 2, 2023 doi: 10.1101/2023.09.29.23296310. MedRxiv 2023.09.29.23296310. [DOI] [Google Scholar]
- 34.Dhingra L.S., Aminorroaya A., Sangha V., et al. An ensemble deep learning algorithm for structural heart disease screening using electrocardiographic images: PRESENT SHD. Preprint. Posted online December 27, 2024 doi: 10.1101/2024.10.06.24314939. MedRxiv 2024.10.06.24314939. [DOI] [Google Scholar]
- 35.Aminorroaya A., Dhingra L.S., Pedroso Camargos A., et al. Development and multinational validation of an ensemble deep learning algorithm for detecting and predicting structural heart disease using noisy single-lead electrocardiograms. Preprint. Posted online October 8, 2024 doi: 10.1101/2024.10.07.24314974. MedRxiv 2024.10.07.24314974. [DOI] [Google Scholar]
- 36.Krishna H., Desai K., Slostad B., et al. Fully automated artificial intelligence assessment of aortic stenosis by echocardiography. J Am Soc Echocardiogr. 2023;36(7):769–777. doi: 10.1016/j.echo.2023.03.008. [DOI] [PubMed] [Google Scholar]
- 37.Nedadur R., Wang B., Tsang W. Artificial intelligence for the echocardiographic assessment of valvular heart disease. Heart. 2022;108(20):1592–1599. doi: 10.1136/heartjnl-2021-319725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vrudhula A., Duffy G., Vukadinovic M., Liang D., Cheng S., Ouyang D. High-throughput deep learning detection of mitral regurgitation. Circulation. 2024;150(12):923–933. doi: 10.1161/CIRCULATIONAHA.124.069047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holste G., Oikonomou E.K., Mortazavi B.J., et al. Severe aortic stenosis detection by deep learning applied to echocardiography. Eur Heart J. 2023;44(43):4592–4604. doi: 10.1093/eurheartj/ehad456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oikonomou E.K., Holste G., Yuan N., et al. A multimodal video-based AI biomarker for aortic stenosis development and progression. JAMA Cardiol. 2024;9(6):534–544. doi: 10.1001/jamacardio.2024.0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sahashi Y., Vukadinovic M., Duffy G., et al. Using deep learning to predict cardiovascular magnetic resonance findings from echocardiography videos. Preprint. Posted online April 19, 2024 doi: 10.1101/2024.04.16.24305936. medRxiv 2024.04.16.24305936. [DOI] [Google Scholar]
- 42.Yuan N., Kwan A.C., Duffy G., et al. Prediction of coronary artery calcium using deep learning of echocardiograms. J Am Soc Echocardiogr. 2023;36(5):474–481.e3. doi: 10.1016/j.echo.2022.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bezerra H.G., Costa M.A., Guagliumi G., Rollins A.M., Simon D.I. Intracoronary optical coherence tomography: a comprehensive review clinical and research applications. J Am Coll Cardiol Intv. 2009;2(11):1035–1046. doi: 10.1016/j.jcin.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Macedo M.M.G., Guimarães W.V.N., Galon M.Z., Takimura C.K., Lemos P.A., Gutierrez M.A. A bifurcation identifier for IV-OCT using orthogonal least squares and supervised machine learning. Comput Med Imaging Graph. 2015;46(2):237–248. doi: 10.1016/j.compmedimag.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 45.Shibutani H., Fujii K., Ueda D., et al. Automated classification of coronary atherosclerotic plaque in optical frequency domain imaging based on deep learning. Atherosclerosis. 2021;328:100–105. doi: 10.1016/j.atherosclerosis.2021.06.003. [DOI] [PubMed] [Google Scholar]
- 46.Wang A., Eggermont J., Reiber J.H.C., Dijkstra J. Fully automated side branch detection in intravascular optical coherence tomography pullback runs. Biomed Opt Express. 2014;5(9):3160–3173. doi: 10.1364/BOE.5.003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prati F., Di Vito L., Biondi-Zoccai G., et al. Angiography alone versus angiography plus optical coherence tomography to guide decision-making during percutaneous coronary intervention: the Centro per la Lotta contro l’Infarto-Optimisation of Percutaneous Coronary Intervention (CLI-OPCI) study. EuroIntervention. 2012;8(7):823–829. doi: 10.4244/EIJV8I7A125. [DOI] [PubMed] [Google Scholar]
- 48.Prati F., Romagnoli E., Burzotta F., et al. Clinical impact of OCT findings during PCI: the CLI-OPCI II study. J Am Coll Cardiol Img. 2015;8(11):1297–1305. doi: 10.1016/j.jcmg.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 49.Alsidawi S., Effat M., Rahman S., Abdallah M., Leesar M. The role of vascular imaging in guiding routine percutaneous coronary interventions: a meta-analysis of bare metal stent and drug-eluting stent trials. Cardiovasc Ther. 2015;33(6):360–366. doi: 10.1111/1755-5922.12160. [DOI] [PubMed] [Google Scholar]
- 50.Ughi G.J., Adriaenssens T., Onsea K., et al. Automatic segmentation of in-vivo intra-coronary optical coherence tomography images to assess stent strut apposition and coverage. Int J Cardiovasc Imaging. 2012;28(2):229–241. doi: 10.1007/s10554-011-9824-3. [DOI] [PubMed] [Google Scholar]
