Abstract
Toll-like receptors (TLRs) are important signal transducers that mediate inflammatory reactions induced by microbes through pattern recognition of virulence molecules such as lipopolysaccharide (LPS) and lipoproteins. We investigated whether proinflammatory cytokine responses induced by certain bacterial protein adhesins may also depend on TLRs. In differentiated THP-1 mononuclear cells stimulated by LPS-free recombinant fimbrillin (rFimA) from Porphyromonas gingivalis, cytokine release was abrogated by monoclonal antibodies (MAbs) to CD14 and TLR4 but not to TLR2. Similar experiments using anti-β2 integrin MAbs suggested that β2 integrins (CD11/CD18) also play a role in cytokine induction by rFimA or native fimbriae. Minor fimbriae (distinct from the fimA-encoded major fimbriae) of P. gingivalis induced proinflammatory cytokine release in a CD14- and TLR2-dependent mode. Cytokine induction by BspA, a leucine-rich repeat protein from Bacteroides forsythus, depended heavily on CD14 and TLR2. We also found that the ability of the streptococcal protein AgI/II to stimulate cytokine release depended partially on CD14 and TLR4, and the AgI/II segment that possibly interacts with these receptors was identified as its N-terminal saliva-binding region. When THP-1 cells were exposed to rFimA for 24 h, surface expression of CD14 and CD18 was decreased and the cells became hyporesponsive to cytokine induction by a second challenge with rFimA. However, tolerance induction was abolished when the THP-1 cells were pretreated with rFimA in the presence of either anti-CD14 MAb or anti-TLR4 MAb. Induction of cross-tolerance between rFimA and LPS correlated with downregulation of the pattern recognition receptors involved. Our data suggest that the CD14-TLR2/4 system is involved in cytokine production and tolerance induction upon interaction with certain proinflammatory bacterial protein adhesins.
Detection of potential pathogens by a nonimmune host has been found to involve recognition of molecular patterns presented on bacterial surfaces by such molecules as lipopolysaccharide (LPS) and peptidoglycan (29, 31). Such pathogen-associated molecular patterns are recognized by germ line-encoded pattern recognition receptors (PRRs) which mediate signals resulting in expression of immunoregulatory genes with the potential to contain the infection and to initiate adaptive immune responses (29, 31). Toll-like receptors (TLRs) have recently been identified as important signal-transducing elements of receptor complexes that alert the host to microbial infection through pattern recognition of chemically diverse microbial products (30). TLRs consist of an extracellular domain which contains leucine-rich repeats (LRRs) and a cytoplasmic domain which is similar to the intracellular portion of the interleukin-1 (IL-1) receptor (9, 21). TLR4 is the signaling receptor linking LPS-CD14 interactions to NF-κB translocation and induction of proinflammatory cytokines (14, 16). TLR2 appears to interact with CD14 in a comparable way, although its agonists are quite distinct from those of TLR4 and include peptidoglycan, lipoteichoic acid, and lipoproteins (25, 45).
It might also be possible that PRRs recognize molecular patterns present on bacterial proteins that can cause tissue damage comparable to that effected by LPS. Examples of such proteins might include fimbriae and flagella which consist of repeated protein subunits or even cell surface proteins that form an array or display a repeated molecular pattern. Although bacterial proteins can readily mutate to avoid immune recognition, molecular motifs that are critical for survival of the pathogen should be relatively invariant (and thus potentially recognizable by germ line-encoded receptors such as TLRs) despite negative selective pressure by the innate immune system. The main objective of this study was to determine whether TLRs can also respond to bacterial virulence proteins.
Fimbriae of Porphyromonas gingivalis have been shown to play an indispensable role in the ability of this oral pathogen to colonize and invade periodontal tissue as well as induce inflammatory bone resorption (20, 26, 33). The functional versatility of fimbriae arises from their ability to interact with various dental or epithelial substrates and extracellular molecules, such as fibrinogen, fibronectin, and lactoferrin (summarized in reference 23). Native fimbriae, derived synthetic peptides, and recombinant fimbrillin (rFimA), the 41-kDa structural subunit of fimbriae, can induce the expression of proinflammatory cytokines such as tumor necrosis factor α (TNF-α) and IL-1β in human and mouse monocytes/macrophages (37, 43). It has been shown that β2 integrins (CD11/CD18) serve as a cellular receptor for fimbria-induced cytokine responses in mouse macrophages (49). The cytoplasmic domains of CD11/CD18, though necessary for transducing phagocytic signals, are not required for NF-κB activation upon stimulation with LPS (17), suggesting that CD11/CD18 is associated with a distinct signal transducer (18). It is not known whether the role of CD11/CD18 in cytokine responses is analogous to that proposed for CD14, which recruits ligands to TLRs (29, 31), and thus whether cytokine induction by fimbriae binding to CD11/CD18 utilizes a downstream signal transducer distinct from the cytoplasmic tails of either CD11 or CD18. Evidence that CD14 interacts with CD11/CD18 (40, 52, 53) and the possibility that the two receptors may share a common signal-transducing system (18, 40) prompted us to investigate whether fimbria-induced cytokine responses depend on a TLR. Inactivation of the fimA gene of P. gingivalis facilitated the discovery of antigenically distinct shorter fimbriae, composed of 67-kDa protein subunits (12). These were termed minor fimbriae to distinguish them from the major fimbriae encoded by the fimA gene (12), and in this study they were compared to the major fimbriae with regard to proinflammatory potential and interactions with the CD14/TLR receptor system.
In addition to these P. gingivalis proteins, we tested proinflammatory proteins from other oral pathogens for possible dependence on TLRs for cytokine induction. BspA is a 98-kDa surface protein adhesin mediating the binding of Bacteroides forsythus to fibronectin and fibrinogen (47). Like CD14 and TLRs (21), BspA is a LRR protein containing fourteen 23-residue LRR motifs (47). LRRs are believed to represent versatile binding motifs involved in protein-protein and protein-lipid interactions, probably due to the resulting amphipathic structures that promote strong hydrophobic interactions (21, 22). To the extent that bacterial LRRs may represent a recognizable pattern, it could be possible that BspA might interact with PRRs. The Streptococcus mutans AgI/II adhesin (167 kDa) contains an N-terminal alanine-rich repeat segment that was termed the saliva-binding region (SBR) due to its ability to bind a salivary receptor (5, 11). AgI/II can also bind to extracellular matrix proteins, such as collagen type I, laminin, and fibronectin (46). This ability of bacterial adhesins to bind diverse host molecules suggests that they might contain versatile structural motifs rather than multiple adhesion epitopes specific for each receptor. Such multifunctional adhesive ability with the potential for alternative colonization possibilities might in turn offer pattern recognition substrates for the innate immune system which could thereby control the colonization of the pathogen. In this report, we investigated whether TLRs and other PRRs on human mononuclear THP-1 cells are involved in proinflammatory cytokine induction by virulence protein adhesins from several pathogens.
MATERIALS AND METHODS
Bacterial molecules.
