Abstract
Nutritional status may have significant importance for the immune system, and particularly, unsaturated fatty acids may serve as modulators of immune functions. Clinical and epidemiological studies have demonstrated that fatty acids are involved in the reduction of the inflammatory processes that occur in diseases characterized by an overactivation of the immune system. At the same time, an increase in susceptibility to infection has also been reported. The importance of immune system modulation by dietary lipids in the presence of an intracellular bacterial pathogen, such as Listeria monocytogenes, was evaluated in the present study. BALB/c mice were divided into four groups which were each fed a low-fat (2.5% by weight) diet, an olive oil (OO; 20% by weight) diet, a fish oil (FO; 20% by weight) diet, or a hydrogenated coconut oil (HCO; 20% by weight) diet for 4 weeks. In each group, lymphocye proliferation was measured, and a reduction in the stimulation index was observed in the FO and HCO groups. Cytotoxicity exerted by L. monocytogenes was increased in the groups fed diets containing OO and FO after 6 h of incubation with the bacterium. An important increase in the production of reactive oxygen species was found in the groups fed the HCO diet after 12 h of incubation with L. monocytogenes. Finally, invasion and adhesion factors were not modified substantially by the action of dietary lipids, although these factors were reduced in cells from mice fed an FO diet. These results underline the importance of several dietary lipids as biological modulators of immune functions and their crucial role in the alteration of host natural resistance.
According to present knowledge, many fatty acids contained in the diets of both animals and humans may exert a modulatory effect on immune functions (5, 12). It is generally assumed that the long-chain n-3 polyunsaturated fatty acids eicosapentaenoic acid (20:5[n-3]) and docosahexaenoic acid (DHA; 22:6[n-3]), contained in fish oil, promote an immunosuppressive function. However, it has also been determined that monounsaturated fatty acids, such as oleic acid (derived from olive oil), rather than the other biological substances contained in this fat, cause an important reduction of numerous immune functions (21). Therefore, as a consequence of these events, many immunological functions, such as lymphocyte proliferation (15, 24), cytokine production (15, 24), expression of cell surface molecules (20), natural killer (NK) cell activity (36), and phagocytosis (7), are modulated by the action of fatty acids. As a result, in recent years, numerous studies have indicated the potential role of several dietary lipids in the development of cancer or in the increase in resistance to tumors (9, 31). Similarly, unsaturated fatty acids have been applied in the resolution of inflammatory disorders associated with diseases characterized by an overreactivation of the immune system, such as rheumatoid arthritis (22, 23), psoriasis (2), and multiple sclerosis (1, 18). However, despite the beneficial effects of several dietary fatty acids in cancer or in the treatment of inflammatory diseases, many fatty acids may exert a detrimental effect because they reduce host immune resistance to bacterial or viral infections. These adverse effects may be of particular interest in individuals susceptible to infection, such as infants or elderly or immunocompromised individuals. Nevertheless, for obvious reasons, very little information is currently available on the clinical relevance of dietary lipids in the resistance of the human immune system to infectious agents, but numerous studies have described the effects of unsaturated fatty acids on the impairment of immunological functions in animals (14). Thus, diets supplemented with n-3 polyunsaturated fatty acids significantly reduce the survival of mice after experimental infection with Listeria monocytogenes (13, 16), as well as the clearance of bacteria from the liver (16) or spleen (13, 16) during the course of infection. Experimental infections with this bacterium induce the production of different cytokines, such as gamma interferon, tumor necrosis factor alpha, and interleukin-6, which play an essential role in antilisterial resistance (26). This fact could explain in part the adverse effects of several dietary lipids on the elimination of L. monocytogenes, because polyunsaturated fatty acids are related to the reduction of cytokine production and lymphocyte proliferation. Hence, we speculate that in addition to the modulation of these factors, other events could be involved in these processes. Thus, alteration of bacterial adhesion, invasion, cytotoxicity, and release of reactive oxygen species (ROS) are examined in the present study, in order to establish the potential effects of different dietary lipids in the modulation of the immune system that contribute to the alteration of susceptibility to L. monocytogenes infection.
MATERIALS AND METHODS
Animals.
BALB/c mice 8 to 10 weeks old were purchased from the University of Jaén (breeding colony of Servicios Técnicos de Investigacíon, University of Jaén, Jaén, Spain). The mice were maintained in cages (five per cage) in an environmentally controlled room at a temperature of 24°C with a 12-h light-dark cycle. The mice were randomly divided into four dietary groups, and each group was allowed access ad libitum to water, as well as to its respective experimental diet, for 4 weeks prior to sacrifice by cervical dislocation.
Experimental-diet preparation.
The diets were identical in composition except for the fat source, which was either olive oil (OO) (20% by weight), fish oil (FO) (20% by weight), or hydrogenated coconut oil (HCO) (20% by weight). The experimental diets were protected from light and kept at 4°C. In addition, the diet containing HCO was supplemented with 1% corn oil in order to prevent essential fatty acid deficiency. The control group consisted of mice fed a low fatty acid (LF) diet (approximately 2.5% lipids by weight). The compositions of the experimental diets are shown in Table 1. The mice were weighed, after being fed their respective diets, at the time they were sacrificed.