- 51.Yang G., Mehanna E., Li C., et al. Stent detection with very thick tissue coverage in intravascular OCT. Biomed Opt Express. 2021;12(12):7500–7516. doi: 10.1364/BOE.444336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van der Hoeven B.L., Liem S.S., Dijkstra J., et al. Stent malapposition after sirolimus-eluting and bare-metal stent implantation in patients with ST-segment elevation myocardial infarction: acute and 9-month intravascular ultrasound results of the MISSION! intervention study. J Am Coll Cardiol Intv. 2008;1(2):192–201. doi: 10.1016/j.jcin.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 53.Im E., Kim B.K., Ko Y.G., et al. Incidences, predictors, and clinical outcomes of acute and late stent malapposition detected by optical coherence tomography after drug-eluting stent implantation. Circ Cardiovasc Interv. 2014;7(1):88–96. doi: 10.1161/CIRCINTERVENTIONS.113.000797. [DOI] [PubMed] [Google Scholar]
- 54.Im E., Hong S.J., Ahn C.M., et al. Long-term clinical outcomes of late stent malapposition detected by optical coherence tomography after drug-eluting stent implantation. J Am Heart Assoc. 2019;8(7) doi: 10.1161/JAHA.118.011817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fujimura T., Matsumura M., Witzenbichler B., et al. Stent expansion indexes to predict clinical outcomes: an IVUS substudy from ADAPT-DES. J Am Coll Cardiol Intv. 2021;14(15):1639–1650. doi: 10.1016/j.jcin.2021.05.019. [DOI] [PubMed] [Google Scholar]
- 56.Kim J.H., Kang D.Y., Ahn J.M., et al. Optimal minimal stent area and impact of stent underexpansion in left main up-front 2-stent strategy. Circ Cardiovasc Interv. 2024;17(1) doi: 10.1161/CIRCINTERVENTIONS.123.013006. [DOI] [PubMed] [Google Scholar]
- 57.Wu P., Gutiérrez-Chico J.L., Tauzin H., et al. Automatic stent reconstruction in optical coherence tomography based on a deep convolutional model. Biomed Opt Express. 2020;11(6):3374–3394. doi: 10.1364/BOE.390113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu H., Lee J., Jakl M., et al. Application and evaluation of highly automated software for comprehensive stent analysis in intravascular optical coherence tomography. Sci Rep. 2020;10(1):2150. doi: 10.1038/s41598-020-59212-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bonnema G.T., Cardinal K.O., Williams S.K., Barton J.K. An automatic algorithm for detecting stent endothelialization from volumetric optical coherence tomography datasets. Phys Med Biol. 2008;53(12):3083–3098. doi: 10.1088/0031-9155/53/12/001. [DOI] [PubMed] [Google Scholar]
- 60.Nam H.S., Kim C.S., Lee J.J., Song J.W., Kim J.W., Yoo H. Automated detection of vessel lumen and stent struts in intravascular optical coherence tomography to evaluate stent apposition and neointimal coverage. Med Phys. 2016;43(4):1662. doi: 10.1118/1.4943374. [DOI] [PubMed] [Google Scholar]
- 61.Lu H., Gargesha M., Wang Z., et al. Automatic stent detection in intravascular OCT images using bagged decision trees. Biomed Opt Express. 2012;3(11):2809–2824. doi: 10.1364/BOE.3.002809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mandelias K., Tsantis S., Spiliopoulos S., et al. Automatic quantitative analysis of in-stent restenosis using FD-OCT in vivo intra-arterial imaging. Med Phys. 2013;40(6) doi: 10.1118/1.4803461. [DOI] [PubMed] [Google Scholar]
- 63.Wang A., Eggermont J., Dekker N., et al. Automatic stent strut detection in intravascular optical coherence tomographic pullback runs. Int J Cardiovasc Imaging. 2013;29(1):29–38. doi: 10.1007/s10554-012-0064-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gharaibeh Y., Lee J., Zimin V.N., et al. Prediction of stent under-expansion in calcified coronary arteries using machine learning on intravascular optical coherence tomography images. Sci Rep. 2023;13(1) doi: 10.1038/s41598-023-44610-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xia Q., Deng C., Yang S., et al. Machine learning constructed based on patient plaque and clinical features for predicting stent malapposition: a retrospective study. Clin Cardiol. 2024;47(8) doi: 10.1002/clc.24332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yoshinaga M., Muramatsu T., Higami H., Nasu K. Pre-procedural virtual reality guiding catheter simulation navigating successful percutaneous coronary intervention of a chronic total occlusion of an anomalous origin of the right coronary artery. Catheter Cardiovasc Interv. 2025;105(1):124–130. doi: 10.1002/ccd.31296. [DOI] [PubMed] [Google Scholar]
- 67.Thalappillil R., Datta P., Datta S., et al. Artificial intelligence for the measurement of the aortic valve annulus. J Cardiothorac Vasc Anesth. 2020;34(1):65–71. doi: 10.1053/j.jvca.2019.06.017. [DOI] [PubMed] [Google Scholar]
- 68.Zhang K., Gao Y., Lv J., Li J., Liu J. Artificial intelligence-based spiral CT 3D reconstruction in transcatheter aortic valve implantation. Comput Math Methods Med. 2022;2022 doi: 10.1155/2022/5794681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang M., Niu G., Chen Y., et al. Development and validation of a deep learning-based fully automated algorithm for pre-TAVR CT assessment of the aortic valvular complex and detection of anatomical risk factors: a retrospective, multicentre study. EBioMedicine. 2023;96 doi: 10.1016/j.ebiom.2023.104794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Toggweiler S., Wyler von Ballmoos M.C., Moccetti F., et al. A fully automated artificial intelligence-driven software for planning of transcatheter aortic valve replacement. Cardiovasc Revasc Med. 2024;65:25–31. doi: 10.1016/j.carrev.2024.03.008. [DOI] [PubMed] [Google Scholar]