FimA was expressed and purified from Escherichia coli BL21(DE3) (Novagen, Madison, Wis.) transformed with the fimA gene of P. gingivalis 381 as previously described (1). After size exclusion and anion-exchange chromatographic purification, fractions that contained rFimA but were negative for contaminating LPS, as judged by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and silver staining, were selected. Moreover, the rFimA preparation was passed through a column containing agarose-immobilized polymyxin B (Detoxi-Gel; Pierce, Rockford, Ill.). Native major fimbriae were purified to homogeneity by size exclusion chromatography of sonic extracts of P. gingivalis 381 (24). Fractions that were negative of any contaminating substances on silver-stained SDS-PAGs were selected. Similar criteria of purity were used to isolate the other proteins. Briefly, minor fimbriae were purified from a fimA mutant strain of P. gingivalis by means of anion-exchange chromatography (12). Recombinant BspA (rBspA) from B. forsythus was expressed as a fusion protein with glutathione S-transferase (pGEX expression system; Pharmacia, Piscataway, N.J.) and purified from sonic extracts of transformed E. coli using a column containing agarose-immobilized glutathione followed by specific cleavage to release rBspA (47). Native AgI/II from S. mutans culture supernatants was purified to homogeneity using size exclusion and anion-exchange chromatography, and its recombinantly expressed SBR was purified using metal chelation chromatography as described previously (51). Although there was no visible contamination with LPS of the selected fractions from the various proteins on silver-stained SDS-PAGs, the Limulus amebocyte lysate assay (BioWhittaker, Walkersville, Md.) showed the presence of LPS traces in certain protein preparations (0.01 to 0.15% [wt/wt]). All assays involving protein stimuli were performed in the presence of LPS inhibitor polymyxin B sulfate (Sigma, St. Louis, Mo.) (10 μg/ml). At this concentration, polymyxin B inhibited at least 98% of TNF-α and IL-1β induction by 1 μg of LPS per ml and completely inhibited cytokine induction by 200 ng of LPS per ml, a much higher concentration than in any of the protein samples tested. Besides, cytokine induction by the various proteins in the presence of polymyxin B was not affected by the exogenous addition of 200 ng of LPS per ml. Furthermore, the findings reported in Results were reproducible in the absence of serum, which is required as a source of LPS-binding protein. P. gingivalis LPS (Pg-LPS) was highly purified as previously described (42). Briefly, phenol-water-extracted Pg-LPS was treated with DNase I, RNase A, and proteinase K, and following chromatographic purification, the purity of the preparation was confirmed by immunodiffusion analysis and SDS-PAGE with silver staining. Highly purified E. coli LPS (Ec-LPS) (14) was used for comparison with Pg-LPS. TNF-α and IL-1β induction by these Pg-LPS and Ec-LPS preparations was found to be inhibited by anti-TLR2 and anti-TLR4 monoclonal antibodies (MAbs), respectively, in accordance with a previous study reporting that they were TLR2 and TLR4 agonists, respectively (15).
Antibodies.
The mouse MAbs used were specific for CD11a (clone MEM-25, immunoglobulin G1 [IgG1]; Caltag, Burlingame, Calif.), CD11b (ICRF44, IgG1; Chemicon, Temecula, Calif.), CD14 (MEM-18, IgG1; Caltag), CD18 (CLB-LFA-1/1, IgG1; Caltag), TLR2 (MAb 2392, IgG1; Genentech, South San Francisco, Calif.), and TLR4 (HTA125, IgG2a; eBioscience, San Diego, Calif.). Fluorescein isothiocyanate (FITC)-conjugated MAbs (Caltag) were specific for CD11b (clone CR3 Bear-1, IgG1), CD14 (Tük 4, IgG2a), CD18 (CLB-LFA-1/1, IgG1). Pure or FITC-labeled mouse IgG1 or IgG2a isotype controls and FITC-conjugated goat anti-mouse IgG were also obtained from Caltag.
Cell culture cytokine induction and inhibition assays.
THP-1 cells (ATCC TIB-202) were differentiated with 10 ng of phorbol myristate acetate per ml for 2 days in 96-well polystyrene culture plates at 37°C in a humidified atmosphere containing 5% CO2. The culture medium consisted of RPMI 1640 (GIBCO BRL) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 2 mM l-glutamine, 10 mM HEPES, 100 U of penicillin G per ml, 100 μg of streptomycin per ml, and 0.05 mM 2-mercaptoethanol. Differentiation into macrophage-like cells correlated with adherence, upregulation of CD14, CD11b, and CD18, and increased responsiveness to rFimA stimulation as assessed by TNF-α induction (data not shown). Differentiated THP-1 cells (106/ml) were used for cytokine induction assays in a total volume of 100 μl per well in the absence or presence of stimulants (rFimA and native major fimbriae were used at 1 μg/ml; minor fimbriae, BspA, AgI/II, and its SBR at 5 μg/ml; Ec-LPS at 200 ng/ml or 1 μg/ml; and Pg-LPS at 1 μg/ml). The doses were chosen on the basis of a previous publication from this group (43) or the results of preliminary dose-response experiments. Culture supernatants were collected after incubation overnight (about 14 h) and stored at −70°C until assayed. None of the stimulants tested was found to affect the viability of the cells. TNF-α or IL-1β release into the culture medium was quantitated using CLB Pelikine enzyme-linked immunosorbent assay kits (obtained through Caltag) according to the protocol suggested by the manufacturer. In experiments designed to block cytokine induction, the cells were pretreated with MAbs to various cell surface receptors (MAbs were used at 10 μg/ml for blocking CD14, TLR2, and TLR4 or at 25 μg/ml for blocking β2 integrin subunits) for 30 min at 37°C prior to addition of the stimulants. In tolerance induction experiments, the cells were pretreated for 24 h with or without various stimulants. Following removal of the culture supernatants and three washes with warm culture medium, the cells were exposed to a second challenge. Recombinant gamma interferon (IFN-γ) used as a priming agent in some experiments was purchased from Caltag. All reported experiments were performed at least twice for verification.
Flow cytometry.
Nonadherent cultures of differentiated THP-1 cells (i.e., differentiated in polypropylene tubes) were used for flow cytometric analysis. Morphological changes, cell surface antigen expression, and cell growth arrest of phorbol myristate acetate-treated THP-1 cells are similar regardless of whether differentiation is induced under conditions which promote or prevent adherence (3). The differentiated cells were treated for 24 h in the absence or presence of stimulants and then washed in ice-cold phosphate-buffered saline containing 3% FBS and 0.05% sodium azide (staining buffer). Approximately 2 × 105 cells were incubated for 45 min on ice with 0.2 μg of FITC-conjugated MAb (or pure MAb followed by a second incubation with FITC-conjugated goat anti-mouse IgG) in a total volume of 100 μl of staining buffer. After the final washes, the cells were analyzed immediately by means of a FACScan flow cytometer (Becton Dickinson).
Statistical analysis.
Data were evaluated by analysis of variance and the Tukey multiple-comparison test using the InStat program (GraphPad Software, San Diego, Calif.) on a Macintosh computer. Differences were considered statistically significant at the level of P < 0.05.
RESULTS
Dependence of virulence proteins on CD14/TLR complex for cytokine induction.
