Abstract
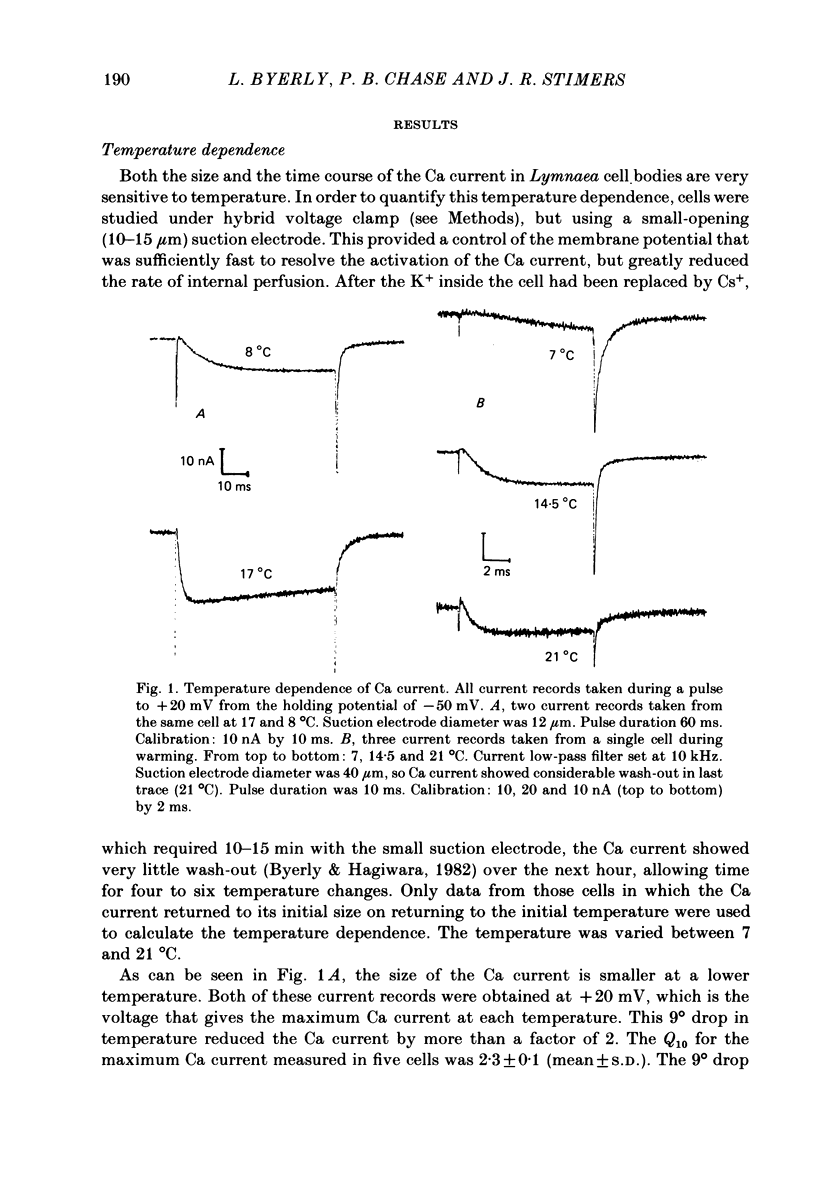
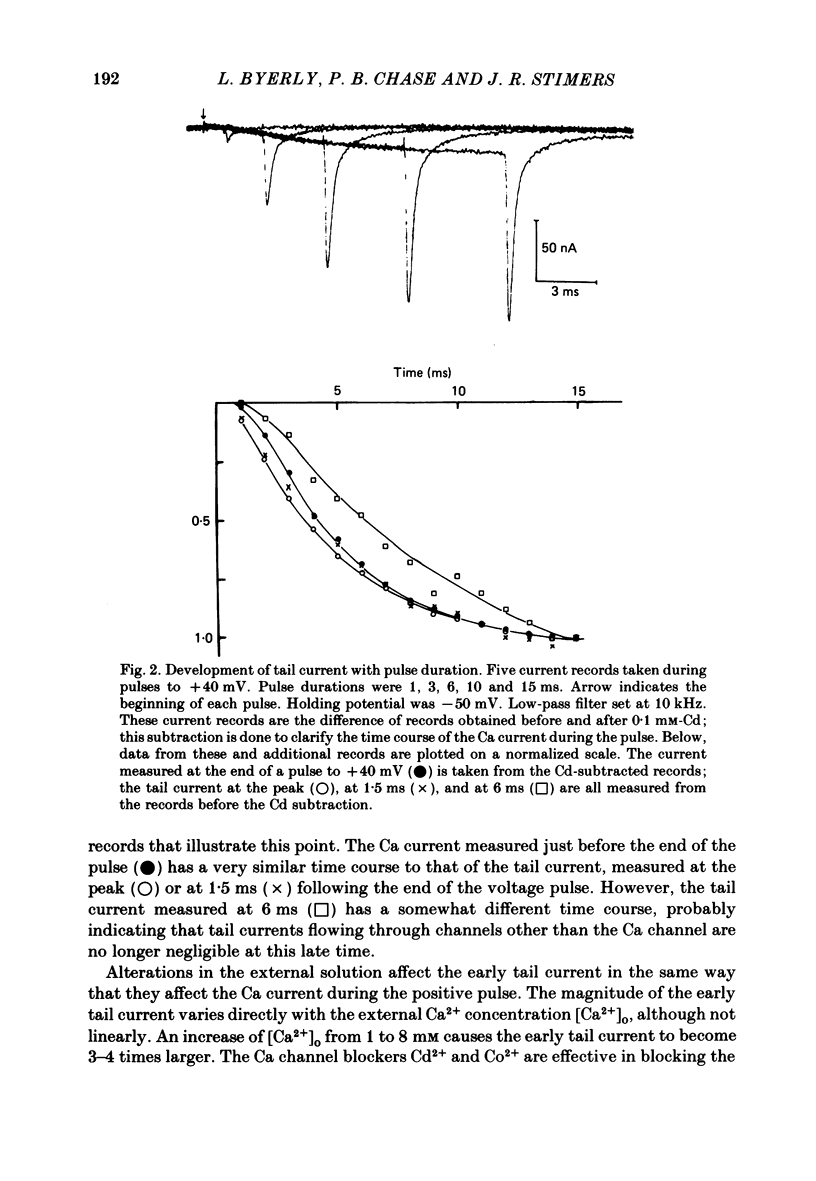
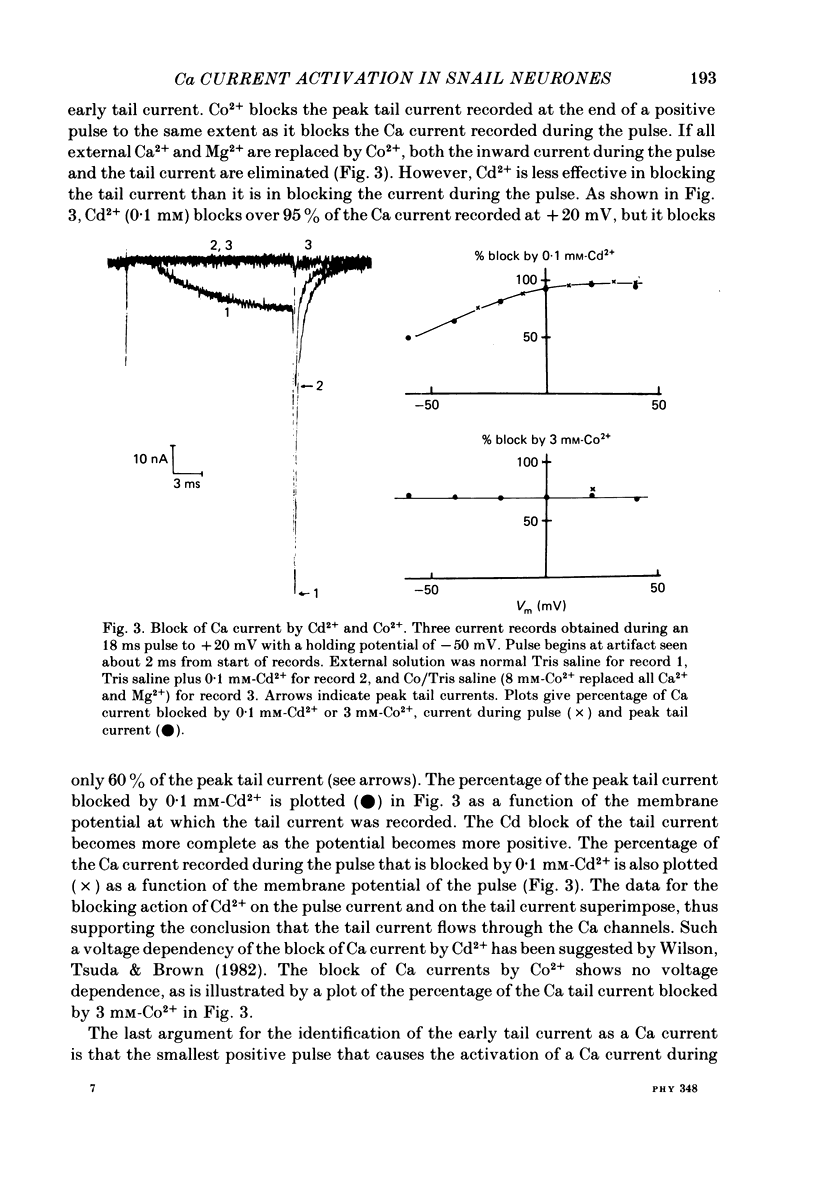
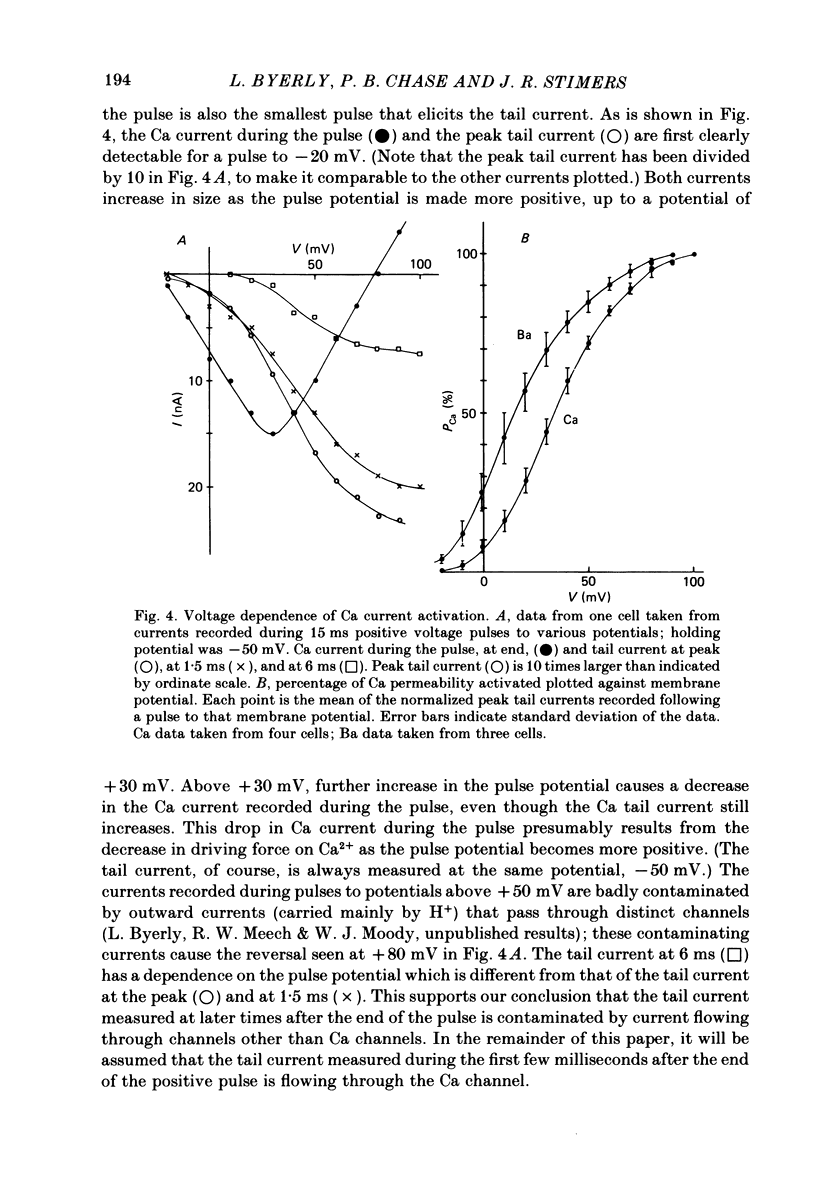
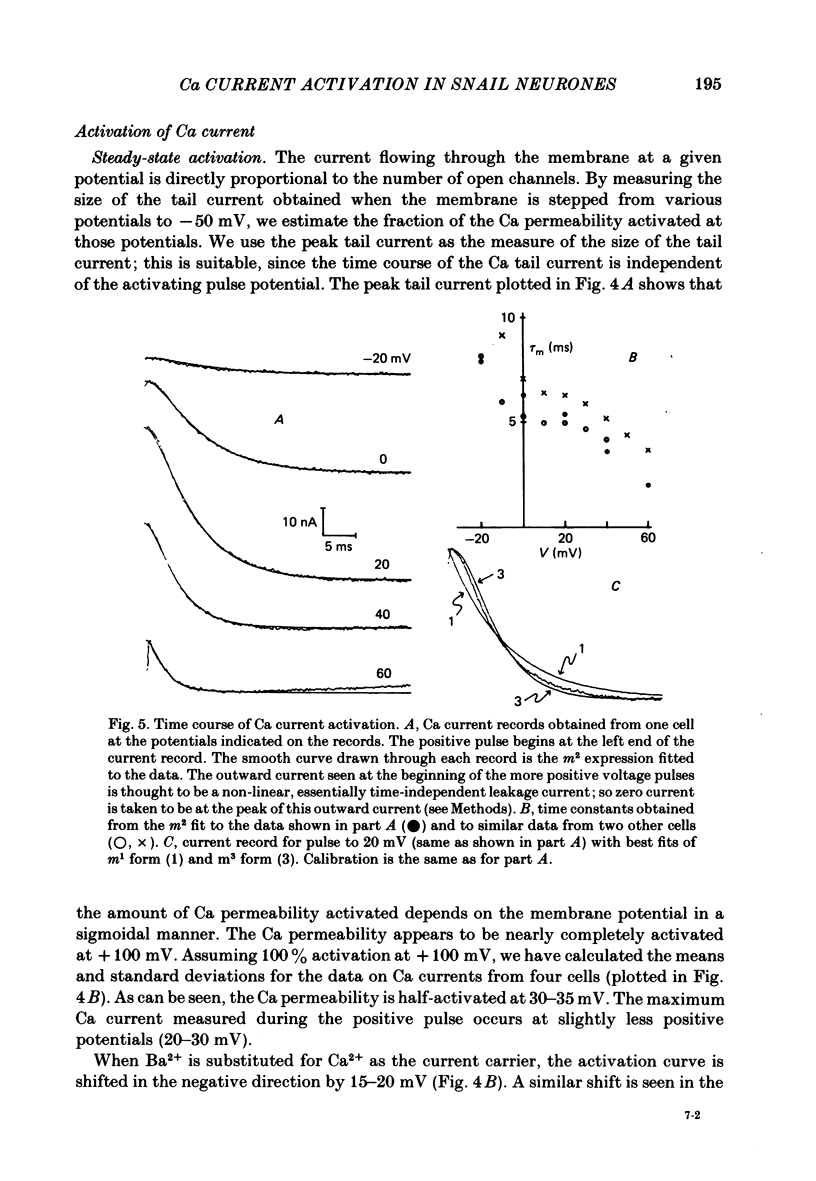
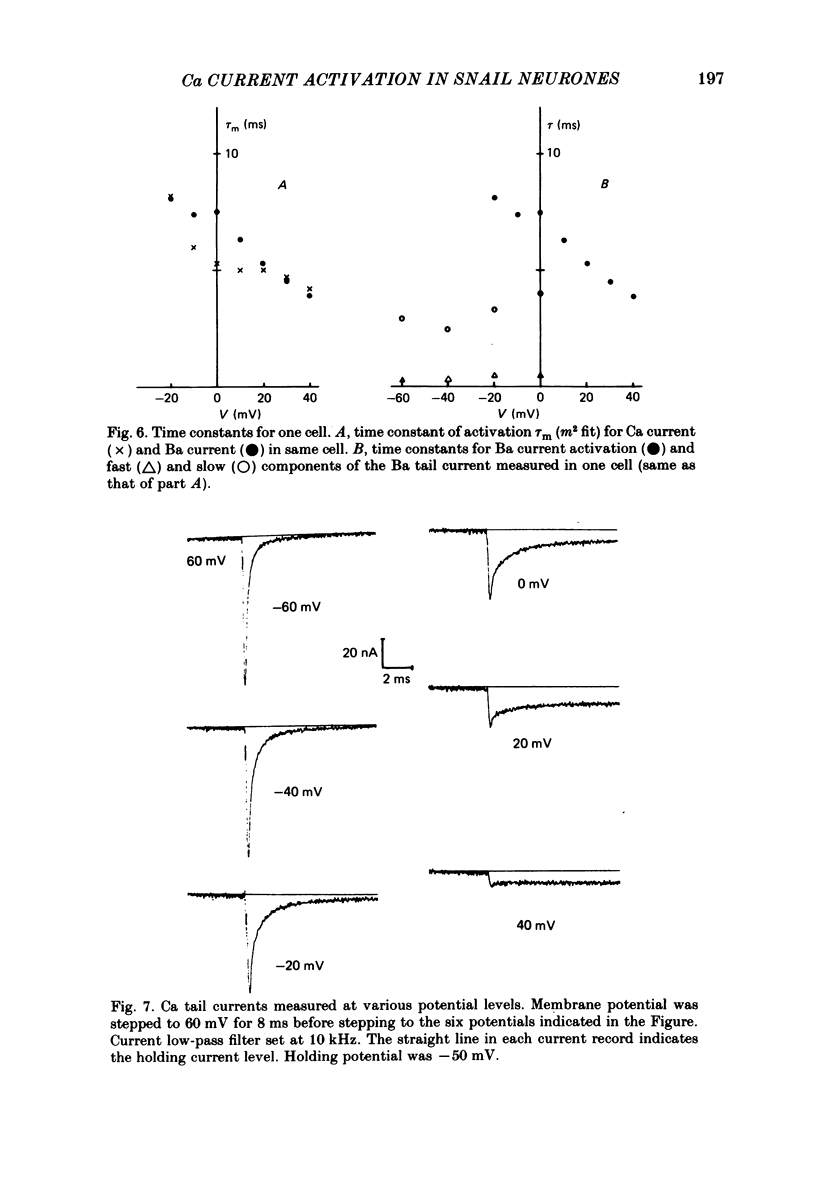
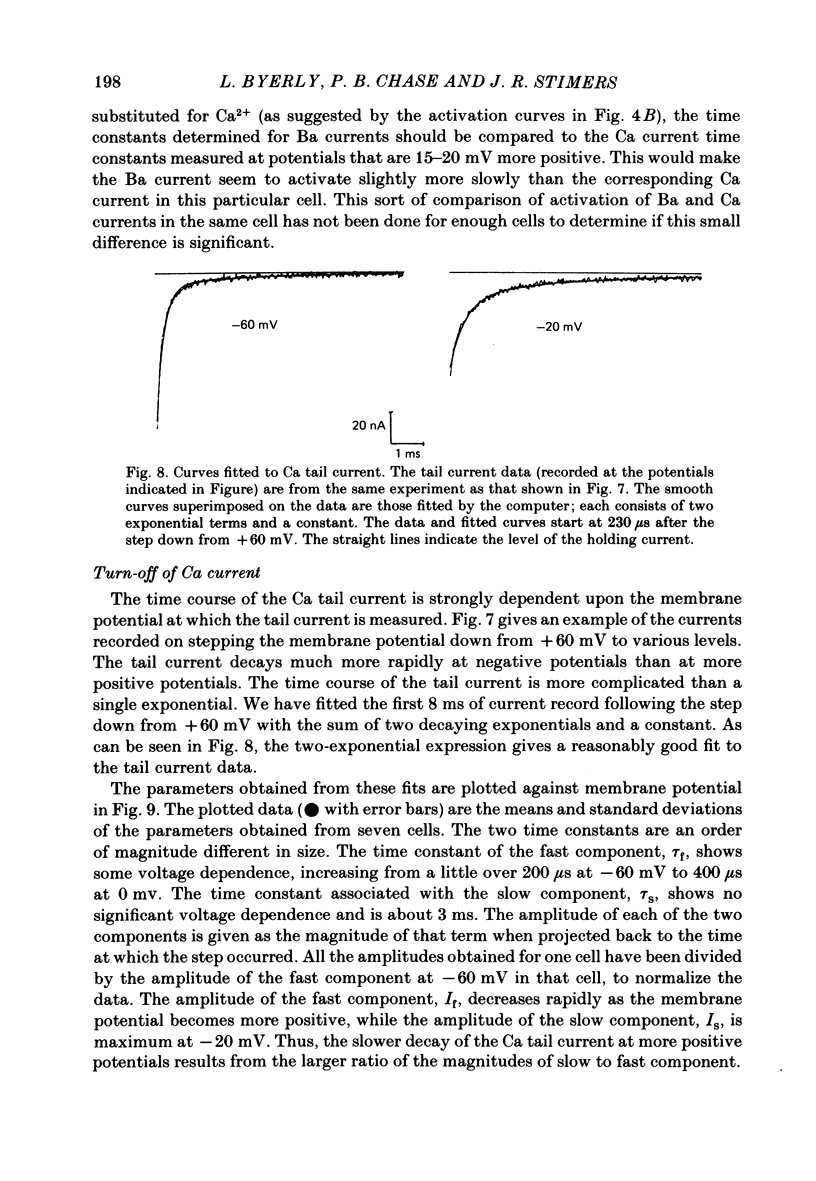
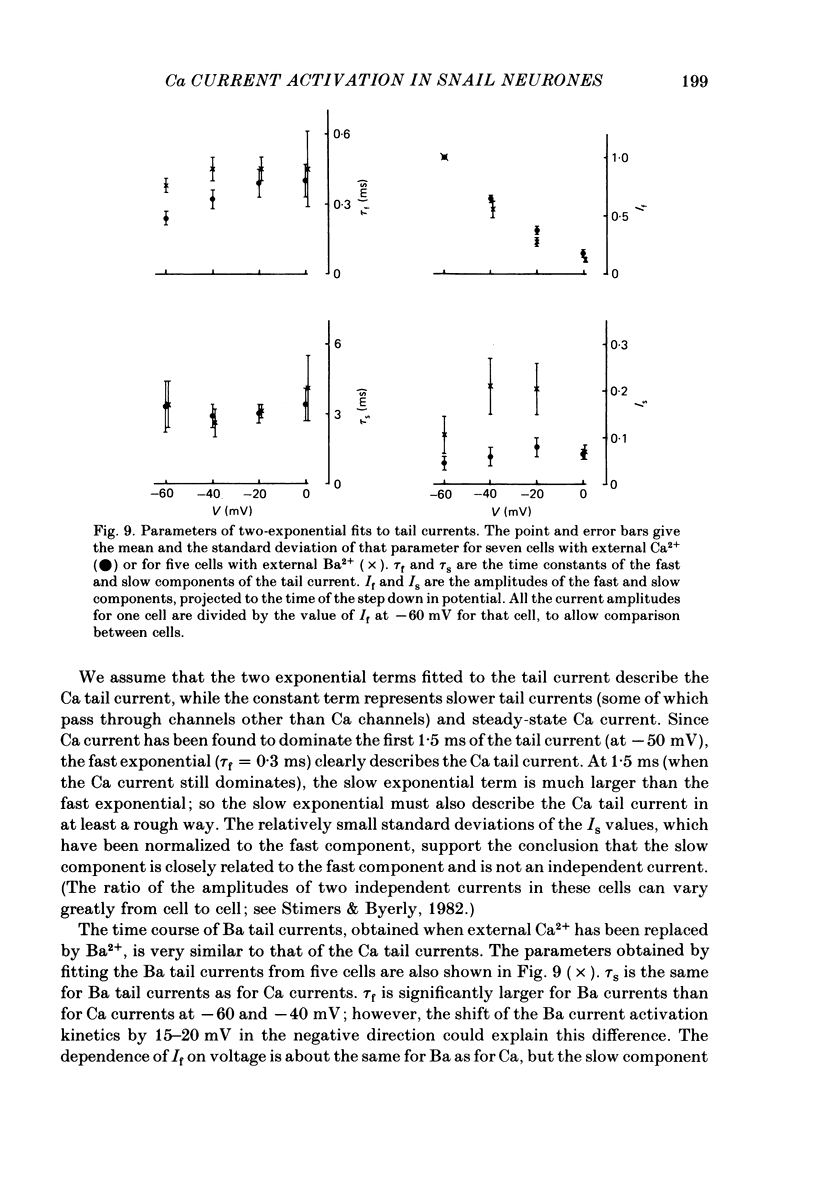
