Abstract
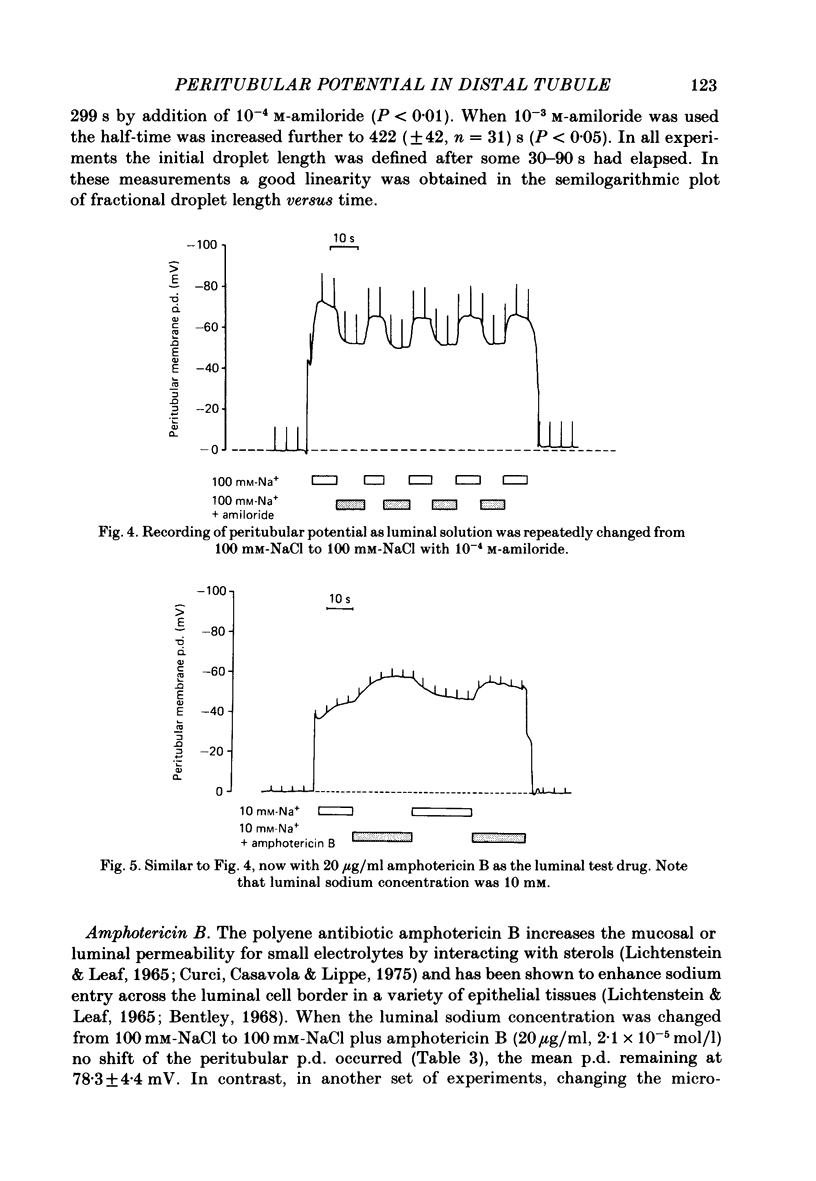
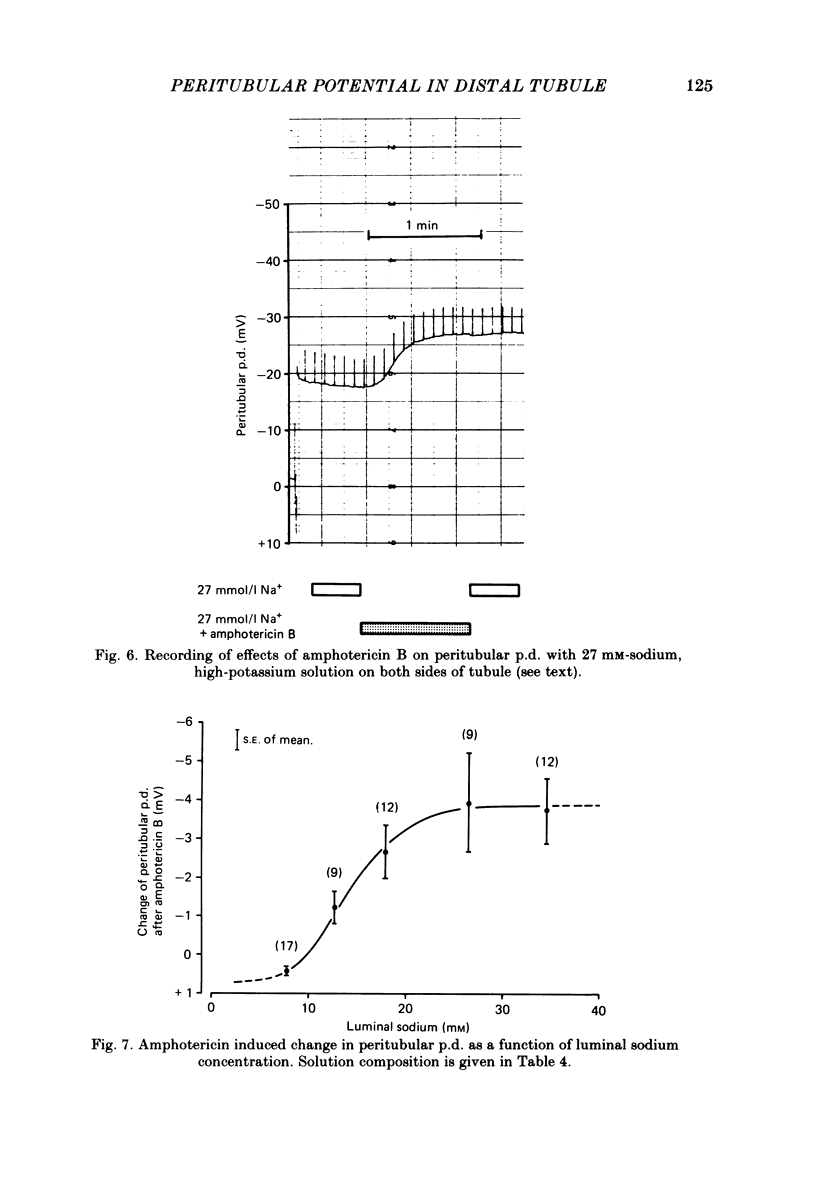
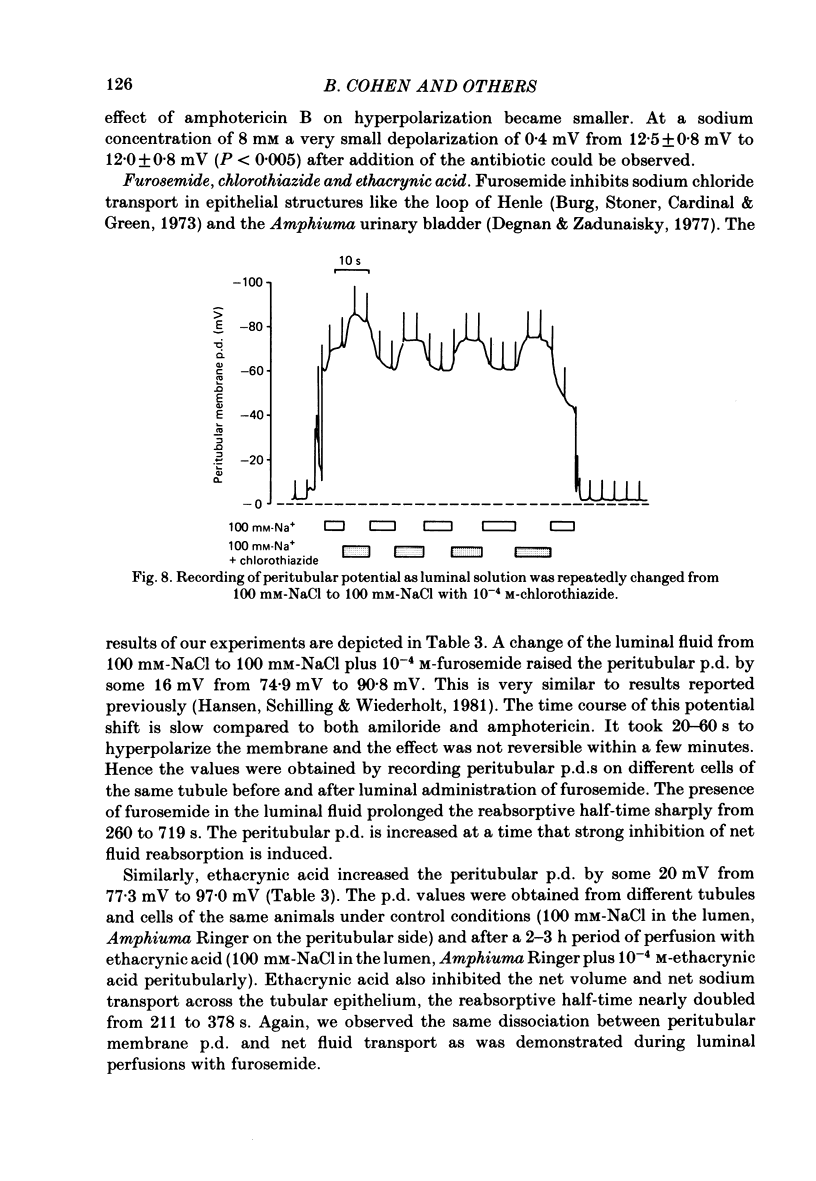
Amphiuma kidneys were isolated and perfused with modified Ringer solution and peritubular and transepithelial membrane potentials (p.d.s) in distal tubules measured with micro-electrodes during rapid changes of luminal electrolyte concentrations. Peritubular membrane potential and net fluid reabsorption (split-oil-droplet method) were also measured with and without application of various drugs known to alter transport. Raising the luminal sodium concentration from 10 to 100 mM reversibly increased the peritubular p.d. The magnitude of the peritubular p.d. was a saturable function of luminal sodium concentration. In the presence of chloride in the lumen the peritubular hyperpolarization following increased luminal sodium could be inhibited by luminal amiloride (10(-4)M). Sodium-induced hyperpolarization of the peritubular p.d. could be completely inhibited by 10(-5)M-ouabain. Adding amiloride (10(-4)M) to the luminal fluid rapidly and reversibly depolarized the peritubular p.d. and inhibited fluid reabsorption. Addition of amphotericin B (20 micrograms/ml) to the luminal perfusion solution had no effect on peritubular p.d. at 100 mM-luminal NaCl but at 10 mM-NaCl, peritubular p.d. hyperpolarized. Fluid reabsorption was stimulated (with 100 mM-NaCl in the lumen). Addition of amphotericin when the tubule was perfused on both sides with solutions containing a constant potassium concentration of 78 mM and a variable sodium concentration ranging from 7.8 to 34.5 mM revealed strong dependence of the peritubular