- 71.Zlahoda-Huzior A., Stanuch M., Witowski J., Dudek D. Automatic aorta and left ventricle segmentation for TAVI procedure planning using convolutional neural networks. Annu Int Conf IEEE Eng Med Biol Soc. 2019;2019:2777–2780. doi: 10.1109/EMBC.2019.8857409. [DOI] [PubMed] [Google Scholar]
- 72.Theriault-Lauzier P., Alsosaimi H., Mousavi N., et al. Recursive multiresolution convolutional neural networks for 3D aortic valve annulus planimetry. Int J Comput Assist Radiol Surg. 2020;15(4):577–588. doi: 10.1007/s11548-020-02131-0. [DOI] [PubMed] [Google Scholar]
- 73.Krüger N., Meyer A., Tautz L., et al. Cascaded neural network-based CT image processing for aortic root analysis. Int J Comput Assist Radiol Surg. 2022;17(3):507–519. doi: 10.1007/s11548-021-02554-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Evertz R., Lange T., Backhaus S.J., et al. Artificial intelligence enabled fully automated CMR function quantification for optimized risk stratification in patients undergoing transcatheter aortic valve replacement. J Interv Cardiol. 2022;2022 doi: 10.1155/2022/1368878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saitta S., Sturla F., Gorla R., et al. A CT-based deep learning system for automatic assessment of aortic root morphology for TAVI planning. Comput Biol Med. 2023;163 doi: 10.1016/j.compbiomed.2023.107147. [DOI] [PubMed] [Google Scholar]
- 76.Boninsegna E., Piffer S., Simonini E., et al. CT angiography prior to endovascular procedures: can artificial intelligence improve reporting? Phys Eng Sci Med. 2024;47(2):643–649. doi: 10.1007/s13246-024-01393-1. [DOI] [PubMed] [Google Scholar]
- 77.Astudillo P., Mortier P., Bosmans J., et al. Enabling automated device size selection for transcatheter aortic valve implantation. J Interv Cardiol. 2019;2019 doi: 10.1155/2019/3591314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Willemink M.J., Noël P.B. The evolution of image reconstruction for CT-from filtered back projection to artificial intelligence. Eur Radiol. 2019;29(5):2185–2195. doi: 10.1007/s00330-018-5810-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang Y., Liu Z., Cheng Y., et al. Deep learning image reconstruction for transcatheter aortic valve implantation planning: image quality, diagnostic performance, contrast volume and radiation dose assessment. Acad Radiol. 2024;31(6):2268–2280. doi: 10.1016/j.acra.2024.02.026. [DOI] [PubMed] [Google Scholar]
- 80.Kojima T., Yamasaki Y., Matsuura Y., et al. The feasibility of deep learning–based reconstruction for low-tube-voltage CT angiography for transcatheter aortic valve implantation. J Comput Assist Tomogr. 2024;48(1):77–84. doi: 10.1097/RCT.0000000000001525. [DOI] [PubMed] [Google Scholar]
- 81.El Sabbagh A., Nishimura R.A. Clinical conundrum of coronary artery disease and aortic valve stenosis. J Am Heart Assoc. 2017;6(2) doi: 10.1161/JAHA.117.005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mehier B., Mahmoudi K., Veugeois A., et al. Diagnostic performance of deep learning to exclude coronary stenosis on CT angiography in TAVI patients. Int J Cardiovasc Imaging. 2024;40(5):981–990. doi: 10.1007/s10554-024-03063-5. [DOI] [PubMed] [Google Scholar]
- 83.Rajiah P., Cummings K.W., Williamson E., Young P.M. CT fractional flow reserve: a practical guide to application, interpretation, and problem solving. Radiographics. 2022;42(2):340–358. doi: 10.1148/rg.210097. [DOI] [PubMed] [Google Scholar]
- 84.Sharma P., Itu L., Zheng X., et al. A framework for personalization of coronary flow computations during rest and hyperemia. Annu Int Conf IEEE Eng Med Biol Soc. 2012;2012:6665–6668. doi: 10.1109/EMBC.2012.6347523. [DOI] [PubMed] [Google Scholar]
- 85.Tesche C., De Cecco C.N., Albrecht M.H., et al. Coronary CT angiography-derived fractional flow reserve. Radiology. 2017;285(1):17–33. doi: 10.1148/radiol.2017162641. [DOI] [PubMed] [Google Scholar]
- 86.Driessen R.S., Danad I., Stuijfzand W.J., et al. Comparison of coronary computed tomography angiography, fractional flow reserve, and perfusion imaging for ischemia diagnosis. J Am Coll Cardiol. 2019;73(2):161–173. doi: 10.1016/j.jacc.2018.10.056. [DOI] [PubMed] [Google Scholar]
- 87.Gohmann R.F., Pawelka K., Seitz P., et al. Combined cCTA and TAVR planning for ruling out significant CAD: added value of ML-based CT-FFR. J Am Coll Cardiol Img. 2022;15(3):476–486. doi: 10.1016/j.jcmg.2021.09.013. [DOI] [PubMed] [Google Scholar]
- 88.Brandt V., Schoepf U.J., Aquino G.J., et al. Impact of machine-learning-based coronary computed tomography angiography-derived fractional flow reserve on decision-making in patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur Radiol. 2022;32(9):6008–6016. doi: 10.1007/s00330-022-08758-8. [DOI] [PubMed] [Google Scholar]
- 89.Lecomte A., Serrand A., Marteau L., et al. Coronary artery assessment on pre transcatheter aortic valve implantation computed tomography may avoid the need for additional coronary angiography. Diagn Interv Imaging. 2023;104(11):547–551. doi: 10.1016/j.diii.2023.06.006. [DOI] [PubMed] [Google Scholar]