TABLE 1.
Compositions of experimental dietsa
| Content of diets | g/kg of diet |
|---|---|
| Casein | 200 |
| d,l-Methionine | 3 |
| Corn starch | 315 |
| Sucrose | 155 |
| Fiber | 80 |
| Fatsb | 200 |
| Mineral mix | 35 |
| Vitamin mix | 10 |
| Choline | 2 |
BALB/c mice were fed their respective diets for 4 weeks.
Oils incorporated in the diets were OO, FO, and HCO. The control group was fed a diet containing 2.5% lipid (by weight) (LF diet).
Preparation of bacterial cells.
A virulent strain of L. monocytogenes was grown in brain heart infusion broth to late exponential phase (optical density [OD] at 550 nm, approximately 1.0) at 37°C for 24 h. Then, the bacteria were washed twice in phosphate-buffered saline (PBS) and suspended in 0.9% NaCl. The number of viable bacteria was determined by plating serial dilutions in triplicate on blood agar plates and incubating them at 37°C for 24 h.
Isolation and preparation of spleen cells.
Spleens were isolated and removed to PBS. Spleen cells were prepared by disrupting the spleen between frosted-glass slides in RPMI 1640. The cells were washed twice in RPMI 1640 at 4°C and 1,200 rpm for 10 min. Mononuclear cells were isolated by density centrifugation on Histopaque (Sigma Chemical Co., St. Louis, Mo.) at 1,200 rpm (Beckman GS-6R; Beckman, Palo Alto, Calif.) for 30 min. Subsequently, the cells were counted and adjusted to the concentration required for each assay. Cellular viability in each experiment was determined by trypan blue exclusion and was always greater than 95%.
Spleen lymphocyte proliferation assay.
The proliferation of lymphocytes isolated from the spleens of mice fed dietary lipids was measured by the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT; Sigma) colorimetric assay. This method uses the tetrazolium salt MTT, which is cleaved by dehydrogenase activity in the mitochondria of viable cells to produce a dark-blue formazan product (25). Briefly, cells were cultured in 96-well microtiter plates, adjusted to a concentration of 105 per well, and incubated in a humidified atmosphere at 37°C for 24 h in the presence of concanavalin A (ConA; Sigma) at a final concentration of 5 μg/ml or lipopolysaccharide (LPS; Sigma) at a final concentration of 50 μg/ml. Then, 20 μl of MTT was added to each well, and the plates were incubated for 3 h. Finally, 150 μl of 0.04 N HCl in isopropyl alcohol was added to each well in order to solubilize the formazan precipitates. The OD of each sample was measured spectrophotometrically (Whittaker 2001; [Salzburg, Austria) in triplicate at 450 nm (test wavelength) and at 550 nm (reference wavelength). The data were expressed as the stimulation index, which was calculated as the OD in the presence of mitogen divided by the OD in the absence of mitogen.
Cytotoxicity analysis.
The measurement of cytotoxic effects exhibited by the bacteria was performed by the colorimetric assay described by Mosmann (25), with minor modifications (10). Splenic cells adjusted to 5 × 104 per well in RPMI 1640 supplemented with 10% fetal calf serum (FCS; Biochrom KG, Berlin, Germany) were added to 96-well microplates. Bacterial suspensions in saline solution were prepared as described above. Bacterial preparations (at a multiplicity of infection of 20 bacteria per cell) were added to the plates immediately or after the bacterial suspension was heated at a temperature of 95°C for 30 min. Positive controls consisted of 1% (vol/vol) Triton X-100 (Sigma), and negative controls consisted of PBS. The microplates were incubated for 3 and 6 h at 37°C in a humidified atmosphere with 5% CO2. After incubation, 20 μl of MTT at a concentration of 5 mg/ml in PBS (pH 7.2) was added to each well. Following 3 h of incubation, the supernatants were decanted and the formazan precipitates were solubilized by the addition of 150 μl of 0.04 N HCl in isopropyl alcohol and placed on a plate shaker. The contents of the wells were examined spectrophotometrically at 620 nm (Whittaker 2001). The results were expressed as the percentage of cytotoxicity [1 − (OD of test well/OD of negative control well) × 100].
Measurement of ROS generation.
To monitor oxidative activity, the cell permeant probe 2′,7′ dichlorodihydrofluorescein diacetate (H2DCFDA; Sigma) was used. H2DCFDA passively diffuses into cells, where intracellular esterases cleave the acetates, and the oxidation of 2′,7′ dichlorodihydrofluorescein by hydrogen peroxide produces a fluorescent response. After preincubation of 2 × 106 cells/ml in RPMI 1640 medium supplemented with 10% FCS in the absence or in the presence of L. monocytogenes for 6 or 12 h (at a multiplicity of infection of 20 bacteria per cell), the cells were stained with H2DCFDA at a concentration of 40 μM at 37°C for 60 min. Then, the cells were washed and removed to 100 μl of PBS. Fluorometric analyses at 480 nm (excitation) and 530 nm (emission) were performed using a fluorescence spectrophotometer (Cary Eclipse; Varian, Mulgrave, Australia). The relative fluorescence intensity was expressed as arbitrary units of fluorescence.