Both the activation kinetics and the magnitude of the Ca current in Lymnaea are strongly dependent on temperature. The Q10 for the reciprocal of the activation time constant is 4.9 +/- 0.2 and the Q10 for the maximum current is 2.3 +/- 0.1. By lowering the temperature to 7-10 degrees C, we have been able to resolve the Ca tail currents. The block of Ca current by Cd2+ is voltage dependent, being more effective at more positive potentials. As determined from the magnitude of the tail currents, the Ca permeability is not maximally activated until the membrane potential is greater than +70 mV. The Ca permeability is half activated in the range 30-35 mV. The open-channel current-voltage relation for the Ca current is in rough agreement with the prediction of the constant-field equation. There is no indication of current saturation at negative potentials for potentials down to -60 mV. The Ca tail current decays with at least two time constants, one 200-400 microseconds and the other 2-4 ms. Although these time constants are not strongly voltage dependent, the ratio of the amplitude of the fast component of the tail current to that of the slow component is much larger at -60 mV than at 0 mV. The time course of the Ba tail current is very similar to that of the Ca tail current. The time course of the activation of the Ca current follows m2 kinetics and does not show evidence for a Cole-Moore-type shift for holding potentials between -50 and -110 mV. During a second positive pulse applied 1 ms after the first, the Ca current activates more rapidly, without the delay characteristic of the Ca current of a single positive pulse. The activation of the Ca current can be represented by a linear sequential model. The simplest model that describes both the turn-on and the turn-off of the Ca current must have at least three closed states, followed by a single open state.