- 90.Brendel J.M., Walterspiel J., Hagen F., et al. Coronary artery disease evaluation during transcatheter aortic valve replacement work-up using photon-counting CT and artificial intelligence. Diagn Interv Imaging. 2024;105(7-8):273–280. doi: 10.1016/j.diii.2024.01.010. [DOI] [PubMed] [Google Scholar]
- 91.Jacquemyn X., Bamps K., Moermans R., et al. Augmented and virtual reality imaging for collaborative planning of structural cardiovascular interventions: a proof-of-concept and validation study. J Med Imaging (Bellingham) 2024;11(6) doi: 10.1117/1.JMI.11.6.062606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ma H., Smal I., Daemen J., van Walsum T.V. Dynamic coronary roadmapping via catheter tip tracking in X-ray fluoroscopy with deep learning based Bayesian filtering. Med Image Anal. 2020;61 doi: 10.1016/j.media.2020.101634. [DOI] [PubMed] [Google Scholar]
- 93.Yabe T., Muramatsu T., Tsukahara R., et al. The impact of percutaneous coronary intervention using the novel dynamic coronary roadmap system. Heart Vessels. 2020;35(3):323–330. doi: 10.1007/s00380-019-01502-1. [DOI] [PubMed] [Google Scholar]
- 94.Quast C., Phinicarides R., Afzal S., et al. Roadmap fusion imaging in percutaneous coronary intervention reduces contrast medium exposure irrespective of investigator’s experience level. J Invasive Cardiol. 2024;36(1) doi: 10.25270/jic/23.00203. [DOI] [PubMed] [Google Scholar]
- 95.Hennessey B., Danenberg H., De Vroey F., et al. Dynamic Coronary Roadmap versus standard angiography for percutaneous coronary intervention: the randomised, multicentre DCR4Contrast trial. EuroIntervention. 2024;20(3):e198–e206. doi: 10.4244/EIJ-D-23-00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hirano S., Yabe T., Oka Y., et al. Clinical outcomes of patients with chronic kidney disease undergoing percutaneous coronary interventions with a novel dynamic coronary roadmap system. Int Heart J. 2023;64(5):823–831. doi: 10.1536/ihj.23-213. [DOI] [PubMed] [Google Scholar]
- 97.Ghoshhajra B.B., Takx R.A.P., Stone L.L., et al. Real-time fusion of coronary CT angiography with x-ray fluoroscopy during chronic total occlusion PCI. Eur Radiol. 2017;27(6):2464–2473. doi: 10.1007/s00330-016-4599-5. [DOI] [PubMed] [Google Scholar]
- 98.Hahn R.T., Little S.H., Monaghan M.J., et al. Recommendations for comprehensive intraprocedural echocardiographic imaging during TAVR. J Am Coll Cardiol Img. 2015;8(3):261–287. doi: 10.1016/j.jcmg.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 99.Grau V., Becher H., Noble J.A. Registration of multiview real-time 3-D echocardiographic sequences. IEEE Trans Med Imaging. 2007;26(9):1154–1165. doi: 10.1109/TMI.2007.903568. [DOI] [PubMed] [Google Scholar]
- 100.Khalil A., Faisal A., Lai K.W., Ng S.C., Liew Y.M. 2D to 3D fusion of echocardiography and cardiac CT for TAVR and TAVI image guidance. Med Biol Eng Comput. 2017;55(8):1317–1326. doi: 10.1007/s11517-016-1594-6. [DOI] [PubMed] [Google Scholar]
- 101.Huang X., Ren J., Guiraudon G., Boughner D., Peters T.M. Rapid dynamic image registration of the beating heart for diagnosis and surgical navigation. IEEE Trans Med Imaging. 2009;28(11):1802–1814. doi: 10.1109/TMI.2009.2024684. [DOI] [PubMed] [Google Scholar]
- 102.Huang X., Moore J., Guiraudon G., et al. Dynamic 2D ultrasound and 3D CT image registration of the beating heart. IEEE Trans Med Imaging. 2009;28(8):1179–1189. doi: 10.1109/TMI.2008.2011557. [DOI] [PubMed] [Google Scholar]
- 103.Li F.P., Rajchl M., White J.A., Goela A., Peters T.M. Ultrasound guidance for beating heart mitral valve repair augmented by synthetic dynamic CT. IEEE Trans Med Imaging. 2015;34(10):2025–2035. doi: 10.1109/TMI.2015.2412465. [DOI] [PubMed] [Google Scholar]
- 104.Dabiri Y., Mahadevan V.S., Guccione J.M., Kassab G.S. A simulation study of the effects of number and location of MitraClips on mitral regurgitation. JACC: Adv. 2022;1(1) doi: 10.1016/j.jacadv.2022.100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Inohara T., Kohsaka S., Yamaji K., et al. Risk stratification model for in-hospital death in patients undergoing percutaneous coronary intervention: a nationwide retrospective cohort study in Japan. BMJ Open. 2019;9(5) doi: 10.1136/bmjopen-2018-026683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Castro-Dominguez Y.S., Wang Y., Minges K.E., et al. Predicting in-hospital mortality in patients undergoing percutaneous coronary intervention. J Am Coll Cardiol. 2021;78(3):216–229. doi: 10.1016/j.jacc.2021.04.067. [DOI] [PubMed] [Google Scholar]
- 107.Al’Aref S.J., Singh G., van Rosendael A.R., et al. Determinants of in-hospital mortality after percutaneous coronary intervention: a machine learning approach. J Am Heart Assoc. 2019;8(5) doi: 10.1161/JAHA.118.011160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kulkarni H., Amin A.P. Artificial intelligence in percutaneous coronary intervention: improved risk prediction of PCI-related complications using an artificial neural network. BMJ Innov. 2021;7(3):564–579. [Google Scholar]
- 109.Singh M., Gulati R., Lewis B.R., et al. Multimorbidity and mortality models to predict complications following percutaneous coronary interventions. Circ Cardiovasc Interv. 2022;15(7) doi: 10.1161/CIRCINTERVENTIONS.121.011540. [DOI] [PubMed] [Google Scholar]