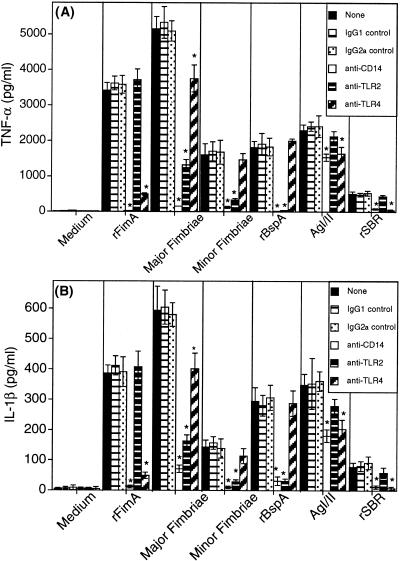
Stimulation of differentiated THP-1 cells with rFimA, native major fimbriae, minor fimbriae, rBspA, AgI/II, or the recombinant SBR (rSBR) of AgI/II, induced TNF-α (Fig. 1A) and IL-1β (Fig. 1B) responses, with major fimbriae being the most potent inducer. Preincubation of the cells with anti-CD14 MAb greatly inhibited cytokine induction by rFimA, major or minor fimbriae, or rBspA and partially suppressed AgI/II-induced cytokine responses (Fig. 1). The segment of AgI/II accounting for its partial CD14 dependence is probably the SBR, since cytokine induction by this segment was almost completely inhibited by anti-CD14 MAb (Fig. 1). Similar treatments with anti-TLR2 or anti-TLR4 MAbs showed that cytokine induction by rFimA or rSBR depended on TLR4, whereas cytokine induction by minor fimbriae or rBspA depended on TLR2 (Fig. 1). Cytokine responses induced by intact AgI/II were partially inhibited by anti-TLR4 but were not significantly inhibited by anti-TLR2 MAb (Fig. 1). Native major fimbriae were dependent on both TLR4 and (especially) TLR2 for stimulation of cytokine responses (Fig. 1). Despite the apparent purity of major fimbriae on silver-stained SDS-PAGs, we examined whether their TLR2 dependence for cytokine induction was attributable to contaminating traces of Pg-LPS, a TLR2 agonist that is relatively resistant to polymyxin B (15). However, when the entire experiment was repeated (data not shown) with or without FBS in the culture medium, cytokine induction by major fimbriae was found to be dependent on TLR2, even under serum-free conditions. Since serum is required as a source of LPS-binding protein for mediating transfer of Pg-LPS (or Ec-LPS) to immobilized CD14 (6), involvement of TLR2 in major fimbria-induced cytokine responses is unlikely to be due to contaminating Pg-LPS. Interestingly, while the repetition of the experiment in the presence or absence of serum did not affect the dependence of protein adhesins on either TLR4 (rFimA, AgI/II, and rSBR) or TLR2 (rBspA and minor fimbriae) for cytokine induction, the ability of rBspA to induce cytokine production was increased by about fourfold in the absence of serum. For example, rBspA-induced IL-1β production was increased from 265 ± 51 pg/ml in the presence of FBS to 1,094 ± 163 pg/ml in the absence of FBS, suggesting the existence of a serum factor that interferes with cytokine induction by rBspA.
FIG. 1.
Dependence of bacterial protein adhesins on the CD14/TLR complex for TNF-α (A) or IL-1β (B) induction. THP-1 cells were pretreated for 30 min with either 10 μg of immunoglobulin isotype controls per ml or with an equal concentration of MAbs to CD14, TLR2, or TLR4 prior to addition of the stimulants (rFimA and native major fimbriae used at 1 μg/ml; minor fimbriae, rBspA, AgI/II, and SBR used at 5 μg/ml). Culture supernatants were collected after overnight incubation and assayed for cytokine content. Results are presented as means ± standard deviations of triplicate determinations. Values that are statistically significantly different (P < 0.05) from those of groups stimulated with protein adhesin in the absence of antibody treatment or in the presence of immunoglobulin isotype control are indicated by an asterisk.
Effects of anti-β2 integrin MAbs on cytokine induction by major fimbriae.
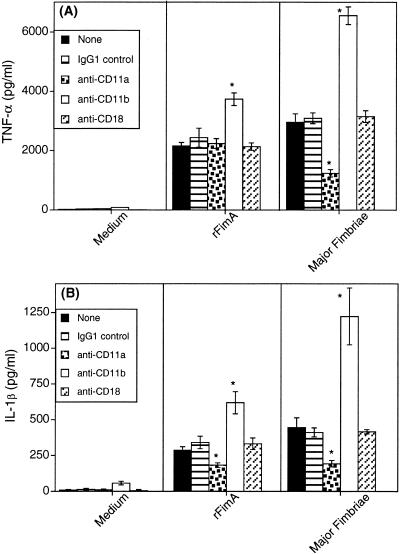
Among anti-β2 integrin MAbs tested, only anti-CD11a MAb inhibited cytokine induction by rFimA or native major fimbriae. Whereas this MAb inhibited both TNF-α and IL-1β induction by native fimbriae (Fig. 2), it inhibited only IL-1β induction by rFimA (Fig. 2B). The anti-CD18 MAb tested was without effect, while the anti-CD11b MAb enhanced the cytokine responses induced by rFimA or native fimbriae (Fig. 2), perhaps by increasing the avidity of β2 integrin for ligand binding. These results showed that β2 integrins play a role in fimbria-induced proinflammatory cytokine induction in human monocytic cells as reported in the mouse system (49).
FIG. 2.
Effects of anti-β2 integrin MAbs on TNF-α (A) or IL-1β (B) induction by fimbriae. THP-1 cells were pretreated for 30 min with either 25 μg of IgG1 isotype control per ml or with an equal concentration of MAbs to CD11a, CD11b, or CD18 prior to addition of the stimulants (1 μg of rFimA or native major fimbriae per ml). Culture supernatants were collected after overnight incubation and assayed for cytokine content. Data are shown as means ± standard deviations of triplicate determinations. Values that are statistically significantly different (P < 0.05) from those of groups stimulated with protein in the absence of antibody treatment or in the presence of immunoglobulin isotype control are indicated by an asterisk.
CD14- and TLR-dependent induction of tolerance to rFimA-mediated cytokine production.
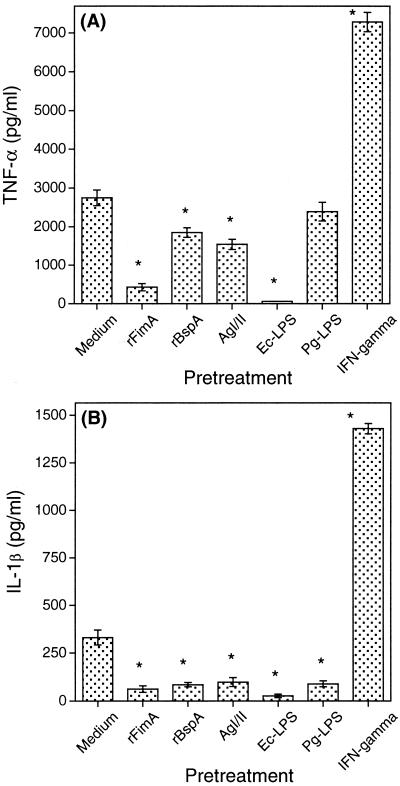
We found that continuous exposure of THP-1 cells to rFimA rendered the cells unresponsive to a secondary challenge with rFimA (Fig. 3), in a mode analogous to tolerance induction by Ec-LPS (28, 34). However, when THP-1 cells were exposed to rFimA in the presence of either anti-CD14 MAb or anti-TLR4 MAb (but not anti-TLR2 or isotype control antibodies), they readily responded to an ensuing challenge with rFimA (Table 1). These data show that rFimA induces tolerance to itself for secondary cytokine induction in a CD14- and TLR4-dependent way. Native major fimbriae behaved similarly to rFimA regarding the ability to induce hyporesponsiveness in THP-1 cells. Pretreatment of the cells with major fimbriae inhibited subsequent cytokine responses to a subsequent challenge with the same protein by about 80% (data not shown).