Full text
PDF





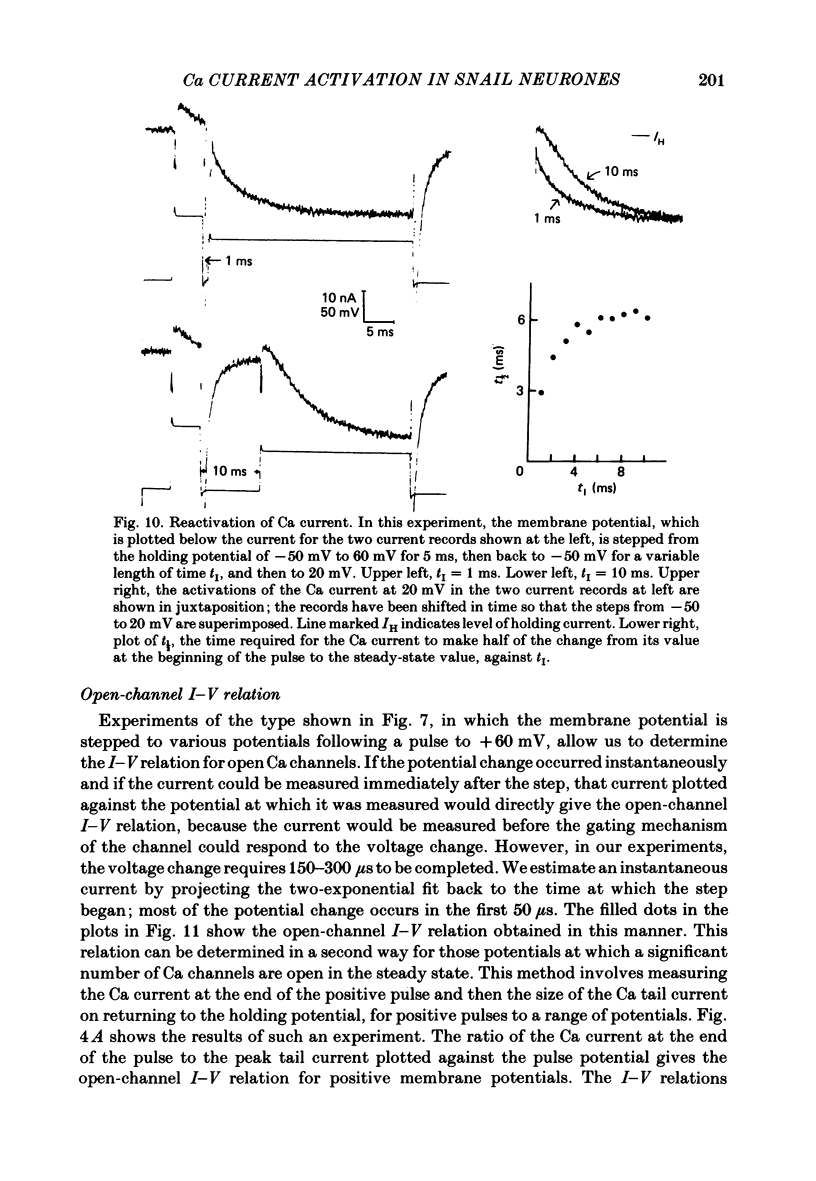
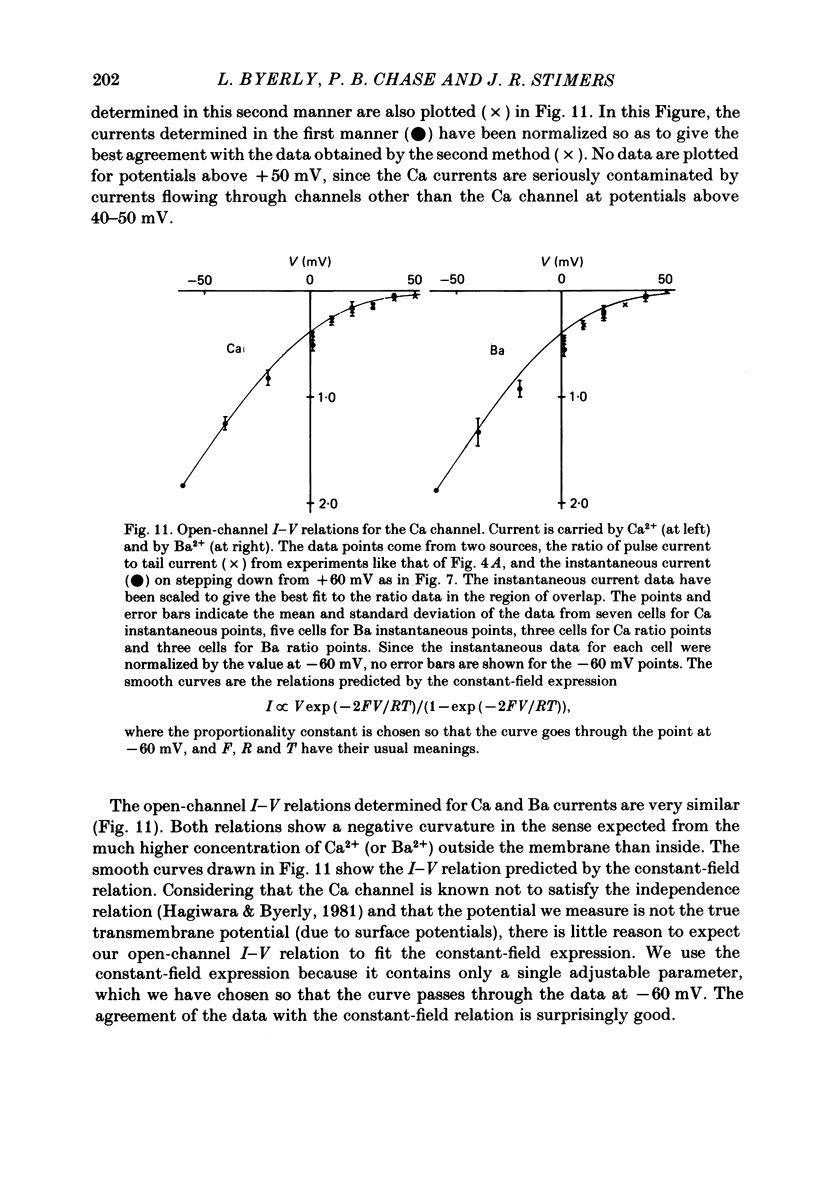





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Byerly L., Hagiwara S. Calcium currents in internally perfused nerve cell bodies of Limnea stagnalis. J Physiol. 1982 Jan;322:503–528. doi: 10.1113/jphysiol.1982.sp014052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLE K. S., MOORE J. W. Potassium ion current in the squid giant axon: dynamic characteristic. Biophys J. 1960 Sep;1:1–14. doi: 10.1016/s0006-3495(60)86871-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R., Tillotson D. L. Calcium-mediated inactivation of the calcium conductance in caesium-loaded giant neurones of Aplysia californica. J Physiol. 1981 May;314:265–280. doi: 10.1113/jphysiol.1981.sp013706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda J., Kawa K. Permeation of manganese, cadmium, zinc, and beryllium through calcium channels of an insect muscle membrane. Science. 1977 Apr 15;196(4287):309–311. doi: 10.1126/science.847472. [DOI] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Ohmori H. Studies of calcium channels in rat clonal pituitary cells with patch electrode voltage clamp. J Physiol. 1982 Oct;331:231–252. doi: 10.1113/jphysiol.1982.sp014371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Ohmori H. Studies of single calcium channel currents in rat clonal pituitary cells. J Physiol. 1983 Mar;336:649–661. doi: 10.1113/jphysiol.1983.sp014603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Kimura J. E., Meves H. The effect of temperature on the asymmetrical charge movement in squid giant axons. J Physiol. 1979 Apr;289:479–500. doi: 10.1113/jphysiol.1979.sp012748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuk P. G., Krishtal O. A., Pidoplichko V. I. Calcium inward current and related charge movements in the membrane of snail neurones. J Physiol. 1981 Jan;310:403–421. doi: 10.1113/jphysiol.1981.sp013557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyuk P. G., Krishtal O. A., Shakhovalov Y. A. Separation of sodium and calcium currents in the somatic membrane of mollusc neurones. J Physiol. 1977 Sep;270(3):545–568. doi: 10.1113/jphysiol.1977.sp011968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryshtal' O. A., Pidoplichko V. I. Vnutrikletochnaia perfuziia gigantskikh neironov ulitki. Neirofiziologiia. 1975;7(3):327–329. [PubMed] [Google Scholar]