hyperpolarization on the sodium concentration. Luminal furosemide (10(-4)M) and chlorothiazide (10(-4)M) and peritubular ethacrynic acid (10(-4)M) all reduced fluid reabsorption but hyperpolarized the peritubular p.d. The data suggest the presence of an electrogenic sodium transport process in the peritubular membrane that directly contributes to the generation of the peritubular potential. In addition, chloride transport has an important role in determining this potential.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benos D. J. Amiloride: a molecular probe of sodium transport in tissues and cells. Am J Physiol. 1982 Mar;242(3):C131–C145. doi: 10.1152/ajpcell.1982.242.3.C131. [DOI] [PubMed] [Google Scholar]
- Bentley P. J. Action of amphotericin B on the toad bladder: evidence for sodium transport along two pathways. J Physiol. 1968 Jun;196(3):703–711. doi: 10.1113/jphysiol.1968.sp008531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagi B., Sohtell M., Giebisch G. Intracellular potassium activity in the rabbit proximal straight tubule. Am J Physiol. 1981 Dec;241(6):F677–F686. doi: 10.1152/ajprenal.1981.241.6.F677. [DOI] [PubMed] [Google Scholar]
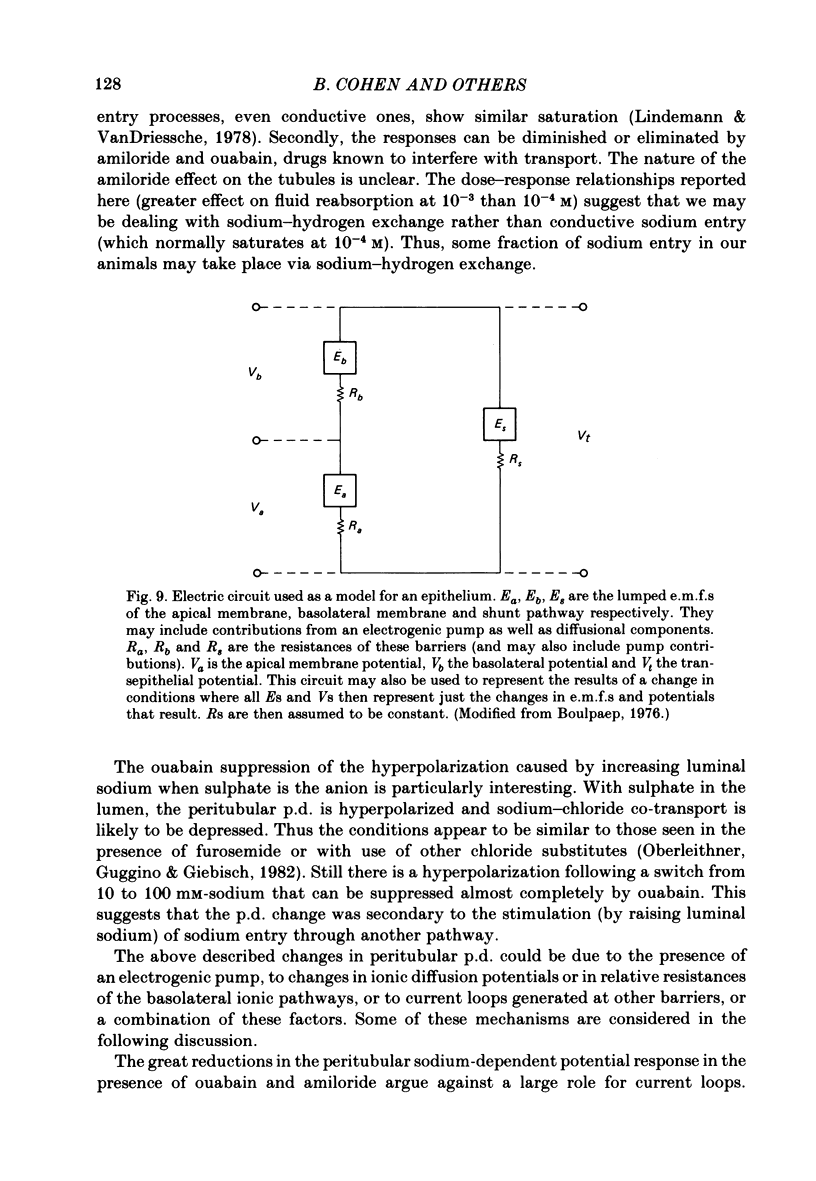
- Boulpaep E. L. Electrical phenomena in the nephron. Kidney Int. 1976 Feb;9(2):88–102. doi: 10.1038/ki.1976.14. [DOI] [PubMed] [Google Scholar]
- Burg M. B. Thick ascending limb of Henle's loop. Kidney Int. 1982 Nov;22(5):454–464. doi: 10.1038/ki.1982.198. [DOI] [PubMed] [Google Scholar]
- Burg M., Stoner L., Cardinal J., Green N. Furosemide effect on isolated perfused tubules. Am J Physiol. 1973 Jul;225(1):119–124. doi: 10.1152/ajplegacy.1973.225.1.119. [DOI] [PubMed] [Google Scholar]