FIG. 3.
Induction of tolerance to rFimA-mediated TNF-α (A) or IL-1β (B) responses. THP-1 cells were pretreated for 24 h with or without various stimulants (rFimA used at 1 μg/ml; rBspA and AgI/II at 5 μg/ml; Ec-LPS and Pg-LPS at 1 μg/ml; IFN-γ at 100 U/ml). Following removal of the culture supernatants and washing, the cells were challenged with 1 μg of rFimA per ml. Culture supernatants were collected after overnight incubation and assayed for cytokine responses. Results are presented as means ± standard deviations of triplicate determinations. Values that are statistically significantly different (P < 0.05) from those of the THP-1 group pretreated with medium only are indicated by an asterisk.
TABLE 1.
Induction of hyporesponsiveness in THP-1 cells to a secondary challenge with rFimA depends on CD14 and TLR4a
| THP-1 pretreatment | Amt (pg/ml) of cytokine measured (mean ± SD) (n = 3)
|
|
|---|---|---|
| TNF-α | IL-1β | |
| Medium only | 2,108 ± 197 | 297 ± 42 |
| rFimA | 365 ± 28 | 57 ± 19 |
| rFimA + IgG1 control | 381 ± 34 | 46 ± 16 |
| rFimA + IgG2a control | 341 ± 54 | 73 ± 37 |
| rFimA + anti-CD14 | 1,920 ± 134* | 218 ± 29* |
| rFimA + anti-TLR2 | 423 ± 46 | 69 ± 34 |
| rFimA + anti-TLR4 | 1,432 ± 158* | 167 ± 28* |
THP-1 cells were exposed for 30 min to 10 μg of immunoglobulin isotype controls per ml or anti-CD14, anti-TLR2, or anti-TLR4 MAb prior to incubation with 1 μg of rFimA per ml. After 24 h, the cells were washed and challenged with 1 μg of rFimA per ml. Culture supernatants were collected after overnight incubation and assayed for cytokine responses. Asterisks * indicate groups for which the MAb treatment significantly (P < 0.05) impaired the ability of rFimA to induce hyporesponsiveness in THP-1 cells to a secondary challenge with rFimA.
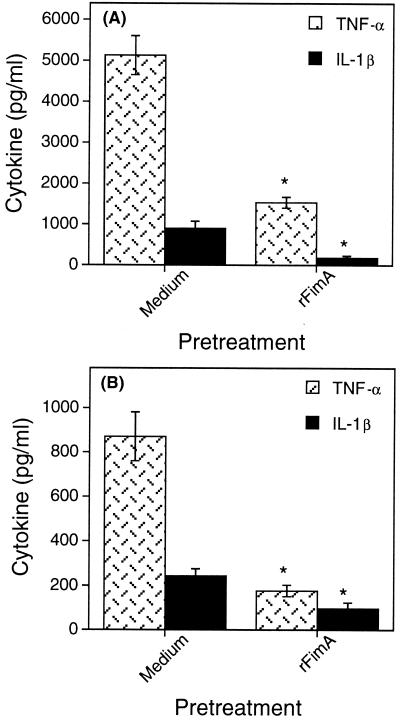
The possibility of cross-tolerance between rFimA and other molecules was then investigated. Various degrees of THP-1 hyporesponsiveness to a secondary challenge with rFimA were observed when the cells were pretreated with Ec-LPS, rBspA, and AgI/II (Fig. 3). Interestingly, Pg-LPS induced hyporesponsiveness to IL-1β (Fig. 3B) but not to TNF-α (Fig. 3A) induction by rFimA. Tolerance induction by Ec-LPS and Pg-LPS to subsequent challenges with rFimA was dependent on TLR4 and TLR2, respectively (Table 2). Conversely, pretreatment of THP-1 cells with rFimA rendered them tolerant to cytokine induction after challenge with either Ec-LPS (Fig. 4A) or Pg-LPS (Fig. 4B). In contrast to these tolerogenic effects, pretreatment of THP-1 cells with IFN-γ had a priming effect on subsequent TNF-α and IL-1β induction by rFimA (Fig. 3). This may, at least partly, be attributable to the ability of IFN-γ to upregulate the expression of CD14, β2-integrins, and TLRs, although these studies involved a variety of cell types (7, 32, 50).
TABLE 2.
Induction of LPS-mediated hyporesponsiveness in THP-1 cells to a subsequent challenge with rFimA depends on TLRsa
| THP-1 pretreatment | Amt (pg/ml) of cytokine measured (mean ± SD) (n = 3)
|
|
|---|---|---|
| TNF-α | IL-1β | |
| Medium only | 2,418 ± 237 | 352 ± 68 |
| Ec-LPS | 49 ± 11 | 31 ± 12 |
| Ec-LPS + anti-TLR2 | 76 ± 26 | 48 ± 16 |
| Ec-LPS + anti-TLR4 | 2,159 ± 183* | 309 ± 44* |
| Pg-LPS | NDb | 98 ± 21 |
| Pg-LPS + anti-TLR2 | ND | 317 ± 55* |
| Pg-LPS + anti-TLR4 | ND | 112 ± 36 |
THP-1 cells were exposed for 30 min to 10 μg of anti-TLR MAbs per ml prior to incubation with 1μg of Ec-LPS or Pg-LPS per ml. After 24 h, the cells were washed and challenged with 1 μg of rFimA per ml. Culture supernatants were collected after overnight incubation and assayed for cytokine responses. Asterisks * indicate groups for which the MAb treatment significantly (P < 0.05) impaired the ability of LPS to induce hyporesponsiveness in THP-1 cells to an ensuing challenge with rFimA.
ND, not determined, as previous experiments showed that Pg-LPS does not cause significant hyporesponsiveness to TNF-α induction by rFimA.
FIG. 4.
Induction of tolerance to Ec-LPS (A)- or Pg-LPS (B)-mediated cytokine responses. THP-1 cells were exposed for 24 h to either medium only or 1 μg of rFimA per ml. The following day, culture supernatants were removed and the cells were rechallenged with 1 μg of either Ec-LPS (A) or Pg-LPS (B) per ml. Culture supernatants were collected after overnight incubation and assayed for cytokine responses. Data are presented as means ± standard deviations of triplicate determinations. Values that are statistically significantly different (P < 0.05) from those of the THP-1 group pretreated with medium only are indicated by an asterisk.
Regulation of PRRs by rFimA.
