Abstract
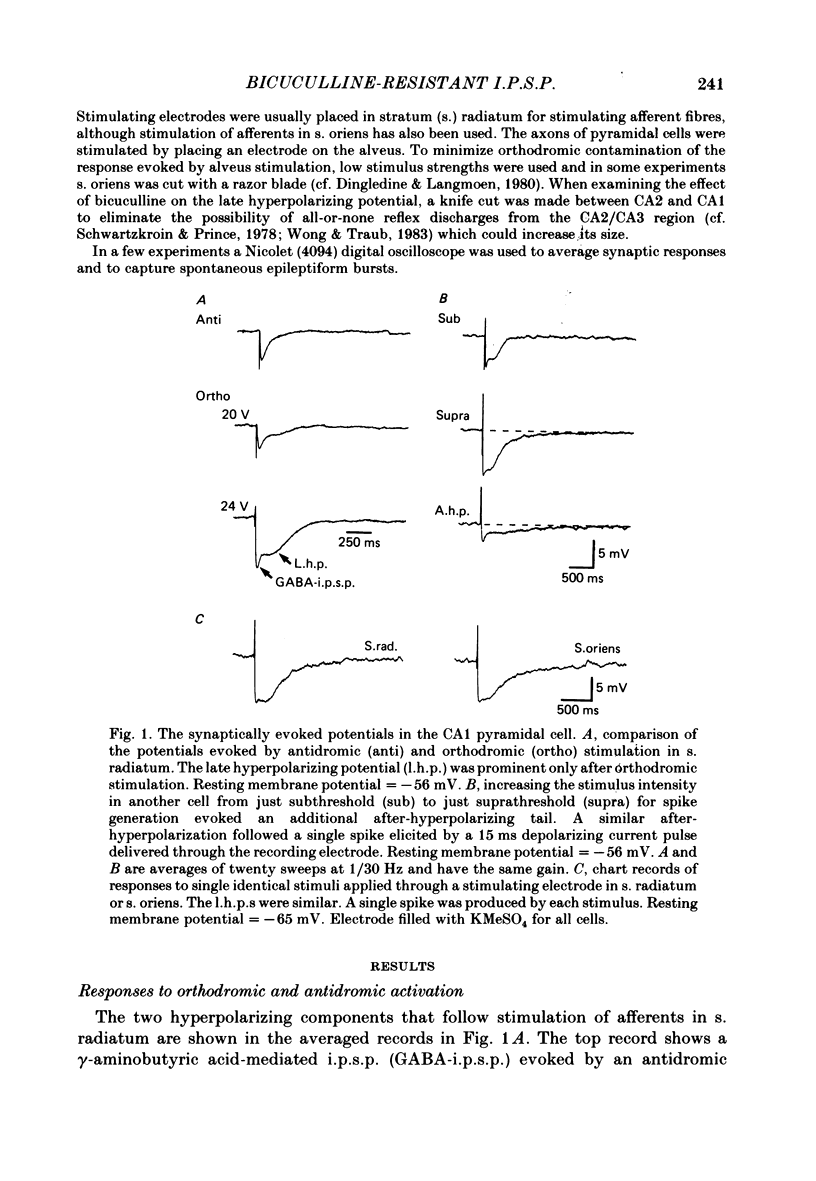
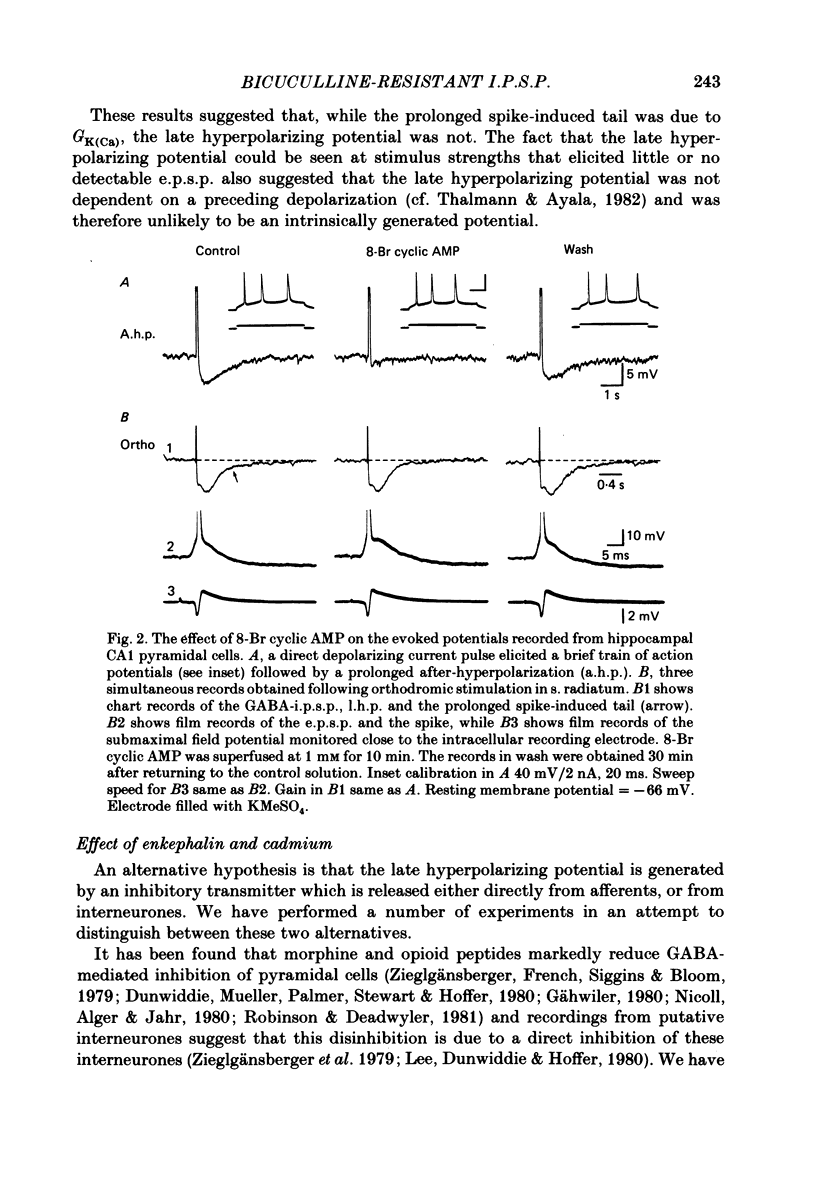
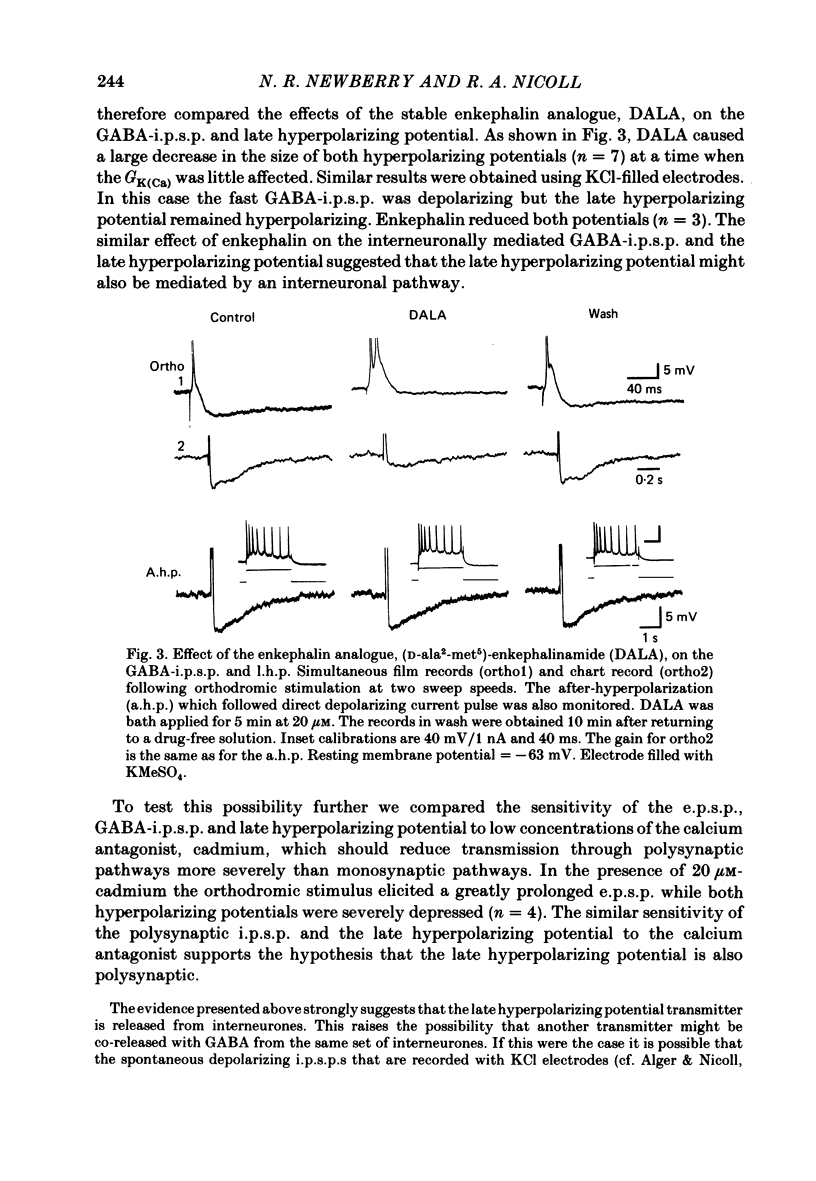
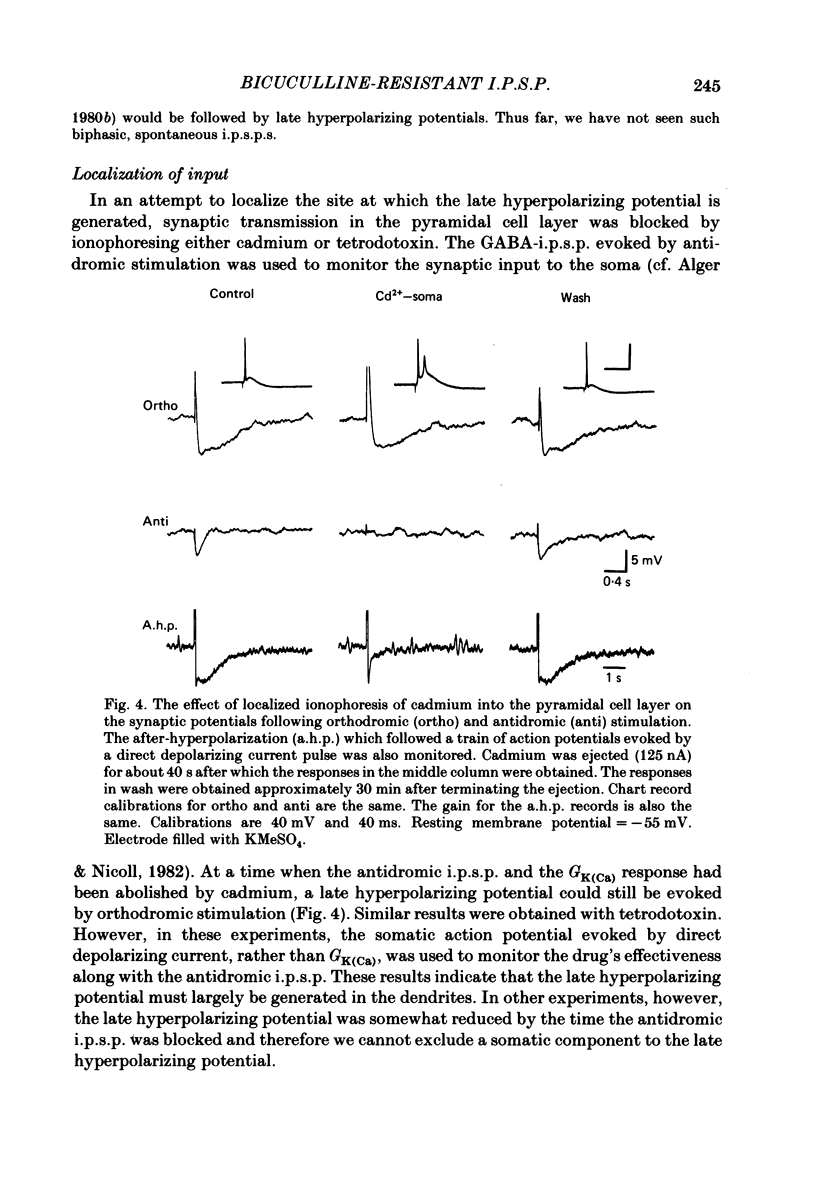
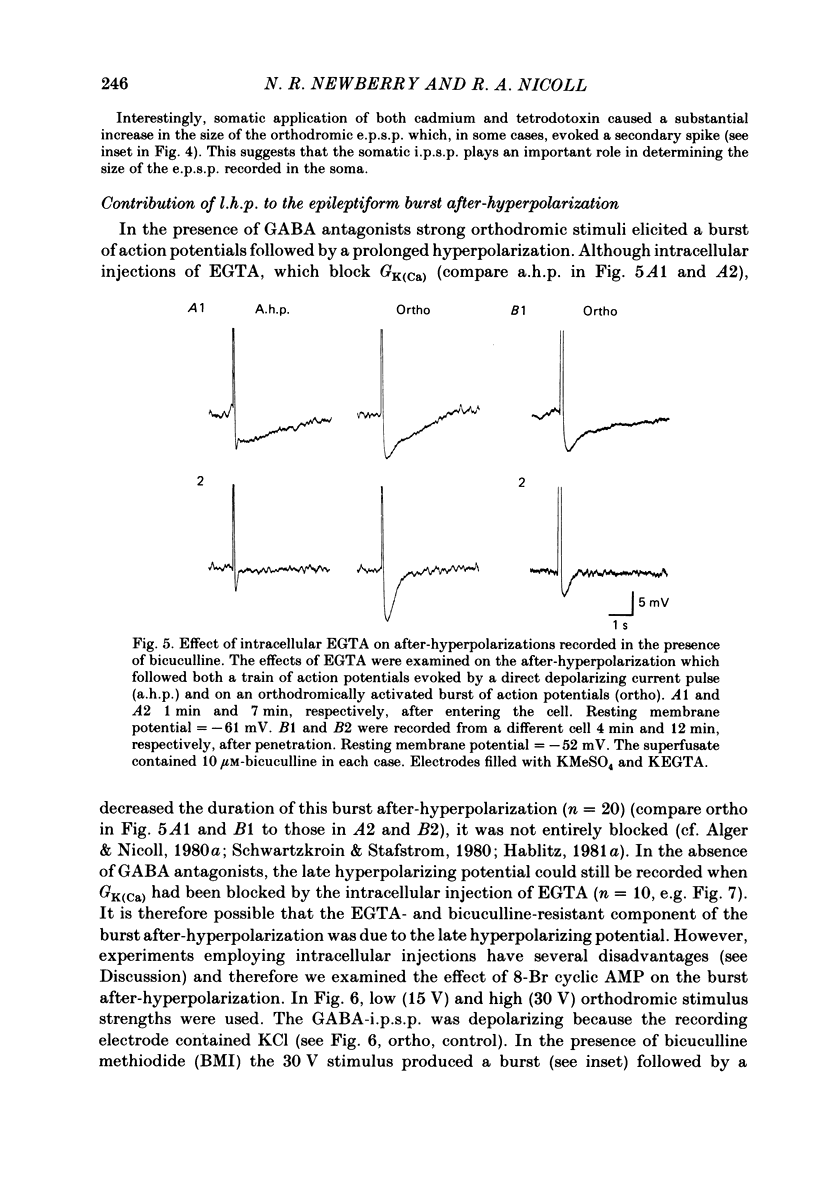
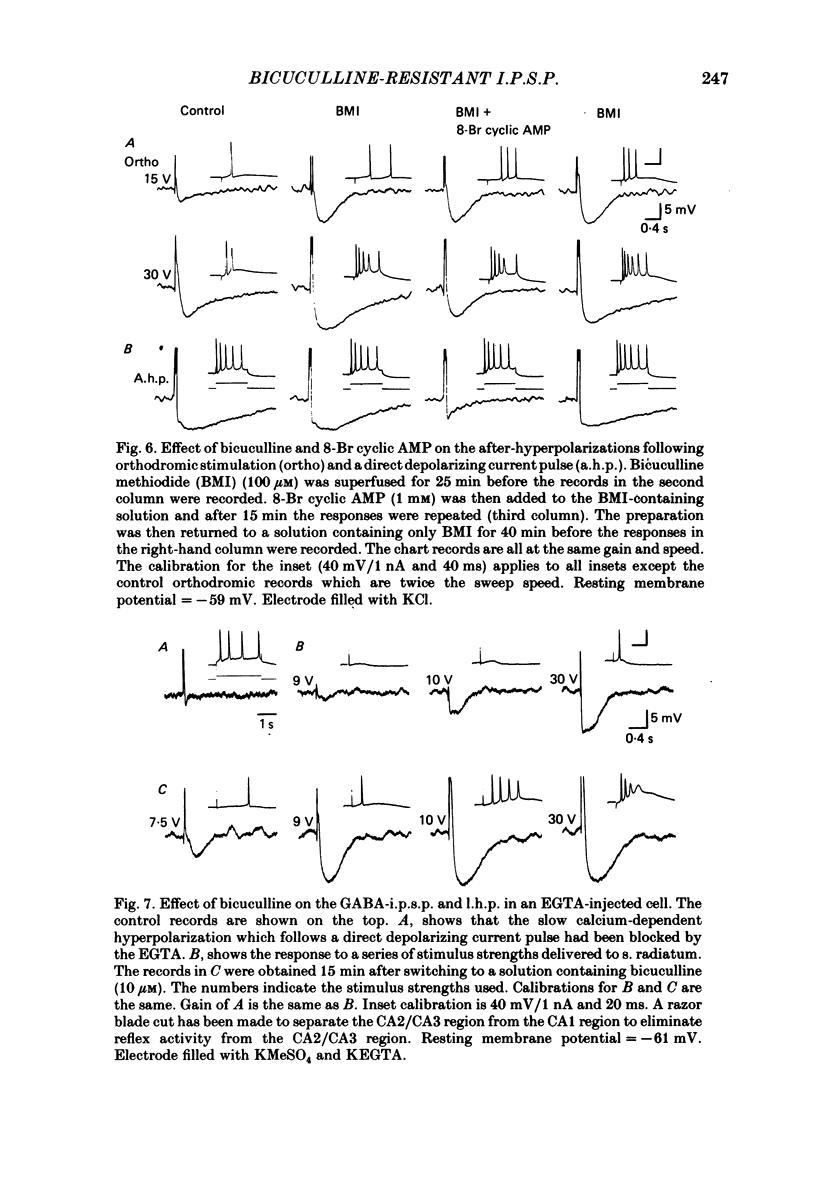
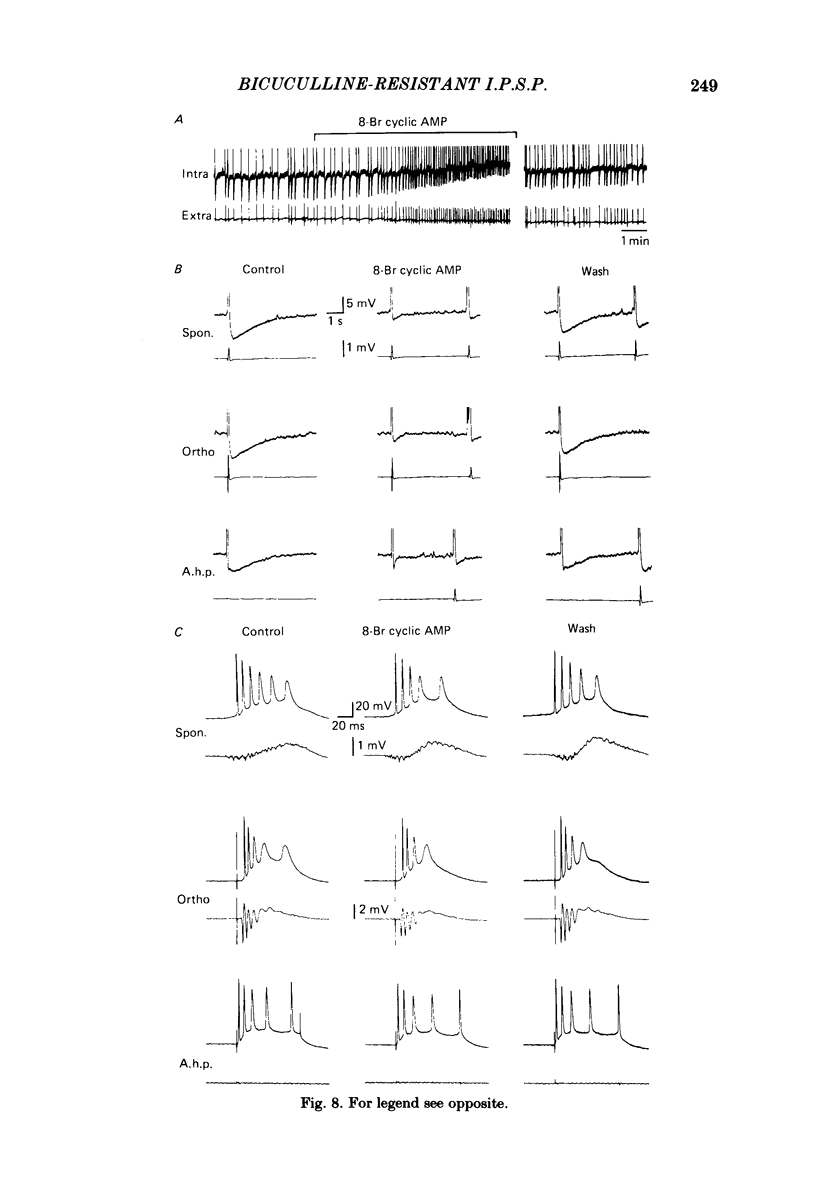
Experiments were performed on rat hippocampal CA1 pyramidal cells in vitro in order to elucidate the origin of the late hyperpolarizing potential, which follows the gamma-aminobutyric acid (GABA)-mediated inhibitory post-synaptic potential (GABA-i.p.s.p.). The late hyperpolarizing potential could be evoked by orthodromic stimulation via stratum radiatum or stratum oriens but not by selective antidromic stimulation. The membrane soluble analogue of cyclic AMP, 8-bromoadenosine 3',5'-cyclic monophosphate (8-Br cyclic AMP), which blocks calcium-activated potassium hyperpolarizations (GK(Ca], did not reduce the late hyperpolarizing potential. The enkephalin analogue, (D-ala2-met5)-enkephalinamide (DALA) reversibly reduced both the GABA-i.p.s.p. and the late hyperpolarizing potential. The late hyperpolarizing potential and GABA-i.p.s.p. were more sensitive to low doses of the calcium antagonist, cadmium, than the excitatory post-synaptic potential (e.p.s.p.). The local application of cadmium to the pyramidal cell layer blocked the antidromic i.p.s.p. but the orthodromically evoked late hyperpolarizing potential was less