Adhesion and invasion assays.
The ability of L. monocytogenes to adhere or to invade different cells was analyzed by previously published protocols (32). Briefly, cells in RPMI 1640 (Sigma) supplemented with 10% FCS were adjusted to a concentration of 3 × 105 per well in 24-well tissue culture plates. For adhesion assays, the cells were infected with 0.1 ml of bacterial culture (at a multiplicity of infection of 20 bacteria per cell) followed by 2, 4, 6, 8, 10, or 12 h of incubation at 37°C in a 5% CO2 atmosphere. After incubation of the cells, the bacteria were removed by three washes with RPMI 1640. The cells were lysed with 1 ml of 1% (vol/vol) Triton X-100 for 5 min at 37°C, followed by serial dilution in 0.9 ml of PBS and subsequent enumeration by the plating of 0.1 ml of 10-fold serial dilutions on blood agar. Finally, the CFU in each plate were counted after incubation for 24 h at 37°C. For invasion assays, 1 ml of RPMI 1640 supplemented with 10% FCS and 10 μg of gentamicin/ml was added to the infected cell culture to kill extracellular bacteria, followed by 2, 4, 6, 8, 10, or 12 h of incubation at 37°C. Then, the cells were washed twice and lysed with 1 ml of 1% Triton X-100 for 5 min at 37°C. The viable bacteria were quantified by the previously described method. Each experiment was carried out in triplicate and repeated three times. The results were expressed as log10 viable bacteria.
Statistical analysis.
Results were expressed as means ± standard errors of the mean (SEM). The experiments were carried out in triplicate. Data were determined by analysis of variance to compare the effects of the experimental diets with the control group (LF diet). When analysis of variance indicated significant differences, the treatment means were compared using Fisher's least-significant-difference test. Statistical significance was established as a P value of <0.05.
RESULTS
Body and spleen weights of mice and cell counts.
The body weights of mice fed the respective dietary lipids did not show any significant difference in comparison to values for mice fed the LF diet (control) (Table 2). Spleen weights from mice fed a diet rich in HCO were significantly increased with respect to the control (Table 2). Cellular counts from spleens after supplementation with each diet did not show any significant difference in comparison to values from the LF group (Table 2).
TABLE 2.
Body and spleen weights and mononuclear-cell counts of mice fed dietary lipidsa
| Experimental group | Body wt (g)b | Spleen wt (g)b,c | Cell count from spleen (106 cells/ml)b |
|---|---|---|---|
| LF (control group) | 21.9 ± 2.6 | 120 ± 2.0 | 86.7 ± 17.7 |
| OO | 26.8 ± 2.6 | 175 ± 5.0 | 79.4 ± 21.0 |
| FO | 21.3 ± 1.3 | 146 ± 6.0 | 88.0 ± 20.4 |
| HCO | 26.0 ± 0.9 | 198 ± 6* | 66.1 ± 13.1 |
BALB/c mice were fed one of four diets that differed only in type of fat.
Means ± SEM of 20 mice.
*, P < 0.05 compared with LF group.
Determination of spleen lymphocyte proliferation.
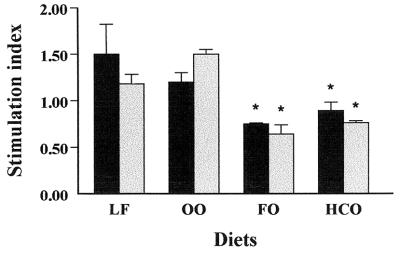
The proliferation of spleen lymphocytes from mice fed dietary lipids was determined by the MTT colorimetric method in order to quantify the stimulation index of spleen lymphocytes from mice fed dietary lipids after 24 h of incubation. The cells were stimulated in the presence of either LPS or ConA. As illustrated in Fig. 1, the results revealed that lymphocyte proliferation of cells from mice fed diets containing FO or HCO and stimulated with both LPS and ConA was significantly reduced in comparison to values for the LF group (P < 0.05). In contrast, it is remarkable that lymphocyte proliferation of cells from mice fed a diet containing OO was not reduced with respect to values from the LF group.
FIG. 1.
Measurement by the MTT colorimetric assay of mitogen-stimulated lymphocyte proliferation in spleens of mice fed dietary lipids. Spleen cells were cultured in the presence of LPS at a concentration of 50 μg/ml (solid bars) or ConA at a concentration of 5 μg/ml (shaded bars) for 24 h. Subsequently, MTT was added to each well, and the cells were incubated for 3 h. Lymphocyte proliferation was measured, and the values were expressed as the stimulation index (n = 5 mice in each group). The data are expressed as the mean + SEM of three independent experiments (calculated as the OD of cells treated in the presence of mitogen divided by the OD of cells incubated in the absence of mitogen). ∗, P < 0.05 compared with the LF group.
Analysis of cytotoxicity.