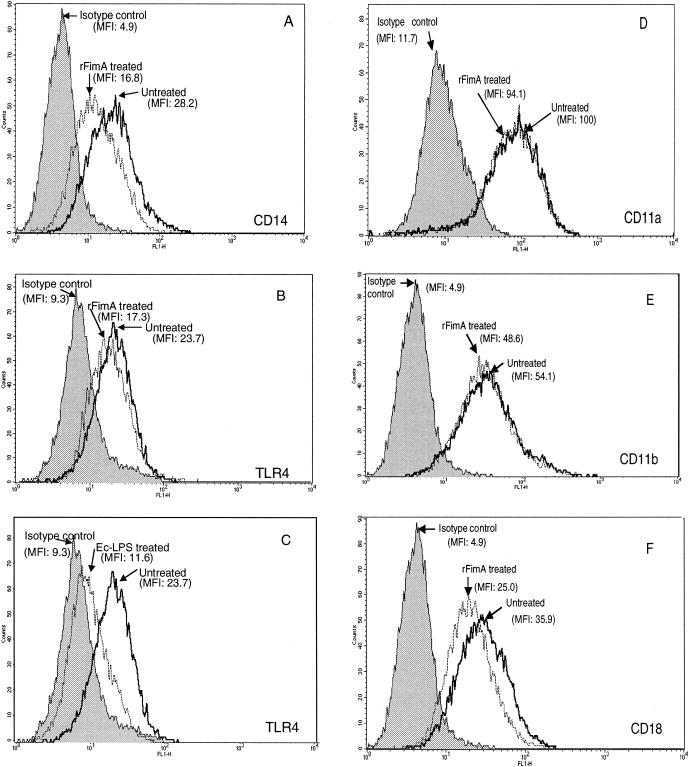
Surface expression of some PRRs was found to be influenced after a 24 h-incubation of THP-1 cells with rFimA. Expression of CD14 was inhibited about 40% by rFimA, as calculated on the basis of mean fluorescence intensities (Fig. 5A). Two additional independent experiments showed CD14 inhibition by 38 and 51%. The expression of TLR4 was slightly downregulated by rFimA (Fig. 5B), in contrast to pronounced Ec-LPS-mediated downregulation of the same receptor (Fig. 5C). However, the rFimA effect (Fig. 5B) was reproducible in two other experiments where TLR4 expression was inhibited by approximately 25 to 30%. In contrast, TLR2 was not affected by rFimA (data not shown). Regarding the effect of rFimA on the surface expression of β2 integrin subunits, CD11a (Fig. 5D) and CD11b (Fig. 5E) were essentially unaffected, whereas CD18 was suppressed by about 30% (Fig. 5F). Similar levels of suppression were observed in two additional experiments. Taken together, the above data suggest that THP-1 cells become hyporesponsive to cytokine production by a secondary challenge with rFimA, at least partly because of downregulation of some of the PRRs involved.
FIG. 5.
Effect of rFimA on surface expression of PRRs. THP-1 cells were treated with 1 μg of rFimA per ml for 24 h and then were FITC stained by means of MAbs specific for CD14 (A), TLR4 (B), CD11a (D), CD11b (E), and CD18 (F). In a similar experiment, THP-1 cells were treated with 1 μg of Ec-LPS per ml and stained by means of anti-TLR4 (C). Receptor expression was analyzed by flow cytometry. Numbers shown on the figure represent mean fluorescence intensity (MFI) values. The results are representative of two (D and E) or three (A, B, C, and F) independent experiments.
DISCUSSION
Numerous bacterial virulence proteins possess repeated motifs or are assembled as multimeric structures that presumably enable them to recognize host cell surfaces for colonization or invasion (19, 23). From the point of view of the host, such molecular patterns may offer conserved structures for recognition by PRRs. Although the exact nature of such interactions is not known, it is likely that they do not depend on specific amino acid sequence recognition but instead may involve (without being limited to) repeating nonpolar motifs. Hydrophobicity drives strong interactions without being too specific in nature, whereas the presence of tandem repeats in bacterial proteins has the potential to cross-link the receptors for effective responses. On the basis of these considerations, we selected a few bacterial proteins to examine whether they can interact with PRRs, as has been reported for LPS, peptidoglycan, lipoproteins, and lipoteichoic acid (25, 30, 45). Indeed, the findings from the studies using blocking MAbs to PRRs (Fig. 1 and 2) suggest that the ability of certain bacterial protein adhesins to induce proinflammatory cytokine release depends on interactions with PRRs, such as CD14 and TLRs. Although several microbial products have been characterized as TLR agonists or ligands, direct binding and estimation of binding affinities have not been demonstrated for any of the reported microbial molecules (reviewed in reference 10). This suggests that direct demonstration of binding is difficult to accomplish with these receptors, possibly because TLRs may detect conformational changes induced by transient association of agonists to molecular complexes comprising several coreceptors.
Our results showed that rFimA is a TLR4 agonist, whereas native major fimbriae appeared to interact with both TLR2 and TLR4 (Fig. 1). The involvement of TLR2 in native fimbria-induced cytokine responses is likely due to a fimbria-associated 12-kDa lipoprotein. This accessory factor has a distinct amino acid composition compared to that of FimA, contains covalently linked fatty acids but lacks sugar moieties, and can be localized by immunogold labeling on P. gingivalis along the entire length of native fimbriae at regular intervals (H. Sojar et al., 72nd Int. Assoc. Dent. Res. session, J. Dent. Res.73:431, abstr. 2633, 1994). Upon dissociation from native fimbriae, purified 12-kDa lipoprotein induces the release of IL-1β from mouse macrophages as potently as rFimA (43). It is thus reasonable to assume that this lipoprotein factor induces proinflammatory cytokine expression via TLR2 activation, as has been reported for other lipoproteins and lipopeptides (25). The relatively recently discovered minor fimbriae of P. gingivalis (12) were found to be a TLR2 agonist (Fig. 1). Although their functional role is not known yet, their involvement in inflammatory reactions warrants further investigation.
Fimbriae are expected to play a significant role in P. gingivalis-induced inflammatory reactions, especially since Pg-LPS is a relatively poor inducer of proinflammatory cytokines compared to enterobacterial LPS (36). In this regard, a fimA mutant of P. gingivalis exhibits significantly reduced ability for proinflammatory cytokine induction in epithelial cells compared to the parent fimA+ strain (44). Although major and minor fimbriae are cell surface structures, they are also shed in the culture fluid. The in vivo environment is anticipated to be proteolytic partly due to the release of cysteine proteinases (gingipains) by P. gingivalis (23). Thus, it is possible that FimA subunits or fragments thereof (some with intact TLR4 agonistic activity) may be present in addition to the polymerized fimbrial form. An interesting future investigation would be to assess the cooperation or even possible antagonistic effects on cellular activation between TLR4 (FimA subunits) and TLR2 (lipoprotein component of major fimbriae as well as Pg-LPS) agonists from P. gingivalis.
The rBspA used in this study represents the N-terminal 70% of the native protein and includes all fourteen 23-residue LRRs (47). These LRRs contain multiple LT motifs which, though not as closely positioned in the primary sequence as the proposed LTXXLT proinflammatory motif (37), are predicted to be close together in the tertiary structure of the protein (21). The amphipathic properties of LRR proteins may explain why such proteins are involved in adherent interactions and why at least half of them participate in signal transduction pathways (21). Both TLR2 and TLR4 are LRR signal-transducing proteins and could potentially interact with BspA via hydrophobic interactions. It would be interesting to know, however, what structural differences account for the selective dependence of BspA on TLR2 for cytokine induction.
Two regions of AgI/II have been previously shown to be involved in TNF-α induction in THP-1 cells (4). The less potent of these segments is the alanine-rich repeat region (4), which is present in the SBR and apparently represents the part of AgI/II that induces proinflammatory cytokines via CD14 and TLR4 (Fig. 1). Its ability to induce TNF-α and IL-1β expression may at least partly be attributable to the presence of two ALTAE motifs that closely resemble the proinflammatory ALTTE and ALT peptides derived from the P. gingivalis FimA sequence (35). The SBR mediates the adherence of S. mutans to dental surfaces via a salivary receptor (5, 11), which has recently been shown to be identical to the lung scavenger receptor gp340 (41). This receptor is a PRR that recognizes at least 14 other bacteria, including Helicobacter pylori, Streptococcus pyogenes, and Streptococcus agalactiae (41). We believe that the SBR may interact with PRRs involved in bacterial colonization and inflammatory responses.