affected. In contrast to the GABA-i.p.s.p., the late hyperpolarizing potential was not reversed by chloride injection and was enhanced, rather than depressed, by bicuculline. We conclude that the late hyperpolarizing potential is a bicuculline-resistant i.p.s.p. The unidentified transmitter for this i.p.s.p. is released from feed-forward interneurones primarily onto the dendrites of the pyramidal cell and may act by increasing the potassium permeability of the membrane. The epileptiform burst after-hyperpolarization evoked in the presence of GABA antagonists is composed of at least two components, a long-duration hyperpolarization mediated GK(Ca) and an earlier and shorter late hyperpolarizing potential. Blockade of the GK(Ca) by 8-Br cyclic AMP did not alter the duration of epileptiform bursts but did markedly increase the frequency of their occurrence. This suggests that GK(Ca) is involved in controlling the interval between bursts.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alger B. E., Nicoll R. A. Epileptiform burst afterhyperolarization: calcium-dependent potassium potential in hippocampal CA1 pyramidal cells. Science. 1980 Dec 5;210(4474):1122–1124. doi: 10.1126/science.7444438. [DOI] [PubMed] [Google Scholar]
- Alger B. E., Nicoll R. A. Feed-forward dendritic inhibition in rat hippocampal pyramidal cells studied in vitro. J Physiol. 1982 Jul;328:105–123. doi: 10.1113/jphysiol.1982.sp014255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger B. E., Nicoll R. A. Spontaneous inhibitory post-synaptic potentials in hippocampus: mechanism for tonic inhibition. Brain Res. 1980 Oct 27;200(1):195–200. doi: 10.1016/0006-8993(80)91108-7. [DOI] [PubMed] [Google Scholar]
- Constanti A., Connor J. D., Galvan M., Nistri A. Intracellularly-recorded effects of glutamate and aspartate on neurones in the guinea-pig olfactory cortex slice. Brain Res. 1980 Aug 18;195(2):403–420. doi: 10.1016/0006-8993(80)90075-x. [DOI] [PubMed] [Google Scholar]
- Dingledine R., Langmoen I. A. Conductance changes and inhibitory actions of hippocampal recurrent IPSPs. Brain Res. 1980 Mar 10;185(2):277–287. doi: 10.1016/0006-8993(80)91068-9. [DOI] [PubMed] [Google Scholar]
- Dodd J., Kelly J. S. The actions of cholecystokinin and related peptides on pyramidal neurones of the mammalian hippocampus. Brain Res. 1981 Feb 2;205(2):337–350. doi: 10.1016/0006-8993(81)90344-9. [DOI] [PubMed] [Google Scholar]
- Dodd J., Kelly S. Is somatostatin an excitatory transmitter in the hippocampus? Nature. 1978 Jun 22;273(5664):674–675. doi: 10.1038/273674a0. [DOI] [PubMed] [Google Scholar]
- Dunwiddie T., Mueller A., Palmer M., Stewart J., Hoffer B. Electrophysiological interactions of enkephalins with neuronal circuitry in the rat hippocampus. I. Effects on pyramidal cell activity. Brain Res. 1980 Feb 24;184(2):311–330. doi: 10.1016/0006-8993(80)90801-x. [DOI] [PubMed] [Google Scholar]