- Curci S., Casavola V., Lippe C. Permeability pathways for non-electrolytes through Bufo bufo gall-bladder. Pflugers Arch. 1975 Mar 26;355(3):267–271. doi: 10.1007/BF00583689. [DOI] [PubMed] [Google Scholar]
- Davis C. W., Finn A. L. Sodium transport effects on the basolateral membrane in toad urinary bladder. J Gen Physiol. 1982 Nov;80(5):733–751. doi: 10.1085/jgp.80.5.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degnan K. J., Zadunaisky J. A. The electrical properties and active ion transport across the urinary bladder of the urodele, Amphiuma means. J Physiol. 1977 Feb;265(1):207–230. doi: 10.1113/jphysiol.1977.sp011713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte C. G., Chomety F., Giebisch G. Effect of amiloride, ouabain, and furosemide on distal tubular function in the rat. Am J Physiol. 1971 Aug;221(2):632–640. doi: 10.1152/ajplegacy.1971.221.2.632. [DOI] [PubMed] [Google Scholar]
- Ehrlich E. N., Crabbé J. The mechanism of action of amipramizide. Pflugers Arch. 1968;302(1):79–96. doi: 10.1007/BF00586783. [DOI] [PubMed] [Google Scholar]
- Frömter E., Gebler B. Electrical properties of amphibian urinary bladder epithelia. III. The cell membrane resistances and the effect of amiloride. Pflugers Arch. 1977 Oct 19;371(1-2):99–108. doi: 10.1007/BF00580777. [DOI] [PubMed] [Google Scholar]
- Frömter E., Gessner K. Effect of inhibitors and diuretics on electrical potential differences in rat kidney proximal tubule. Pflugers Arch. 1975 Jun 26;357(3-4):209–224. doi: 10.1007/BF00585976. [DOI] [PubMed] [Google Scholar]