Our findings support the involvement of human β2 integrins in FimA-mediated inflammatory reactions (49). The ability of MAbs to activate β2 integrins is not unprecedented: an anti-CD18 MAb induces a conformational change on CD18 and promotes adhesion of CD11b/CD18 to natural ligands such as iC3b and ICAM-1 (2). Another mechanism of CD11b/CD18 activation involves the participation of CD14. Binding of LPS to CD14 leads to enhanced binding of iC3b-coated erythrocytes by CD11b/CD18, and an anti-CD14 MAb almost completely blocks the ability of CD11b/CD18 to engage its ligand (52). This raises the possibility that CD14-FimA interactions may cooperatively enhance the FimA-binding capacity of CD11/CD18.
Induction of LPS tolerance in monocytic cells after initial LPS exposure is believed to be a defense mechanism by which the innate immune system attempts to downregulate an excessive inflammatory reaction, as in the case of sepsis with gram-negative bacteria. LPS tolerance can be induced at various levels ranging from reprogramming of signal transduction pathways (28) to downregulation of surface TLR4 expression (34). Our data suggest that similar tolerance mechanisms may operate in virulence protein-induced inflammation (Fig. 3). Moreover, the findings that BspA and AgI/II induced hyporesponsiveness to subsequent challenge with rFimA suggest the possibility of cross-tolerance between different bacterial species. Downregulation of CD14 (but apparently not of TLR4) during primary exposure of cells to rFimA may partially contribute to induction of cytokine hyporesponsiveness to secondary challenges with rFimA, though this does not rule out postreceptor tolerogenic mechanisms. Conversely, when macrophages were pretreated with Ec-LPS, subsequent tolerance to Ec-LPS could be correlated with alterations in TLR4 but not with CD14 expression (34). It is intriguing that Ec-LPS and FimA induce partly different outcomes despite using the same TLR. However, this is not unprecedented; it was recently shown that glucuronoxylomannan from Cryptococcus neoformans induces distinct cellular responses from those of Ec-LPS, although both molecules exert their activity through TLR4 (48). Specific antagonists at the TLR4 level may elucidate which TLR4 agonists utilize the same or distinct TLR4 interfaces for cellular activation. It was suggested recently that LPS tolerance may occur independently of PRR expression levels (27). In this respect, we have just shown that upon FimA restimulation of THP-1 cells, the p65 (but not the p50) subunit of NF-κB is downregulated (unpublished observations). Since p50 molecules (but not p65) lack a transactivation domain, the relative p50 abundance upon FimA secondary stimulation may result in predominantly inactive NF-κB homodimers of p50 and reduced transcription of proinflammatory genes, in a manner analogous with a LPS tolerance mechanism (54). Thus, induction of tolerance may be the combined effect of mechanisms acting at different levels of the signaling process.
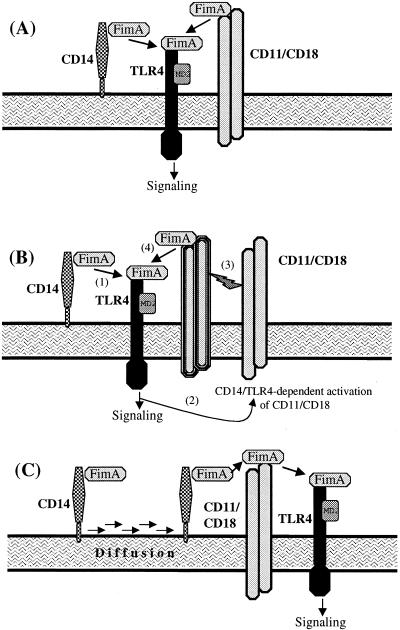
On the basis of our present results and published reports regarding the properties of CD14, TLR4, and β2 integrins (18, 40, 52, 53), we propose three models to explain how FimA may interact with these PRRs. In the simplest model (Fig. 6A), CD14 and CD11/CD18 bind and present FimA to TLR4. Figure 6B depicts the possibility that CD11/CD18 may require CD14-dependent activation for enhanced binding of pathogen-associated molecular patterns (52). Finally, Fig. 6C shows glycophosphatidylinositol-linked CD14 as a rapidly diffusing “scout” for CD11/CD18 (53). The two models shown in Fig. 6B and C are consistent with our findings that an anti-CD14 MAb almost completely inhibited rFimA-induced cytokine responses in the absence of additional treatment interfering with CD11/CD18. Regardless of the particular way FimA is presented to the innate immune system, the downstream signal transducer should be TLR4, according to our findings.
FIG. 6.
Interaction of PRRs with FimA for cytokine induction. (A) FimA may be presented independently by CD14 and CD11/CD18 to TLR4, or (B) CD11/CD18 may largely depend on CD14-mediated activation for enhanced ligand binding. (C) CD14 is shown as a readily diffusing “scout” transferring FimA to CD11/CD18 prior to TLR4-dependent cellular activation. These models are based on findings from this paper and published properties of PRRs (18, 40, 52, 53).
Several instances of bacterial or host proteins interacting with TLRs have now been reported (8, 13, 38, 39). Our findings suggest that PRRs detect the presence of certain virulence bacterial proteins and can either respond with expression of proinflammatory cytokines or mediate downregulation of the inflammatory responses upon continuous exposure to the microbial stimuli.
Acknowledgments
We thank Rose Parkhill and Denise Nicosia for expert assistance in preparing the manuscript.
This work was supported in part by Public Health Service grants DE07034, DE06746, and DE09691.