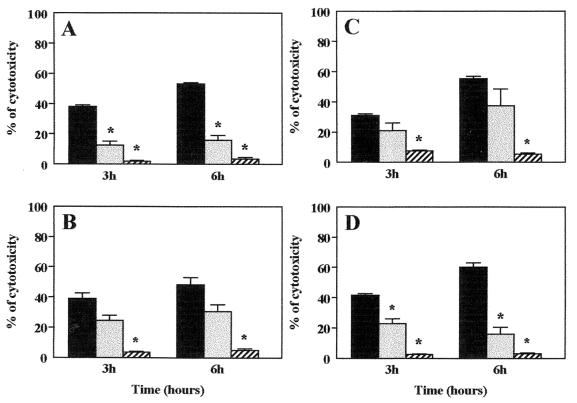
Cytotoxicity promoted by L. monocytogenes was quantified by the MTT colorimetric assay. The cytotoxic activity due to the presence of L. monocytogenes was measured after 3 and 6 h of incubation. The most remarkable results, compared to the positive control, were observed in the groups fed diets containing OO and FO, in which the percentage of cytotoxicity increased to 31 and 38%, respectively (Fig. 2B and C) after 6 h of splenic cell incubation in the presence of L. monocytogenes, whereas in the other groups this percentage was lower than 17%. Thus, the percentage of cytotoxicity was significantly reduced in the groups fed diets containing LF (Fig. 2A) and HCO (Fig. 2D) after 6 h of incubation with the bacterium (P < 0.05). The increase of cytotoxicity produced by L. monocytogenes after 3 to 6 h of cellular incubation was maintained in all of the groups, with the exception of the HCO group, in which this percentage was markedly reduced. On the other hand, heat-killed bacteria did not cause any cytotoxic effect on cellular cultures.
FIG. 2.
Analysis of cytotoxicity from splenic cells of mice fed dietary lipids. Cytotoxicity was quantified by the MTT colorimetric assay. Cells were cultured with a positive control, such as Triton X-100 (solid columns), in the presence of viable (shaded bars) or heat-killed (hatched bars) L. monocytogenes for 3 or 6 h. Shown are percentages of cytotoxicity of L. monocytogenes in cells from mice fed an LF (A), OO (B), FO (C), or HCO (D) diet. The data are expressed as the mean + SEM of three independent determinations (n = 3 mice in each group). ∗, P < 0.05 compared with the percentage of positive control in each dietary group.
Production of ROS.
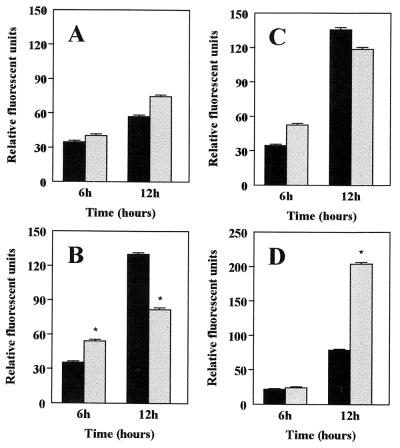
Analysis of ROS production was carried out in the absence or in the presence of L. monocytogenes. Both splenic cells and bacteria were incubated for 6 and 12 h. At first glance, the measurement of ROS production in the presence of L. monocytogenes indicated a marked effect of dietary lipids on the production of ROS compared to the results from cells cultured in the absence of L. monocytogenes. ROS production in the presence of L. monocytogenes did not increase significantly in the group fed an LF diet (Fig. 3A). Conversely, the production of ROS by cells incubated with L. monocytogenes was markedly reduced in the groups fed an OO or FO diet (P < 0.05) (Fig. 3B and C). The most significant alteration in the production of ROS was observed in the group fed HCO, in which the production of these reactive substances was increased in the presence of L. monocytogenes after 12 h of incubation (P < 0.05) (Fig. 3D).
FIG. 3.
Measurement of ROS production in spleen cells isolated from mice fed dietary lipids. Cellular ROS production was quantified by the method of intracellular deacylation and oxidation of H2DCFDA to the fluorescent substance 2′,7′ dichlorodihydrofluorescein. The measurement of ROS production was carried out in the absence (solid bars) or in the presence (shaded bars) of L. monocytogenes. Shown is ROS production in cells from mice fed LF (A), OO (B), FO (C), and HCO (D) diets. Each experiment was carried out in triplicate and repeated three times. The data are expressed as the mean + SEM of three independent determinations (n = 3 in each dietary group). ∗, P < 0.05 compared with the values in the absence of L. monocytogenes.
Measurement of adhesion and invasion by L. monocytogenes.
The ability of L. monocytogenes to adhere to and invade cells from the spleens of mice fed dietary lipids was established in the present study, as illustrated in Tables 3 and 4. All of the dietary groups showed an increase of adhesion and invasion by L. monocytogenes during the course of infection. Thus, levels of bacterial adhesion in the groups fed an LF or OO diet were identical, and the values did not differ substantially during the process of infection by L. monocytogenes. However, it is important to note that adhesion in cells from mice fed an FO diet was lower than adhesion in cells from mice fed an LF diet. In fact, these values were significantly reduced with respect to those from cells of mice fed an LF diet during the course of the infection from 2 to 12 h (P < 0.05). The levels of invasion by L. monocytogenes in cells from mice fed a diet containing HCO were significantly lower than those found in the LF group after 10 and 12 h of incubation with the bacterium.
TABLE 3.