- 110.Hamilton D.E., Albright J., Seth M., et al. Merging machine learning and patient preference: a novel tool for risk prediction of percutaneous coronary interventions. Eur Heart J. 2024;45(8):601–609. doi: 10.1093/eurheartj/ehad836. [DOI] [PubMed] [Google Scholar]
- 111.Niimi N., Shiraishi Y., Sawano M., et al. Machine learning models for prediction of adverse events after percutaneous coronary intervention. Sci Rep. 2022;12:6262. doi: 10.1038/s41598-022-10346-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zack C.J., Senecal C., Kinar Y., et al. Leveraging machine learning techniques to forecast patient prognosis after percutaneous coronary intervention. J Am Coll Cardiol Intv. 2019;12(14):1304–1311. doi: 10.1016/j.jcin.2019.02.035. [DOI] [PubMed] [Google Scholar]
- 113.McAllister K.S.L., Ludman P.F., Hulme W., et al. A contemporary risk model for predicting 30-day mortality following percutaneous coronary intervention in England and Wales. Int J Cardiol. 2016;210:125–132. doi: 10.1016/j.ijcard.2016.02.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Doll J.A., O’Donnell C.I., Plomondon M.E., Waldo S.W. Contemporary clinical and coronary anatomic risk model for 30-day mortality after percutaneous coronary intervention. Circ Cardiovasc Interv. 2021;14(12) doi: 10.1161/CIRCINTERVENTIONS.121.010863. [DOI] [PubMed] [Google Scholar]
- 115.Tacey M., Dinh D.T., Andrianopoulos N., et al. Risk-adjusting key outcome measures in a clinical quality PCI registry: development of a highly predictive model without the need to exclude high-risk conditions. J Am Coll Cardiol Intv. 2019;12(19):1966–1975. doi: 10.1016/j.jcin.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 116.Hosseini K., Behnoush A.H., Khalaji A., et al. Machine learning prediction of one-year mortality after percutaneous coronary intervention in acute coronary syndrome patients. Int J Cardiol. 2024;409 doi: 10.1016/j.ijcard.2024.132191. [DOI] [PubMed] [Google Scholar]
- 117.Ngew K.Y., Tay H.Z., Yusof A.K.M. Development and validation of a predictive models for predicting the cardiac events within one year for patients underwent percutaneous coronary intervention procedure at IJN. BMC Cardiovasc Disord. 2023;23(1):545. doi: 10.1186/s12872-023-03536-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mamas M.A., Roffi M., Fröbert O., et al. Predicting target lesion failure following percutaneous coronary intervention through machine learning risk assessment models. Eur Heart J Digit Health. 2023;4(6):433–443. doi: 10.1093/ehjdh/ztad051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mortazavi B.J., Bucholz E.M., Desai N.R., et al. Comparison of machine learning methods with National Cardiovascular Data Registry models for prediction of risk of bleeding after percutaneous coronary intervention. JAMA Netw Open. 2019;2(7) doi: 10.1001/jamanetworkopen.2019.6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Galimzhanov A., Matetic A., Tenekecioglu E., Mamas M.A. Prediction of clinical outcomes after percutaneous coronary intervention: machine-learning analysis of the National Inpatient Sample. Int J Cardiol. 2023;392 doi: 10.1016/j.ijcard.2023.131339. [DOI] [PubMed] [Google Scholar]
- 121.Behnoush A.H., Shariatnia M.M., Khalaji A., et al. Predictive modeling for acute kidney injury after percutaneous coronary intervention in patients with acute coronary syndrome: a machine learning approach. Eur J Med Res. 2024;29(1):76. doi: 10.1186/s40001-024-01675-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Huang C., Murugiah K., Mahajan S., et al. Enhancing the prediction of acute kidney injury risk after percutaneous coronary intervention using machine learning techniques: a retrospective cohort study. PLOS Med. 2018;15(11) doi: 10.1371/journal.pmed.1002703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hernandez-Suarez D.F., Kim Y., Villablanca P., et al. Machine learning prediction models for in-hospital mortality after transcatheter aortic valve replacement. J Am Coll Cardiol Intv. 2019;12(14):1328–1338. doi: 10.1016/j.jcin.2019.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Russo M.J., Elmariah S., Kaneko T., et al. Machine learning identification of modifiable predictors of patient outcomes after transcatheter aortic valve replacement. JACC: Adv. 2024;3(8) doi: 10.1016/j.jacadv.2024.101116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Agasthi P., Ashraf H., Pujari S.H., et al. Artificial intelligence trumps TAVI2-SCORE and CoreValve Score in predicting 1-year mortality post-transcatheter aortic valve replacement. Cardiovasc Revasc Med. 2021;24:33–41. doi: 10.1016/j.carrev.2020.08.010. [DOI] [PubMed] [Google Scholar]
- 126.Zisiopoulou M., Berkowitsch A., Redlich L., Walther T., Fichtlscherer S., Leistner D.M. Personalised preinterventional risk stratification of mortality, length of stay and hospitalisation costs in transcatheter aortic valve implantation using a machine learning algorithm: a pilot trial. Open Heart. 2024;11(1) doi: 10.1136/openhrt-2023-002540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Truong V.T., Beyerbach D., Mazur W., et al. Machine learning method for predicting pacemaker implantation following transcatheter aortic valve replacement. Pacing Clin Electrophysiol. 2021;44(2):334–340. doi: 10.1111/pace.14163. [DOI] [PubMed] [Google Scholar]