- Finley J. C., Maderdrut J. L., Roger L. J., Petrusz P. The immunocytochemical localization of somatostatin-containing neurons in the rat central nervous system. Neuroscience. 1981;6(11):2173–2192. doi: 10.1016/0306-4522(81)90006-3. [DOI] [PubMed] [Google Scholar]
- Fujita Y. Evidence for the existence of inhibitory postsynaptic potentials in dendrites and their functional significance in hippocampal pyramidal cells of adult rabbits. Brain Res. 1979 Oct 12;175(1):59–69. doi: 10.1016/0006-8993(79)90514-6. [DOI] [PubMed] [Google Scholar]
- Greenwood R. S., Godar S. E., Reaves T. A., Jr, Hayward J. N. Cholecystokinin in hippocampal pathways. J Comp Neurol. 1981 Dec 10;203(3):335–350. doi: 10.1002/cne.902030303. [DOI] [PubMed] [Google Scholar]
- Gähwiler B. H. Excitatory action of opioid peptides and opiates on cultured hippocampal pyramidal cells. Brain Res. 1980 Jul 21;194(1):193–203. doi: 10.1016/0006-8993(80)91328-1. [DOI] [PubMed] [Google Scholar]
- Haas H. L., Konnerth A. Histamine and noradrenaline decrease calcium-activated potassium conductance in hippocampal pyramidal cells. 1983 Mar 31-Apr 6Nature. 302(5907):432–434. doi: 10.1038/302432a0. [DOI] [PubMed] [Google Scholar]
- Hablitz J. J. Altered burst responses in hippocampal CA3 neurons injected with EGTA. Exp Brain Res. 1981;42(3-4):483–485. doi: 10.1007/BF00237513. [DOI] [PubMed] [Google Scholar]