Alteration of adhesion factor in splenic cells from mice fed dietary lipids and cultured in the presence of L. monocytogenes
| Diet | Adhesiona
|
|||||
|---|---|---|---|---|---|---|
| 2b | 4 | 6 | 8 | 10 | 12 | |
| LF | 6.30 ± 0.12 | 6.47 ± 0.13 | 7.18 ± 0.04 | 7.27 ± 0.03 | 7.48 ± 0.02 | 7.49 ± 0.05 |
| OO | 6.01 ± 0.03 | 6.50 ± 0.16 | 7.19 ± 0.02 | 7.23 ± 0.05 | 7.30 ± 0.08 | 7.35 ± 0.06 |
| FO | 5.60 ± 0.15* | 5.75 ± 0.12* | 6.02 ± 0.2* | 6.43 ± 0.09* | 6.62 ± 0.20* | 6.96 ± 0.20* |
| HCO | 6.50 ± 0.18 | 6.67 ± 0.20 | 7.28 ± 0.06 | 7.33 ± 0.10 | 7.59 ± 0.15 | 7.91 ± 0.25* |
Log10 viable bacteria; means ± SEM of three independent determinations (n = 3 in each dietary group). *, P < 0.05 compared with LF group quantified at the same time.
Hours of incubation with L. monocytogenes.
TABLE 4.
Alteration of invasion factor in splenic cells from mice fed dietary lipids and cultured in the presence of L. monocytogenes
| Diet | Invasiona
|
|||||
|---|---|---|---|---|---|---|
| 2b | 4 | 6 | 8 | 10 | 12 | |
| LF | 4.21 ± 0.06a | 4.20 ± 0.03 | 5.53 ± 0.06 | 6.00 ± 0.02 | 6.68 ± 0.02 | 7.25 ± 0.08 |
| OO | 4.90 ± 0.35* | 4.60 ± 0.30 | 5.70 ± 0.17 | 5.84 ± 0.10 | 5.93 ± 0.08* | 6.30 ± 0.09* |
| FO | 4.32 ± 0.20 | 4.61 ± 0.20 | 5.20 ± 0.05* | 5.92 ± 0.10 | 6.45 ± 0.15 | 7.01 ± 0.12 |
| HCO | 3.91 ± 0.25 | 4.20 ± 0.04 | 5.60 ± 0.05 | 5.80 ± 0.15 | 5.95 ± 0.20* | 6.10 ± 0.20* |
Log10 viable bacteria; means ± SEM of three independent determinations (n = 3 in each dietary group). *, P < 0.05 compared with LF group quantified at the same time.
Hours of incubation with L. monocytogenes.
DISCUSSION
As a consequence of dietary lipid administration, several changes that alter the ability of cells to eliminate many pathogenic agents are detected in the immune system functions of both humans and animals (5, 12). The present study shows the potential role of dietary fatty acids in the alteration of immune cell functions that may modulate adhesion and invasion by L. monocytogenes, the cytotoxic effects promoted by this bacterium in cells from mice fed dietary lipids, and its capacity to stimulate the cells to release ROS. In past years, several lines of evidence have indicated that the immunosuppressive effects of many dietary lipids increase the susceptibility of the host immune system to infectious agents. As a consequence, this process may produce a detrimental effect that can acquire critical relevance in immunocompromised patients. Based on these premises, we have attempted to demonstrate the state of immune cells from animals fed dietary lipids in the presence of an intracellular microorganism, such as L. monocytogenes. In fact, the study of this intracellular pathogen has increased our knowledge of the diverse interactions between this biological mediator and the innate and adaptive immune responses. Moreover, other investigations have also demonstrated the reduction of host immune resistance to different bacteria or viruses in animals fed polyunsaturated fatty acids (3, 4, 8, 28, 29).