- Lee K. S., Akaike N., Brown A. M. Properties of internally perfused, voltage-clamped, isolated nerve cell bodies. J Gen Physiol. 1978 May;71(5):489–507. doi: 10.1085/jgp.71.5.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Presynaptic calcium currents in squid giant synapse. Biophys J. 1981 Mar;33(3):289–321. doi: 10.1016/S0006-3495(81)84898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux H. D., Nagy K. Single channel Ca2+ currents in Helix pomatia neurons. Pflugers Arch. 1981 Sep;391(3):252–254. doi: 10.1007/BF00596179. [DOI] [PubMed] [Google Scholar]
- Oxford G. S. Some kinetic and steady-state properties of sodium channels after removal of inactivation. J Gen Physiol. 1981 Jan;77(1):1–22. doi: 10.1085/jgp.77.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant T. D., Standen N. B., Ward T. A. The effects of injection of calcium ions and calcium chelators on calcium channel inactivation in Helix neurones. J Physiol. 1983 Jan;334:189–212. doi: 10.1113/jphysiol.1983.sp014489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983 Feb 17;301(5901):569–574. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- Reuter H., Stevens C. F., Tsien R. W., Yellen G. Properties of single calcium channels in cardiac cell culture. Nature. 1982 Jun 10;297(5866):501–504. doi: 10.1038/297501a0. [DOI] [PubMed] [Google Scholar]
- Stimers J. R., Byerly L. Slowing of sodium current inactivation by ruthenium red in snail neurons. J Gen Physiol. 1982 Oct;80(4):485–497. doi: 10.1085/jgp.80.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Yoshii M. Effects of internal free calcium upon the sodium and calcium channels in the tunicate egg analysed by the internal perfusion technique. J Physiol. 1978 Jun;279:519–549. doi: 10.1113/jphysiol.1978.sp012360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillotson D. Inactivation of Ca conductance dependent on entry of Ca ions in molluscan neurons. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1497–1500. doi: 10.1073/pnas.76.3.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]