- Hablitz J. J. Effects of intracellular injections of chloride and EGTA on postepileptiform-burst hyperpolarizations in hippocampal neurons. Neurosci Lett. 1981 Mar 10;22(2):159–163. doi: 10.1016/0304-3940(81)90080-x. [DOI] [PubMed] [Google Scholar]
- Handelmann G., Meyer D. K., Beinfeld M. C., Oertel W. H. CCK-containing terminals in the hippocampus are derived from intrinsic neurons: an immunohistochemical and radioimmunological study. Brain Res. 1981 Nov 9;224(1):180–184. doi: 10.1016/0006-8993(81)91130-6. [DOI] [PubMed] [Google Scholar]
- Hotson J. R., Prince D. A. A calcium-activated hyperpolarization follows repetitive firing in hippocampal neurons. J Neurophysiol. 1980 Feb;43(2):409–419. doi: 10.1152/jn.1980.43.2.409. [DOI] [PubMed] [Google Scholar]
- Jahr C. E., Nicoll R. A. An intracellular analysis of dendrodendritic inhibition in the turtle in vitro olfactory bulb. J Physiol. 1982 May;326:213–234. doi: 10.1113/jphysiol.1982.sp014187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferys J. G., Haas H. L. Synchronized bursting of CA1 hippocampal pyramidal cells in the absence of synaptic transmission. Nature. 1982 Dec 2;300(5891):448–450. doi: 10.1038/300448a0. [DOI] [PubMed] [Google Scholar]
- KANDEL E. R., SPENCER W. A., BRINLEY F. J., Jr Electrophysiology of hippocampal neurons. I. Sequential invasion and synaptic organization. J Neurophysiol. 1961 May;24:225–242. doi: 10.1152/jn.1961.24.3.225. [DOI] [PubMed] [Google Scholar]