- GERTZ K. H. [Transtubular sodium chloride transport and permeability for nonelectrolytes in the proximal and distal convolution of the rat kidney]. Pflugers Arch Gesamte Physiol Menschen Tiere. 1963;276:336–356. [PubMed] [Google Scholar]
- Grandchamp A., Boulpaep E. L. Pressure control of sodium reabsorption and intercellular backflux across proximal kidney tubule. J Clin Invest. 1974 Jul;54(1):69–82. doi: 10.1172/JCI107751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greger R. Chloride reabsorption in the rabbit cortical thick ascending limb of the loop of Henle. A sodium dependent process. Pflugers Arch. 1981 Apr;390(1):38–43. doi: 10.1007/BF00582708. [DOI] [PubMed] [Google Scholar]
- Greger R., Schlatter E., Lang F. Evidence for electroneutral sodium chloride cotransport in the cortical thick ascending limb of Henle's loop of rabbit kidney. Pflugers Arch. 1983 Mar;396(4):308–314. doi: 10.1007/BF01063936. [DOI] [PubMed] [Google Scholar]
- Greger R., Schlatter E. Properties of the basolateral membrane of the cortical thick ascending limb of Henle's loop of rabbit kidney. A model for secondary active chloride transport. Pflugers Arch. 1983 Mar;396(4):325–334. doi: 10.1007/BF01063938. [DOI] [PubMed] [Google Scholar]
- Greger R., Schlatter E. Properties of the lumen membrane of the cortical thick ascending limb of Henle's loop of rabbit kidney. Pflugers Arch. 1983 Mar;396(4):315–324. doi: 10.1007/BF01063937. [DOI] [PubMed] [Google Scholar]
- Györy A. Z. Reexamination of the split oil droplet method as applied to kidney tubules. Pflugers Arch. 1971;324(4):328–343. doi: 10.1007/BF00592461. [DOI] [PubMed] [Google Scholar]