Recently, several studies have reported that cultivation of monocytes with both eicosapentaenoic acid and DHA reduces the surface expression of major histocompatibility complex class II molecules and some adhesion molecules on human monocytes (19, 20). These results appear to indicate that administration of these fatty acids, contained in FO, may impair the intercellular interaction between T cells and antigen-presenting cells developed to recognize the antigen. As a consequence, a detrimental effect may occur, because host defense against bacterial and other antigens could be compromised (33). Likewise, a significant reduction of the expression of CD4 or CD8 on cell surfaces has recently been documented after dietary supplementation with DHA (34). The present results have confirmed that some dietary lipids are related to a reduction of lymphocyte proliferation stimulated by ConA or LPS. Thus, diets containing FO (which consists of polyunsaturated fatty acids) or HCO (which consists of saturated fatty acids) are responsible for a significant reduction of lymphocyte proliferation compared to values from an LF group. Nevertheless, cytotoxicity associated with L. monocytogenes growth was increased in the groups fed OO (which consists of monounsaturated fatty acids) or FO, whereas a significant reduction of cytotoxic effects was observed in the groups fed LF and HCO after 6 h of incubation in the presence of L. monocytogenes. These data suggest that the potential effects of an FO diet on lymphocyte proliferation or cytotoxicity could be related to the previously described reduction in the survival of mice after experimental infection with L. monocytogenes or to the reduction of the resistance of mice and their capacity to eliminate this microorganism from the liver or spleen (13, 16). Similarly, the effects produced by an HCO diet on lymphocyte proliferation or the cytotoxicity of L. monocytogenes may be associated with the beneficial action of this diet on the survival of mice as well as on the recovery of bacteria from the spleen (13). In vitro assays have demonstrated a marked reduction of bactericidal activity in peritoneal cells treated with linolenic acid or linoleic acid (n-3 or n-6 polyunsaturated fatty acid, respectively) and cultured in the presence of L. monocytogenes. Similarly, ex vivo assays have also shown that the bactericidal activity of these cells was significantly reduced after FO administration in mice when peritoneal cells were cultured in the presence of L. monocytogenes (30). However, it has been reported recently that administration of dietary conjugated linoleic acid does not modify the immunological resistance of mice to L. monocytogenes infection because this treatment diminishes the release of tumor necrosis factor and other inflammatory cytokines (35), which are able to reduce resistance to listeriosis in mice (11). It appears clear that the cytotoxic effects of L. monocytogenes on cells from mice fed dietary lipids may be different, depending on the type of fat contained in the diet. Thus, a possible explanation of this effect could be a marked production of ROS, which has been investigated in the present study. ROS production was increased in all of the dietary groups assayed with the exception of the OO and FO groups at 12 h after infection with L. monocytogenes. In these groups, the lower ROS production was probably due to an increase of cytotoxic effects, and as a consequence, the cellular viability of these cells was also disminished. Nevertheless, in cells from mice fed an HCO diet in the absence of L. monocytogenes, low ROS production was also observed. By contrast, in cells from mice fed this diet and incubated in the presence of L. monocytogenes, a massive production of ROS was quantified after 12 h of infection. However, previous studies have determined that there is not a direct relationship between ROS and listeria elimination, because ROS was demonstrated to be relatively ineffective in the destruction of this bacterium in activated macrophages (17). In fact, a recent study has reported that ROS release does not play a crucial role in the direct eradication of L. monocytogenes, although it is interesting that ROS may be involved in triggering the cellular response (27). In general, these biological events cannot be associated with the susceptibility of these cells to bacterial invasion. In other words, the levels of cytotoxicity and the capacity to produce ROS do not affect the ability of the cells to be invaded by L. monocytogenes, so these properties are independent. Although membrane fluidity is another important factor modulated by dietary lipid manipulation that could contribute to the increase of cellular susceptibility to infection (6, 7), the results of these studies have not demonstrated that adherence or invasion by L. monocytogenes depends on the composition of dietary lipids, because the OO group showed values similar to those of the HCO group. However, it is important to remark that after the administration of an FO diet (the most immunosuppressive diet), the lowest levels of adhesion were determined. Therefore, this contradictory finding indicates that factors other than adhesion and invasion allow the reduction of immune resistance to L. monocytogenes shown by cells from mice fed an FO diet.
Concluding remarks.
This study suggests that some dietary lipids, particularly unsaturated fatty acids, modulate many functions of the immune system, such as lymphocyte proliferation, response to bacterial cytotoxic effects, and release of ROS. Modulation of lymphocyte proliferation and cytokine synthesis are mainly involved in the regulation of immune system functions by dietary lipids. Nevertheless, in the present investigation, three important factors, response to cytotoxic effects, ROS production, and adhesion or invasion, explain in part the alteration of host natural resistance described when animals fed dietary lipids are experimentally infected with an infectious agent. The first factor, based on the cellular response to bacterial cytotoxic effects, indicates that unsaturated fatty acids, contained in OO and FO, lead to an increase in the percentage of cytotoxicity in the presence of L. monocytogenes. The second factor, ROS production, suggests that these substances participate in the elimination of L. monocytogenes, because a reduction in ROS production has been quantified in FO and OO groups (the most immunosuppressive diets). Finally, the third factor, based on the measurement of bacterial adhesion and invasion, has demonstrated that dietary lipids (with the exception of an FO diet) do not alter the susceptibility of cells to adhesion or invasion by L. monocytogenes. This study also explains the previously reported beneficial effects of an HCO diet on the survival of mice as well as on the recovery of L. monocytogenes from the spleen (13), because a significant reduction in cytotoxic effects has been observed in these cell cultures in the presence of L. monocytogenes. Therefore, irrespective of the application of FO in the reduction of inflammatory processes, the above arguments may serve to confirm that an immunosuppressive state induced by polyunsaturated or monounsaturated fatty acids impairs host natural resistance. Further research will help to establish other potential mechanisms that will increase our knowledge of the interactions among dietary lipid manipulation, the immune system, and infectious processes promoted by an alteration of immune functions.