- Lee H. K., Dunwiddie T., Hoffer B. Electrophysiological interactions of enkephalins with neuronal circuitry in the rat hippocampus. II. Effects on interneuron excitability. Brain Res. 1980 Feb 24;184(2):331–342. doi: 10.1016/0006-8993(80)90802-1. [DOI] [PubMed] [Google Scholar]
- Madison D. V., Nicoll R. A. Noradrenaline blocks accommodation of pyramidal cell discharge in the hippocampus. Nature. 1982 Oct 14;299(5884):636–638. doi: 10.1038/299636a0. [DOI] [PubMed] [Google Scholar]
- Mori K., Nowycky M. C., Shepherd G. M. Analysis of a long-duration inhibitory potential in mitral cells in the isolated turtle olfactory bulb. J Physiol. 1981 May;314:311–320. doi: 10.1113/jphysiol.1981.sp013709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller A. L., Dunwiddie T. V. Anticonvulsant and proconvulsant actions of alpha- and beta-noradrenergic agonists on epileptiform activity in rat hippocampus in vitro. Epilepsia. 1983 Feb;24(1):57–64. doi: 10.1111/j.1528-1157.1983.tb04866.x. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Alger B. E. A simple chamber for recording from submerged brain slices. J Neurosci Methods. 1981 Aug;4(2):153–156. doi: 10.1016/0165-0270(81)90049-2. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Alger B. E., Jahr C. E. Enkephalin blocks inhibitory pathways in the vertebrate CNS. Nature. 1980 Sep 4;287(5777):22–25. doi: 10.1038/287022a0. [DOI] [PubMed] [Google Scholar]