- 128.Tsushima T., Al-Kindi S., Nadeem F., et al. Machine learning algorithms for prediction of permanent pacemaker implantation after transcatheter aortic valve replacement. Circ Arrhythm Electrophysiol. 2021;14(3) doi: 10.1161/CIRCEP.120.008941. [DOI] [PubMed] [Google Scholar]
- 129.Agasthi P., Ashraf H., Pujari S.H., et al. Prediction of permanent pacemaker implantation after transcatheter aortic valve replacement: the role of machine learning. World J Cardiol. 2023;15(3):95–105. doi: 10.4330/wjc.v15.i3.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bansal A., Jaber W., Krishnaswamy A., et al. Predicting infective endocarditis after transcatheter aortic valve implantation via a risk model. Am J Cardiol. 2021;150:131–132. doi: 10.1016/j.amjcard.2021.04.007. [DOI] [PubMed] [Google Scholar]
- 131.Wang Z.H., Lahoti G., Wang K., et al. 2018 IEEE 15th International Symposium on Biomedical Imaging (ISBI 2018) April 4-7, 2018. Prediction of paravalvular leak post transcatheter aortic valve replacement using a convolutional neural network. Paper presented at. Washington, DC, USA. [DOI] [Google Scholar]
- 132.Theis M., Block W., Luetkens J.A., Attenberger U.I., Nowak S., Sprinkart A.M. Direct deep learning-based survival prediction from pre-interventional CT prior to transcatheter aortic valve replacement. Eur J Radiol. 2023;168 doi: 10.1016/j.ejrad.2023.111150. [DOI] [PubMed] [Google Scholar]
- 133.Okuno T., Overtchouk P., Asami M., et al. Deep learning-based prediction of early cerebrovascular events after transcatheter aortic valve replacement. Sci Rep. 2021;11(1) doi: 10.1038/s41598-021-98265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Brahier M.S., Kochi S., Huang J., et al. Machine learning of cardiac anatomy and the risk of new-onset atrial fibrillation after TAVR. J Am Coll Cardiol EP. 2024;10(8):1873–1884. doi: 10.1016/j.jacep.2024.04.006. [DOI] [PubMed] [Google Scholar]
- 135.Cruz E.O., Sakowitz S., Mallick S., et al. Application of machine learning to predict in-hospital mortality after transcatheter mitral valve repair. Surgery. 2024;176(5):1442–1449. doi: 10.1016/j.surg.2024.07.011. [DOI] [PubMed] [Google Scholar]
- 136.Hernandez-Suarez D.F., Ranka S., Kim Y., et al. Machine-learning-based in-hospital mortality prediction for transcatheter mitral valve repair in the United States. Cardiovasc Revasc Med. 2021;22:22–28. doi: 10.1016/j.carrev.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hausleiter J., Lachmann M., Stolz L., et al. Artificial intelligence-derived risk score for mortality in secondary mitral regurgitation treated by transcatheter edge-to-edge repair: the EuroSMR risk score. Eur Heart J. 2024;45(11):922–936. doi: 10.1093/eurheartj/ehad871. [DOI] [PubMed] [Google Scholar]
- 138.Sulaiman S., Kawsara A., El Sabbagh A., et al. Machine learning vs. conventional methods for prediction of 30-day readmission following percutaneous mitral edge-to-edge repair. Cardiovasc Revasc Med. 2023;56:18–24. doi: 10.1016/j.carrev.2023.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zweck E., Spieker M., Horn P., et al. Machine learning identifies clinical parameters to predict mortality in patients undergoing transcatheter mitral valve repair. J Am Coll Cardiol Intv. 2021;14(18):2027–2036. doi: 10.1016/j.jcin.2021.06.039. [DOI] [PubMed] [Google Scholar]
- 140.Lachmann M., Rippen E., Schuster T., et al. Artificial intelligence-enabled phenotyping of patients with severe aortic stenosis: on the recovery of extra-aortic valve cardiac damage after transcatheter aortic valve replacement. Open Heart. 2022;9(2) doi: 10.1136/openhrt-2022-002068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lachmann M., Rippen E., Schuster T., et al. Subphenotyping of patients with aortic stenosis by unsupervised agglomerative clustering of echocardiographic and hemodynamic data. J Am Coll Cardiol Intv. 2021;14(19):2127–2140. doi: 10.1016/j.jcin.2021.08.034. [DOI] [PubMed] [Google Scholar]
- 142.Abdul Ghffar Y., Osman M., Shrestha S., et al. Usefulness of semisupervised machine-learning-based phenogrouping to improve risk assessment for patients undergoing transcatheter aortic valve implantation. Am J Cardiol. 2020;136:122–130. doi: 10.1016/j.amjcard.2020.08.048. [DOI] [PubMed] [Google Scholar]
- 143.Trenkwalder T., Lachmann M., Stolz L., et al. Machine learning identifies pathophysiologically and prognostically informative phenotypes among patients with mitral regurgitation undergoing transcatheter edge-to-edge repair. Eur Heart J Cardiovasc Imaging. 2023;24(5):574–587. doi: 10.1093/ehjci/jead013. [DOI] [PubMed] [Google Scholar]
- 144.Galimzhanov A., Sabitov Y., Guclu E., Tenekecioglu E., Mamas M.A. Phenotyping for percutaneous coronary intervention and long-term recurrent weighted outcomes. Int J Cardiol. 2023;374:12–19. doi: 10.1016/j.ijcard.2022.12.035. [DOI] [PubMed] [Google Scholar]
- 145.Baladrón C., Amat-Santos I.J., San Román A. Machine learning is no magic: put a rabbit into the hat before pulling it out. J Am Coll Cardiol Intv. 2019;12(20):2112–2113. doi: 10.1016/j.jcin.2019.08.018. [DOI] [PubMed] [Google Scholar]