REFERENCES
- 1.Amano, A., A. Sharma, J.-Y. Lee, H. T. Sojar, P. A. Raj, and R. J. Genco. 1996. Structural domains of Porphyromonas gingivalis recombinant fimbrillin that mediate binding to salivary proline-rich protein and statherin. Infect. Immun. 64:1631-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrew, D., A. Shock, E. Ball, S. Ortlepp, J. Bell, and M. Robinson. 1993. KIM185, a monoclonal antibody to CD18 which induces a change in the conformation of CD18 and promotes both LFA-1 and CR3-dependent adhesion. Eur. J. Immunol. 23:2217-2222. [DOI] [PubMed] [Google Scholar]
- 3.Auwerx, J., B. Staels, F. Van Vaeck, and J. L. Ceuppens. 1992. Changes in IgG Fc receptor expression induced by phorbol 12-myristate 13-acetate treatment of THP-1 monocytic leukemia cells. Leukoc. Res. 16:317-327. [DOI] [PubMed] [Google Scholar]
- 4.Chatenay-Rivauday, C., I. Yamodo, M. A. Sciotti, J. A. Ogier, and J. P. Klein. 1998. The A and the extended V N-terminal regions of streptococcal protein I/IIf mediate the production of tumour necrosis factor alpha in the monocyte cell line THP-1. Mol. Microbiol. 29:39-48. [DOI] [PubMed] [Google Scholar]
- 5.Crowley, P. J., L. J. Brady, D. A. Piacentini, and A. S. Bleiweis. 1993. Identification of a salivary agglutinin-binding domain within cell surface adhesin P1 of Streptococcus mutans. Infect. Immun. 61:1547-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunningham, M. D., C. Seachord, K. Ratcliffe, B. Bainbridge, A. Aruffo, and R. P. Darveau. 1996. Helicobacter pylori and Porphyromonas gingivalis lipopolysaccharides are poorly transferred to recombinant soluble CD14. Infect. Immun. 64:3601-3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faure, E., L. Thomas, H. Xu, A. E. Medvedev, O. Equils, and M. Arditi. 2001. Bacterial lipopolysaccharide and IFN-γ induce Toll-like receptor 2 and Toll-like receptor 4 expression in human endothelial cells: role of NF-κB activation. J. Immunol. 166:2018-2024. [DOI] [PubMed] [Google Scholar]
- 8.Frendéus, B., C. Wachtler, M. Hedlund, H. Fischer, P. Samuelsson, M. Svensson, and C. Svanborg. 2001. Escherichia coli P fimbriae utilize the Toll-like receptor 4 pathway for cell activation. Mol. Microbiol. 40:37-51. [DOI] [PubMed] [Google Scholar]
- 9.Gay, N. J., and F. J. Keith. 1991. Drosophila Toll and IL-1 receptor. Nature 351:355-356. [DOI] [PubMed] [Google Scholar]
- 10.Golenbock, D. T., and M. J. Fenton. 2001. Extolling the diversity of bacterial endotoxins. Nat. Immunol. 2:286-288. [DOI] [PubMed] [Google Scholar]
- 11.Hajishengallis, G., T. Koga, and M. W. Russell. 1994. Affinity and specificity of the interactions between Streptococcus mutans antigen I/II and salivary components. J. Dent. Res. 73:1493-1502. [DOI] [PubMed] [Google Scholar]
- 12.Hamada, N., H. T. Sojar, M.-I. Cho, and R. J. Genco. 1996. Isolation and characterization of a minor fimbria from Porphyromonas gingivalis. Infect. Immun. 64:4788-4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayashi, F., K. D. Smith, A. Ozinsky, T. R. Hawn, E. C. Yi, D. R. Goodlett, J. K. Eng, S. Akira, D. M. Underhill, and A. Aderem. 2001. The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 410:1099-1103. [DOI] [PubMed] [Google Scholar]
- 14.Hirschfeld, M., Y. Ma, J. H. Weis, S. N. Vogel, and J. J. Weis. 2000. Cutting edge: repurification of lipopolysaccharide eliminates signaling through both human and murine Toll-like receptor 2. J. Immunol. 165:618-622. [DOI] [PubMed] [Google Scholar]
- 15.Hirschfeld, M., J. J. Weis, V. Toshchakov, C. A. Salkowski, M. J. Cody, D. C. Ward, N. Qureshi, S. M. Michalek, and S. N. Vogel. 2000. Signaling by Toll-like receptor 2 and 4 agonists results in differential gene expression in murine macrophages. Infect. Immun. 69:1477-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoshino, K., O. Takeuchi, T. Kawai, H. Sanjo, T. Ogawa, Y. Takeda, K. Takeda, and S. Akira. 1999. Cutting edge: Toll-like receptor 4 (TLR4)-deficient mice are hyporesponsive to lipopolysaccharide: evidence for TLR4 as the Lps gene product. J. Immunol. 162:3749-3752. [PubMed] [Google Scholar]
- 17.Ingalls, R. R., M. A. Arnaout, and D. T. Golenbock. 1997. Outside-in signaling by lipopolysaccharide through a tailless integrin. J. Immunol. 159:433-438. [PubMed] [Google Scholar]
- 18.Ingalls, R. R., B. G. Monks, R. J. Savedra, W. J. Christ, R. L. Delude, A. E. Medvedev, T. Espevik, and D. T. Golenbock. 1998. CD11/CD18 and CD14 share a common lipid A signaling pathway. J. Immunol. 161:5413-5420. [PubMed] [Google Scholar]
- 19.Jenkinson, H. F., and R. J. Lamont. 1997. Streptococcal adhesion and colonization. Crit. Rev. Oral Biol. Med. 8:175-200. [DOI] [PubMed] [Google Scholar]
- 20.Kawata, Y., S. Hanazawa, S. Amano, Y. Murakami, T. Matsumoto, K. Nishida, and S. Kitano. 1994. Porphyromonas gingivalis fimbriae stimulate bone resorption in vitro. Infect. Immun. 62:3012-3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kobe, B., and J. Deisenhofer. 1994. The leucine-rich repeat: a versatile binding motif. Trends Biochem. Sci. 19:415-421. [DOI] [PubMed] [Google Scholar]
- 22.Krantz, D. D., R. Zidovetski, B. L. Kagan, and S. L. Zipursky. 1991. Amphipathic beta structure of a leucine-rich repeat peptide. J. Biol. Chem. 266:16801-16807. [PubMed] [Google Scholar]
- 23.Lamont, R. J., and H. F. Jenkinson. 1998. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol. Mol. Biol. Rev. 62:1244-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee, J.-Y., H. T. Sojar, A. Amano, and R. J. Genco. 1995. Purification of major fimbrial proteins of Porphyromonas gingivalis. Protein Expr. Purific. 6:496-500. [DOI] [PubMed] [Google Scholar]
- 25.Lien, E., T. J. Sellati, A. Yoshimura, T. H. Flo, G. Rawadi, R. W. Finberg, J. D. Carroll, T. Espevik, R. R. Ingalls, J. D. Radolf, and D. T. Golenbock. 1999. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J. Biol. Chem. 274:33419-33425. [DOI] [PubMed] [Google Scholar]
- 26.Malek, R., J. G. Fisher, A. Caleca, M. Stinson, C. J. van Oss, J.-Y. Lee, M.-I. Cho, R. J. Genco, R. T. Evans, and D. W. Dyer. 1994. Inactivation of the Porphyromonas gingivalis fimA gene blocks periodontal damage in gnotobiotic rats. J. Bacteriol. 176:1052-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medvedev, A. E., P. Henneke, A. Schromm, E. Lien, R. Ingalls, M. J. Fenton, D. T. Golenbock, and S. N. Vogel. 2001. Induction of tolerance to lipopolysaccharide and mycobacterial components in Chinese hamster ovary/CD14 cells is not affected by overexpression of Toll-like receptors 2 or 4. J. Immunol. 167:2257-2267. [DOI] [PubMed] [Google Scholar]
- 28.Medvedev, A. E., K. M. Kopydlowski, and S. N. Vogel. 2000. Inhibition of lipopolysaccharide-induced signal transduction in endotoxin-tolerized mouse macrophages: dysregulation of cytokine, chemokine, and toll-like receptor 2 and 4 gene expression. J. Immunol. 164:5564-5574. [DOI] [PubMed] [Google Scholar]