- Hansen L. L., Schilling A. R., Wiederholt M. Effect of calcium, furosemide and chlorothiazide on net volume reabsorption and basolateral membrane potential of the distal tubule. Pflugers Arch. 1981 Jan;389(2):121–126. doi: 10.1007/BF00582101. [DOI] [PubMed] [Google Scholar]
- LICHTENSTEIN N. S., LEAF A. EFFECT OF AMPHOTERICIN B ON THE PERMEABILITY OF THE TOAD BLADDER. J Clin Invest. 1965 Aug;44:1328–1342. doi: 10.1172/JCI105238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenfant C., Johansen K. Respiratory adaptations in selected amphibians. Respir Physiol. 1967 May;2(3):247–260. doi: 10.1016/0034-5687(67)90030-8. [DOI] [PubMed] [Google Scholar]
- Lewis S. A., Wills N. K., Eaton D. C. Basolateral membrane potential of a tight epithelium: ionic diffusion and electrogenic pumps. J Membr Biol. 1978 Jun 28;41(2):117–148. doi: 10.1007/BF01972629. [DOI] [PubMed] [Google Scholar]
- Lindenmayer G. E., Schwartz A., Thompson H. K., Jr A kinetic description for sodium and potassium effects on (Na+ plus K+)-adenosine triphosphatase: a model for a two-nonequivalent site potassium activation and an analysis of multiequivalent site models for sodium activation. J Physiol. 1974 Jan;236(1):1–28. doi: 10.1113/jphysiol.1974.sp010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberleithner H., Giebisch G., Lang F., Wang W. Cellular Mechanism of the furosemide sensitive transport system in the kidney. Klin Wochenschr. 1982 Oct 1;60(19):1173–1179. doi: 10.1007/BF01716719. [DOI] [PubMed] [Google Scholar]
- Oberleithner H., Guggino W., Giebisch G. Mechanism of distal tubular chloride transport in Amphiuma kidney. Am J Physiol. 1982 Apr;242(4):F331–F339. doi: 10.1152/ajprenal.1982.242.4.F331. [DOI] [PubMed] [Google Scholar]