- 146.Boulares M., Alotaibi R., AlMansour A., Barnawi A. Cardiovascular disease recognition based on heartbeat segmentation and selection process. Int J Environ Res Public Health. 2021;18(20) doi: 10.3390/ijerph182010952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Xu C., Li X., Zhang X., et al. Cardiac murmur grading and risk analysis of cardiac diseases based on adaptable heterogeneous-modality multi-task learning. Health Inf Sci Syst. 2024;12(1):2. doi: 10.1007/s13755-023-00249-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Netto A.N., Abraham L., Philip S. HBNET: a blended ensemble model for the detection of cardiovascular anomalies using phonocardiogram. Technol Health Care. 2024;32(3):1925–1945. doi: 10.3233/THC-231290. [DOI] [PubMed] [Google Scholar]
- 149.Al-Issa Y., Alqudah A.M. A lightweight hybrid deep learning system for cardiac valvular disease classification. Sci Rep. 2022;12 doi: 10.1038/s41598-022-18293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jamil S., Roy A.M. An efficient and robust Phonocardiography (PCG)-based Valvular Heart Diseases (VHD) detection framework using Vision Transformer (ViT) Comput Biol Med. 2023;158 doi: 10.1016/j.compbiomed.2023.106734. [DOI] [PubMed] [Google Scholar]
- 151.Pathak A., Mandana K., Saha G. Ensembled transfer learning and multiple kernel learning for phonocardiogram based atherosclerotic coronary artery disease detection. IEEE J Biomed Health Inform. 2022;26(6):2804–2813. doi: 10.1109/JBHI.2022.3140277. [DOI] [PubMed] [Google Scholar]
- 152.Yin C., Zheng Y., Ding X., Shi Y., Qin J., Guo X. Detection of coronary artery disease based on clinical phonocardiogram and multiscale attention convolutional compression network. IEEE J Biomed Health Inform. 2024;28(3):1353–1362. doi: 10.1109/JBHI.2024.3354832. [DOI] [PubMed] [Google Scholar]
- 153.Li H., Wang X., Liu C., Li P., Jiao Y. Integrating multi-domain deep features of electrocardiogram and phonocardiogram for coronary artery disease detection. Comput Biol Med. 2021;138 doi: 10.1016/j.compbiomed.2021.104914. [DOI] [PubMed] [Google Scholar]
- 154.Sangha V., Oikonomou E.K., Khunte A., Gupta K., Mortazavi B., Khera R. Smart-as: a novel artificial intelligence tool to detect severe aortic stenosis from electrocardiographic images. J Am Coll Cardiol. 2023;81(8):2409. (suppl) [Google Scholar]
- 155.Aminorroaya A., Dhingra L.S., Camargos A.P., et al. Study protocol for the artificial intelligence-driven evaluation of structural heart diseases using wearable electrocardiogram (ID-SHD) Preprint. Posted online March 19. 2024 doi: 10.1101/2024.03.18.24304477. medRxiv 2024.03.18.24304477. [DOI] [Google Scholar]
- 156.Oikonomou E.K., Holste G., Coppi A., et al. Artificial intelligence-guided detection of under-recognized cardiomyopathies on point-of-care cardiac ultrasound: a multi-center study. Preprint. Posted online June 29. 2024 doi: 10.1101/2024.03.10.24304044. MedRxiv 2024.03.10.24304044. [DOI] [Google Scholar]
- 157.Huang W., Koh T., Tromp J., et al. Point-of-care AI-enhanced novice echocardiography for screening heart failure (PANES-HF) Sci Rep. 2024;14 doi: 10.1038/s41598-024-62467-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kim S., Fischetti C., Guy M., Hsu E., Fox J., Young S.D. Artificial intelligence (AI) applications for point of care ultrasound (POCUS) in low-resource settings: a scoping review. Diagnostics (Basel) 2024;14(15):1669. doi: 10.3390/diagnostics14151669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tsampasian V., Grafton-Clarke C., Gracia Ramos A.E., et al. Management of asymptomatic severe aortic stenosis: a systematic review and meta-analysis. Open Heart. 2022;9(1) doi: 10.1136/openhrt-2022-001982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Frey N., Steeds R.P., Rudolph T.K., et al. Symptoms, disease severity and treatment of adults with a new diagnosis of severe aortic stenosis. Heart. 2019;105(22):1709–1716. doi: 10.1136/heartjnl-2019-314940. [DOI] [PubMed] [Google Scholar]
- 161.Coisne A., Montaigne D., Aghezzaf S., et al. Association of mortality with aortic stenosis severity in outpatients: results from the VALVENOR study. JAMA Cardiol. 2021;6(12):1424–1431. doi: 10.1001/jamacardio.2021.3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Collins G.S., Moons K.G.M., Dhiman P., et al. TRIPOD+AI statement: updated guidance for reporting clinical prediction models that use regression or machine learning methods. BMJ. 2024;385 doi: 10.1136/bmj-2023-078378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Norgeot B., Quer G., Beaulieu-Jones B.K., et al. Minimum information about clinical artificial intelligence modeling: the MI-CLAIM checklist. Nat Med. 2020;26(9):1320–1324. doi: 10.1038/s41591-020-1041-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sounderajah V., Ashrafian H., Aggarwal R., et al. Developing specific reporting guidelines for diagnostic accuracy studies assessing AI interventions: the STARD-AI Steering Group. Nat Med. 2020;26(6):807–808. doi: 10.1038/s41591-020-0941-1. [DOI] [PubMed] [Google Scholar]