- 29.Medzhitov, R., and C. Janeway. 2000. Innate immunity. N. Engl. J. Med. 343:338-344. [DOI] [PubMed] [Google Scholar]
- 30.Medzhitov, R., and C. Janeway. 2000. The Toll receptor family and microbial recognition. Trends Microbiol. 10:452-456. [DOI] [PubMed] [Google Scholar]
- 31.Modlin, R. L., H. D. Brightbill, and P. J. Godowski. 1999. The Toll of innate immunity on microbial pathogens. N. Engl. J. Med. 340:1834-1835. [DOI] [PubMed] [Google Scholar]
- 32.Molina, I. J., and B. T. Huber. 1991. Regulation of macrophage activation markers by IL-4 and IFN-γ is subpopulation-specific. Cell. Immunol. 134:241-248. [DOI] [PubMed] [Google Scholar]
- 33.Njoroge, T., R. J. Genco, H. T. Sojar, N. Hamada, and C. A. Genco. 1997. A role for fimbriae in Porphyromonas gingivalis invasion of oral epithelial cells. Infect. Immun. 65:1980-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nomura, F., S. Akashi, Y. Sakao, S. Sato, T. Kawai, M. Matsumoto, K. Nakanishi, M. Kimoto, K. Miyake, K. Takeda, and S. Akira. 2000. Endotoxin tolerance in mouse macrophages correlates with down-regulation of surface Toll-like receptor 4 expression. J. Immunol. 164:3476-3479. [DOI] [PubMed] [Google Scholar]
- 35.Ogawa, T., and H. Uchida. 1995. A peptide, ALTTE, within the fimbrial subunit protein from Porphyromonas gingivalis, induces production of interleukin 6, gene expression and protein phosphorylation in human peripheral blood mononuclear cells. FEMS Immunol. Med. Microbiol. 11:197-206. [DOI] [PubMed] [Google Scholar]
- 36.Ogawa, T., H. Uchida, and K. Amino. 1994. Immunobiological activities of chemically defined lipid A from lipopolysaccharides of Porphyromonas gingivalis. Microbiology 140:1209-1216. [DOI] [PubMed] [Google Scholar]
- 37.Ogawa, T., H. Uchida, and S. Hamada. 1994. Porphyromonas gingivalis fimbriae and their synthetic peptides induce proinflammatory cytokines in human peripheral blood monocyte cultures. FEMS Microbiol. Lett. 116:237-242. [DOI] [PubMed] [Google Scholar]
- 38.Ohashi, K., V. Burkart, S. Flohé, and H. Kolb. 2000. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the Toll-like receptor-4 complex. J. Immunol. 164:558-561. [DOI] [PubMed] [Google Scholar]
- 39.Okamura, Y., M. Watari, E. S. Jerud, D. W. Young, S. T. Ishizaka, J. Rose, J. C. Chow, and J. F. Strauss. 2001. The extra domain A of fibronectin activates Toll-like receptor 4. J. Biol. Chem. 276:10229-10233. [DOI] [PubMed] [Google Scholar]
- 40.Perera, P.-Y., T. N. Mayadas, O. Takeuchi, S. Akira, M. Zaks-Zilberman, S. M. Goyert, and S. N. Vogel. 2001. CD11b/CD18 acts in concert with CD14 and Toll-like receptor (TLR) 4 to elicit full lipopolysaccharide and taxol-inducible gene expression. J. Immunol. 166:574-581. [DOI] [PubMed] [Google Scholar]
- 41.Prakobphol, A., F. Xu, V. M. Hoang, T. Larsson, J. Bergstrom, I. Johansson, L. Frangsmyr, U. Holmskov, H. Leffler, C. Nilsson, T. Boren, J. R. Wright, N. Stromberg, and S. J. Fisher. 2000. Salivary agglutinin, which binds Streptococcus mutans and Helicobacter pylori, is the lung scavenger receptor cysteine-rich protein gp340. J. Biol. Chem. 275:39860-39866. [DOI] [PubMed] [Google Scholar]
- 42.Preshaw, P. M., R. E. Schifferle, and J. D. Walters. 1999. Porphyromonas gingivalis lipopolysaccharide delays human polymorphonuclear leukocyte apoptosis in vitro. J. Periodontal Res. 34:197-202. [DOI] [PubMed] [Google Scholar]
- 43.Saito, A., H. T. Sojar, and R. J. Genco. 1996. Porphyromonas gingivalis surface components induce interleukin-1 release and tyrosine phosphorylation in macrophages. FEMS Immunol. Med. Microbiol. 15:51-57. [DOI] [PubMed] [Google Scholar]
- 44.Sandros, J., C. Karlsson, D. F. Lappin, P. N. Madianos, D. F. Kinane, and P. N. Papapanou. 2000. Cytokine responses of oral epithelial cells to Porphyromonas gingivalis infection. J. Dent. Res. 79:1808-1814. [DOI] [PubMed] [Google Scholar]
- 45.Schwandner, R., R. Dziarski, H. Wesche, M. Rothe, and C. J. Kirschning. 1999. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by Toll-like receptor 2. J. Biol. Chem. 274:17406-17409. [DOI] [PubMed] [Google Scholar]
- 46.Sciotti, M.-A., I. Yamodo, J.-P. Klein, and J. A. Ogier. 1997. The N-terminal half part of the oral streptococcal antigen I/IIf contains two distinct binding domains. FEMS Microbiol. Lett. 153:439-445. [DOI] [PubMed] [Google Scholar]
- 47.Sharma, A., H. T. Sojar, I. Glurich, K. Honma, H. K. Kuramitsu, and R. J. Genco. 1998. Cloning, expression, and sequencing of a cell surface antigen containing a leucine-rich repeat motif from Bacteroides forsythus ATCC 43037. Infect. Immun. 66:5703-5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shoham, S., C. Huang, J. M. Chen, D. T. Golenbock, and S. M. Levitz. 2001. Toll-like receptor 4 mediates intracellular signalling without TNF-α release in response to Cryptococcus neoformans polysaccharide capsule. J. Immunol. 166:4620-4626. [DOI] [PubMed] [Google Scholar]
- 49.Takeshita, A., Y. Murakami, Y. Yamashita, M. Ishida, S. Fujisawa, S. Kitano, and S. Hanazawa. 1998. Porphyromonas gingivalis fimbriae use β2 integrin (CD11/CD18) on mouse peritoneal macrophages as a cellular receptor, and the CD18 β chain plays a functional role in fimbrial signaling. Infect. Immun. 66:4056-4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takeshita, S., K. Nakatani, Y. Takata, H. Kawase, I. Sekine, and S. Yoshioka. 1998. Interferon-γ (IFN-γ) and tumor necrosis factor-α (TNF-α) enhance lipopolysaccharide binding to neutrophils via CD14. Inflamm. Res. 47:101-103. [DOI] [PubMed] [Google Scholar]
- 51.Toida, N., G. Hajishengallis, H.-Y. Wu, and M. W. Russell. 1997. Oral immunization with the saliva-binding region of Streptococcus mutans AgI/II genetically coupled to the cholera toxin B subunit elicits T helper cell responses in gut-associated lymphoid tissues. Infect. Immun. 65:909-915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wright, S. D., R. A. Ramos, A. Hermanowski-Vosatka, P. Rockwell, and P. A. Detmers. 1991. Activation of the adhesive capacity of CR3 on neutrophils by endotoxin: dependence on lipopolysaccharide binding protein and CD14. J. Exp. Med. 173:1281-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zarewych, D. M., A. L. Kindzelskii, R. F. Todd, and H. R. Petty. 1996. LPS induces CD14 association with complement receptor 3, which is reversed by neutrophil adhesion. J. Immunol. 156:430-433. [PubMed] [Google Scholar]
- 54.Ziegler-Heitbrock, H. W. L., A. Wedel, W. Schraut, M. Ströbel, P. Wendelgass, T. Sternsdorf, P. A. Bäuerle, J. G. Haas, and G. Riethmüller. 1994. Tolerance to lipopolysaccharide involves mobilization of nuclear factor κB with predominance of p50 homodimers. J. Biol. Chem. 269:17001-17004. [PubMed] [Google Scholar]