REFERENCES
- 1.Bates, D., P. R. W. Fawcett, D. A. Shaw, and D. Weightman. 1978. Polyunsaturated fatty acids in the treatment of acute remitting multiple sclerosis. Br. Med. J. 2:1390-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bittiner, S. B., W. F. G. Tucker, I. Cartwright, and S. S. Bleehen. 1988. A double blind, randomised, placebo-controlled trial of fish oil in psoriasis. Lancet i:378-380. [DOI] [PubMed] [Google Scholar]
- 3.Byleveld, M., G. T. Pang, R. L. Clancy, and D. C. Roberts. 2000. Fish oil feeding enhances lymphocyte proliferation but impairs virus-specific T lymphocyte cytotoxicity in mice following challenge with influenza virus. Clin. Exp. Immunol. 119:287-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byleveld, M., G. T. Pang, R. L. Clancy, and D. C. K. Roberts. 1999. Fish oil feeding delays influenza virus clearance and impairs production of interferon-γ and virus-specific immunoglobulin A in the lungs of mice. J. Nutr. 129:328-335. [DOI] [PubMed] [Google Scholar]
- 5.Calder, P. C. 1998. Fat chance of immunomodulation. Immunol. Today 19:244-247. [DOI] [PubMed] [Google Scholar]
- 6.Calder, P. C., P. Yaqoob, D. J. Harvey, A. Watts, and E. A. Newsholme. 1994. Incorporation of fatty acids by concanavalin A-stimulated lymphocytes and the effect on fatty acid composition and membrane fluidity. Biochem. J. 300:509-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calder, P. C., J. A. Bond, D. J. Harvey, S. Gordon, and E. A. Newsholme. 1990. Uptake and incorporation of saturated and unsaturated fatty acids into macrophage lipids and their effect upon macrophage adhesion and phagocytosis. Biochem. J. 269:807-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang, H. R., A. G. Dullo, I. R. Vladoianu, P. F. Piguet, D. Arsenijevic, L. Girardier, and J. C. Pechere. 1992. Fish oil decreases natural resistance of mice to infection with Salmonella typhimurium. Metabolism 41:1-2. [DOI] [PubMed] [Google Scholar]
- 9.Cohen, L. A., D. O. Thompson, Y. Maeura, K. Choi, M. E. Blank, and D. P. Rose. 1986. Dietary fat and mammary cancer. I. Promoting effects of different fats on N-nitrosomethylurea-induced rat mammary tumorigenesis. J. Natl. Cancer Inst. 77:33-42. [PubMed] [Google Scholar]
- 10.Coote, J. G., and T. Arain. 1996. A rapid, colorimetric assay for cytotoxin activity in Campylobacter jejuni. FEMS Immunol. Med. Microbiol. 13:65-70. [DOI] [PubMed] [Google Scholar]
- 11.Czuprynski, C. J., and M. Haak-Frendscho. 1997. Non-specific resistance mechanism to listeriosis: implications for experimental and naturally occurring infection. Immunol. Rev. 158:47-56. [DOI] [PubMed] [Google Scholar]
- 12.de Pablo, M. A., and G. Álvarez de Cienfuegos. 2000. Modulatory effects of dietary lipids on immune system functions. Immunol. Cell. Biol. 78:31-39. [DOI] [PubMed] [Google Scholar]
- 13.de Pablo, M. A., M. A. Puertollano, A. Galvez, E. Ortega, J. J. Gaforio, and G. Álvarez de Cienfuegos. 2000. Determination of natural resistance of mice fed dietary lipids to experimental infection induced by Listeria monocytogenes. FEMS Immunol. Med. Microbiol. 27:127-133. [DOI] [PubMed] [Google Scholar]
- 14.de Pablo, M. A., M. A. Puertollano, and G. Alvarez de Cienfuegos. 2000. Immune cell functions, lipids and host natural resistance. FEMS Immunol. Med. Microbiol. 29:323-328. [DOI] [PubMed] [Google Scholar]
- 15.Endres, S., R. Ghorbani, V. E. Kelley, K. Georgilis, G. Lonnemann, J. M. W. van der Meer, J. G. Cannon, T. S. Rogers, M. S. Klempner, P. C. Weber, E. J. Schaeffer, S. M. Wolff, and C. A. Dinarello. 1989. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N. Engl. J. Med. 320: 265-271. [DOI] [PubMed] [Google Scholar]
- 16.Fritsche, K. L., L. M. Shahbazian, C. Feng, and J. N. Berg. 1997. Dietary fish oil reduces survival and impairs bacterial clearance in C3H/Hen mice challenged with Listeria monocytogenes. Clin. Sci. 92:95-101. [DOI] [PubMed] [Google Scholar]
- 17.Godfrey, R. W., and M. S. Wilder. 1984. Relationships between oxidative metabolism, macrophage activation, and antilisterial activity. J. Leukoc. Biol. 36:533-543. [DOI] [PubMed] [Google Scholar]
- 18.Harbige, L. S., L. Layward, M. M. Morris-Downes, D. C. Dumonde, and S. Amor. 2000. The protective effects of omega-6 fatty acids in experimental autoimmune encephalomyelitis (EAE) in relation to transforming growth factor-beta 1 (TGF-β1) up-regulation and increased prostaglandin E2 (PGE2) production. Clin. Exp. Immunol. 122:445-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hughes, D. A., and A. C. Pinder. 2000. n-3 polyunsaturated fatty acids inhibit the antigen-presenting function of human monocytes. Am. J. Clin. Nutr. 71:357S-360S. [DOI] [PubMed]