- 165.Liu X., Cruz Rivera S., Moher D., Calvert M.J., Denniston A.K. SPIRIT-AI and CONSORT-AI Working Group. Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI extension. Nat Med. 2020;26(9):1364–1374. doi: 10.1038/s41591-020-1034-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Vasey B., Nagendran M., Campbell B., et al. Reporting guideline for the early-stage clinical evaluation of decision support systems driven by artificial intelligence: DECIDE-AI. Nat Med. 2022;28(5):924–933. doi: 10.1038/s41591-022-01772-9. [DOI] [PubMed] [Google Scholar]
- 167.Khera R., Butte A.J., Berkwits M., et al. AI in medicine-JAMA’s focus on clinical outcomes, patient-centered care, quality, and equity. JAMA. 2023;330(9):818–820. doi: 10.1001/jama.2023.15481. [DOI] [PubMed] [Google Scholar]
- 168.Flanagin A., Pirracchio R., Khera R., Berkwits M., Hswen Y., Bibbins-Domingo K. Reporting use of AI in research and scholarly publication-JAMA network guidance. JAMA. 2024;331(13):1096–1098. doi: 10.1001/jama.2024.3471. [DOI] [PubMed] [Google Scholar]
- 169.Collins G.S., Dhiman P., Andaur Navarro C.L., et al. Protocol for development of a reporting guideline (TRIPOD-AI) and risk of bias tool (PROBAST-AI) for diagnostic and prognostic prediction model studies based on artificial intelligence. BMJ Open. 2021;11(7) doi: 10.1136/bmjopen-2020-048008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Attia I.Z., Tseng A.S., Benavente E.D., et al. External validation of a deep learning electrocardiogram algorithm to detect ventricular dysfunction. Int J Cardiol. 2021;329:130–135. doi: 10.1016/j.ijcard.2020.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Obermeyer Z., Emanuel E.J. Predicting the future - big data, machine learning, and clinical medicine. N Engl J Med. 2016;375(13):1216–1219. doi: 10.1056/NEJMp1606181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Aminorroaya A., Dhingra L.S., Oikonomou E.K., et al. Development and multinational validation of an algorithmic strategy for high Lp(a) screening. Nat Cardiovasc Res. 2024;3(5):558–566. doi: 10.1038/s44161-024-00469-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Merkel D. Docker: lightweight Linux containers for consistent development and deployment. Linux J. 2014;2014(239):2. [Google Scholar]
- 174.Kaur D., Hughes J.W., Rogers A.J., et al. Race, sex, and age disparities in the performance of ECG deep learning models predicting heart failure. Circ Heart Fail. 2024;17(1) doi: 10.1161/CIRCHEARTFAILURE.123.010879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yang Y., Zhang H., Gichoya J.W., Katabi D., Ghassemi M. The limits of fair medical imaging AI in real-world generalization. Nat Med. 2024;30(10):2838–2848. doi: 10.1038/s41591-024-03113-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Khera R., Simon M.A., Ross J.S. Automation bias and assistive AI: risk of harm from AI-driven clinical decision support. JAMA. 2023;330(23):2255–2257. doi: 10.1001/jama.2023.22557. [DOI] [PubMed] [Google Scholar]
- 177.Sangha V., Nargesi A.A., Dhingra L.S., et al. Detection of left ventricular systolic dysfunction from electrocardiographic images. Circulation. 2023;148(9):765–777. doi: 10.1161/CIRCULATIONAHA.122.062646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Attia Z.I., Kapa S., Lopez-Jimenez F., et al. Screening for cardiac contractile dysfunction using an artificial intelligence-enabled electrocardiogram. Nat Med. 2019;25(1):70–74. doi: 10.1038/s41591-018-0240-2. [DOI] [PubMed] [Google Scholar]
- 179.Shankar S.V., Oikonomou E.K., Khera R. CarDS-Plus ECG platform: development and feasibility evaluation of a multiplatform artificial intelligence toolkit for portable and wearable device electrocardiograms. Preprint. Posted October 3, 2023 doi: 10.1101/2023.10.02.23296404. medRxiv 2023.10.02.23296404. [DOI] [Google Scholar]
- 180.Bajwa J., Munir U., Nori A., Williams B. Artificial intelligence in healthcare: transforming the practice of medicine. Future Healthc J. 2021;8(2):e188–e194. doi: 10.7861/fhj.2021-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Topol E.J. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. 2019;25(1):44–56. doi: 10.1038/s41591-018-0300-7. [DOI] [PubMed] [Google Scholar]
- 182.Artificial Intelligence and Machine Learning (AI/ML)-Enabled Medical Devices. U.S. Food and Drug Administration (FDA) 2024. https://www.fda.gov/medical-devices/software-medical-device-samd/artificial-intelligence-and-machine-learning-aiml-enabled-medical-devices
- 183.U.S. Food and Drug Administration (FDA) 510(k) clearance for FFRangio System. 2019. https://www.accessdata.fda.gov/cdrh_docs/pdf19/K192442.pdf
- 184.U.S. Food and Drug Administration (FDA) 510(k) clearance for FFRangio TM System. 2018. https://www.accessdata.fda.gov/cdrh_docs/pdf18/K182149.pdf
- 185.U.S. Food and Drug Administration (FDA) Device classification under section 513(f)(2)(de novo) for FEops HEARTguide. 2021. https://www.accessdata.fda.gov/cdrh_docs/reviews/DEN200030.pdf
- 186.Al Nooryani A., Aboushokka W. Rotate-on-Retract procedural automation for robotic-assisted percutaneous coronary intervention: first clinical experience. Case Rep Cardiol. 2018;2018 doi: 10.1155/2018/6086034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.U.S. Food and Drug Administration (FDA) 510(k) clearance for technIQ Smart Procedural Automation Series for Corindus’ CorPath GRX System. 2020. https://www.accessdata.fda.gov/cdrh_docs/pdf20/K202275.pdf