- Oberleithner H., Guggino W., Giebisch G. Potassium transport in the early distal tubule of Amphiuma kidney. Effects of potassium adaptation. Pflugers Arch. 1983 Mar 1;396(3):185–191. doi: 10.1007/BF00587854. [DOI] [PubMed] [Google Scholar]
- Oberleithner H., Lang F., Wang W., Giebisch G. Effects of inhibition of chloride transport on intracellular sodium activity in distal amphibian nephron. Pflugers Arch. 1982 Jul;394(1):55–60. doi: 10.1007/BF01108308. [DOI] [PubMed] [Google Scholar]
- Sackin H., Boulpaep E. L. Isolated perfused salamander proximal tubule. II. Monovalent ion replacement and rheogenic transport. Am J Physiol. 1981 Nov;241(5):F540–F555. doi: 10.1152/ajprenal.1981.241.5.F540. [DOI] [PubMed] [Google Scholar]
- Spring K. R., Giebisch G. Kinetics of Na+ transport in Necturus proximal tubule. J Gen Physiol. 1977 Sep;70(3):307–328. doi: 10.1085/jgp.70.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroup R. F., Weinman E., Hayslett J. P., Kashgarian M. Effect of luminal permeability on net transport across the amphibian proximal tubule. Am J Physiol. 1974 May;226(5):1110–1116. doi: 10.1152/ajplegacy.1974.226.5.1110. [DOI] [PubMed] [Google Scholar]
- Sullivan W. J. Electrical potential differences across distal renal tubules of Amphiuma. Am J Physiol. 1968 May;214(5):1096–1103. doi: 10.1152/ajplegacy.1968.214.5.1096. [DOI] [PubMed] [Google Scholar]
- Wiederholt M., Sullivan W. J., Giebisch G. Potassium and sodium transport across single distal tubules of Amphiuma. J Gen Physiol. 1971 May;57(5):495–525. doi: 10.1085/jgp.57.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]