- 20.Hughes, D. A., A. C. Pinder, Z. Piper, I. T. Johnson, and E. K. Lund. 1996. Fish oil supplementation inhibits the expression of major histocompatibility complex class II molecules and adhesion molecules on human monocytes. Am. J. Clin. Nutr. 63:267-272. [DOI] [PubMed] [Google Scholar]
- 21.Jeffery, N. M., P. Yaqoob, E. A. Newsholme, and P. C. Calder. 1996. The effects of olive oil upon rat serum lipid levels and lymphocyte functions appear to be due to oleic acid. Ann. Nutr. Metab. 40:71-80. [DOI] [PubMed] [Google Scholar]
- 22.Kremer, J. M., D. A. Lawrence, W. Jubiz, R. DiGiacomo, R. Rynes, L. E. Bartholomew, and M. Sherman. 1990. Dietary fish oil and olive oil supplementation in patients with rheumatoid arthritis. Clinical and immunologic effects. Arthritis Rheum. 33:810-820. [DOI] [PubMed] [Google Scholar]
- 23.Linos, A., E. Kaklamanis, A. Kontomerkos, Y. Koumantaki, S. Gazi, G. Vaiopoulos, G. C. Tsokos, and P. Kaklamanis. 1991. The effect of olive oil and fish consumption on rheumatoid arthritis—a case control study. Scand. J. Rheumatol. 20:419-426. [DOI] [PubMed] [Google Scholar]
- 24.Meydani, S. N., S. Endres, M. M. Woods, B. R. Goldin, C. Soo, A. Morrill-Labrode, C. Dinarello, and S. L. Gorbach. 1991. Oral (n-3) fatty acids supplementation suppresses cytokine production and lymphocyte proliferation: comparison between young and older women. J. Nutr. 121:547-555. [DOI] [PubMed] [Google Scholar]
- 25.Mosmann, T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65:55-63. [DOI] [PubMed] [Google Scholar]
- 26.Nakane, A., A. Numata, and T. Minagawa. 1992. Endogenous tumor necrosis factor, interleukin-6, and gamma interferon levels during Listeria monocytogenes infection in mice. Infect. Immun. 60:523-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogawa, R., R. Pacelli, M. G. Espey, K. M. Miranda, N. Friedman, S. M. Kim, G. Cox, J. B. Mitchell, D. A. Wink, and A. Russo. 2001. Comparison of control of Listeria by nitric oxide redox chemistry from murine macrophages and NO donors: insights into listeriocidal activity of oxidative and nitrosactive stress. Free Radic. Biol. Med. 30:268-276. [DOI] [PubMed] [Google Scholar]
- 28.Paul, K. P., M. Leichsenring, M. Pfisterer, E. Mayatepek, D. Wagner, M. Domann, H. G. Sonntag, and H. J. Bremer. 1997. Influence of n-6 and n-3 polyunsaturated fatty acids on the resistance to experimental tuberculosis. Metabolism 46:619-624. [DOI] [PubMed] [Google Scholar]
- 29.Peck, M. D., J. W. Alexander, C. K. Ogle, and G. F. Babcock. 1990. The effect of dietary fatty acids on response to Pseudomonas infection in burned mice. J. Trauma 30:445-452. [PubMed] [Google Scholar]
- 30.Puertollano, M. A., M. A. de Pablo, and G. Alvarez de Cienfuegos. 2001. Immunomodulatory effects of dietary lipids alter host natural resistance of mice to Listeria monocytogenes infection. FEMS Immunol. Med. Microbiol. 32:47-52. [DOI] [PubMed]
- 31.Reddy, B. S., and H. Maruyama. 1986. Effect of dietary fish oil on azoxymethane-induced colon carcinogenesis in male F344 rats. Cancer Res. 46:3367-3370. [PubMed] [Google Scholar]
- 32.Rowan, N. J., A. A. G. Candlish, A. Bubert, J. G. Anderson, K. Kramer, and J. McLauchlin. 2000. Virulent rough filaments of Listeria monocytogenes from clinical and food samples secreting wild-type levels of cell-free p60 protein. J. Clin. Microbiol. 38:2643-2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanderson, P., G. G. MacPherson, C. H. Jenkins, and P. C. Calder. 1997. Dietary fish oil diminishes the antigen presentation activity of rat dendritic cells. J. Leukoc. Biol. 62:771-777. [DOI] [PubMed] [Google Scholar]
- 34.Sasaki, T., Y. Kanke, M. Nagashaki, M. Toyokawa, M. Matsuda, J. Shimizu, Y. Misawa, and T. Takita. 2000. Dietary docosahexaenoic acid can alter the surface expression of CD4 and CD8 on T cells in peripheral blood. J. Agric. Food Chem. 48:1047-1049. [DOI] [PubMed] [Google Scholar]
- 35.Turnock, L., M. Cook, H. Steinberg, and C. Czuprynski. 2001. Dietary supplementation with conjugated linoleic acid does not alter the resistance of mice to Listeria monocytogenes infection. Lipids 36:135-138. [DOI] [PubMed] [Google Scholar]
- 36.Yaqoob, P., E. A. Newsholme, and P. C. Calder. 1994. Inhibition of natural killer cell activity by dietary lipids. Immunol. Lett. 41:241-247. [DOI] [PubMed] [Google Scholar]