- Nicoll R. A., Alger B. E. Synaptic excitation may activate a calcium-dependent potassium conductance in hippocampal pyramidal cells. Science. 1981 May 22;212(4497):957–959. doi: 10.1126/science.6262912. [DOI] [PubMed] [Google Scholar]
- Pittman Q. J., Siggins G. R. Somatostatin hyperpolarizes hippocampal pyramidal cells in vitro. Brain Res. 1981 Sep 28;221(2):402–408. doi: 10.1016/0006-8993(81)90791-5. [DOI] [PubMed] [Google Scholar]
- Pong S. F., Graham L. T., Jr A simple preparation of bicuculline methiodide, a water-soluble GABA antagonist. Brain Res. 1973 Aug 17;58(1):266–267. doi: 10.1016/0006-8993(73)90844-5. [DOI] [PubMed] [Google Scholar]
- Robinson J. H., Deadwyler S. A. Intracellular correlates of morphine excitation in the hippocampal slice preparation. Brain Res. 1981 Nov 16;224(2):375–387. doi: 10.1016/0006-8993(81)90867-2. [DOI] [PubMed] [Google Scholar]
- Satou M., Mori K., Tazawa Y., Takagi S. F. Two types of postsynaptic inhibition in pyriform cortex of the rabbit: fast and slow inhibitory postsynaptic potentials. J Neurophysiol. 1982 Nov;48(5):1142–1156. doi: 10.1152/jn.1982.48.5.1142. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Mathers L. H. Physiological and morphological identification of a nonpyramidal hippocampal cell type. Brain Res. 1978 Nov 17;157(1):1–10. doi: 10.1016/0006-8993(78)90991-5. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Prince D. A. Cellular and field potential properties of epileptogenic hippocampal slices. Brain Res. 1978 May 19;147(1):117–130. doi: 10.1016/0006-8993(78)90776-x. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin P. A., Stafstrom C. E. Effects of EGTA on the calcium-activated afterhyperpolarization in hippocampal CA3 pyramidal cells. Science. 1980 Dec 5;210(4474):1125–1126. doi: 10.1126/science.6777871. [DOI] [PubMed] [Google Scholar]
- Thalmann R. H., Ayala G. F. A late increase in potassium conductance follows synaptic stimulation of granule neurons of the dentate gyrus. Neurosci Lett. 1982 Apr 26;29(3):243–248. doi: 10.1016/0304-3940(82)90324-x. [DOI] [PubMed] [Google Scholar]
- Traub R. D., Wong R. K. Synchronized burst discharge in disinhibited hippocampal slice. II. Model of cellular mechanism. J Neurophysiol. 1983 Feb;49(2):459–471. doi: 10.1152/jn.1983.49.2.459. [DOI] [PubMed] [Google Scholar]
- Wong R. K., Traub R. D. Synchronized burst discharge in disinhibited hippocampal slice. I. Initiation in CA2-CA3 region. J Neurophysiol. 1983 Feb;49(2):442–458. doi: 10.1152/jn.1983.49.2.442. [DOI] [PubMed] [Google Scholar]
- Zieglgänsberger W., French E. D., Siggins G. R., Bloom F. E. Opioid peptides may excite hippocampal pyramidal neurons by inhibiting adjacent inhibitory interneurons. Science. 1979 Jul 27;205(4404):415–417. doi: 10.1126/science.451610. [DOI] [PubMed] [Google Scholar]


