Abstract
T-cell immune responses in patients with cutaneous leishmaniasis (CL) and mucosal leishmaniasis (ML) were studied during the active disease, at the end of therapy, and 1 to 17 years posttherapy (long-term follow-up). Lymphocyte proliferative responses, phenotypic characterization of CD4+ and CD8+ Leishmania-reactive T cells, and cytokine production were assayed. Patients with active ML and CL showed higher proportions of CD4+ than CD8+ T cells. In CL, the healing process was associated with a decrease of CD4+ and an increase of CD8+, leading to similar CD4+ and CD8+ proportions. This pattern was only seen in ML after long-term therapy. Long-term follow-up of patients with CL showed a positive CD4+/CD8+ ratio as observed during the active disease, although the percentages of these T cell subsets were significantly lower. Patients with CL did not show significant differences between gamma interferon (IFN-γ) and interleukin-5 (IL-5) production during the period of study. Patients with active ML presented higher IFN-γ and IL-5 levels compared to patients with active CL. IL-4 was only detected during active disease. Patients long term after cure from ML showed increasing production of IFN-γ, significant decrease of IL-5, and no IL-4 production. Two apparently beneficial immunological parameters were detected in tegumentary leishmaniasis: (i) decreasing proportions of CD4+ Leishmania-reactive T cells in the absence of IL-4 production associated with cure of CL and ML and (ii) decreasing levels of IL-5 long after cure, better detected in patients with ML. The observed T-cell responses maintained for a long period in healed patients could be relevant for immunoprotection against reinfection and used as a parameter for determining the prognosis of patients and selecting future vaccine preparations.
American tegumentary leishmaniasis (ATL) is produced in Brazil mainly by Leishmania (Viannia) braziliensis, Leishmania (Leishmania amazonensis, and Leishmania (Viannia) guyanensis. Nowadays ATL is often found near metropolitan regions, where the life cycle of its causative organism is apparently maintained by domestic animal reservoirs and anthropophilic sand fly species. In the area of Rio de Janeiro the only species that has been detected as infecting humans and dogs is L. braziliensis (15).
Many experimental studies using mice infected by Leishmania major (reviewed in reference 30) have shown that the T-cell-mediated immune responses play a pivotal role in the processes either for cure or aggravation of the disease. In the first case, as observed in mouse strains resistant to L. major infection, the Th1 CD4+ T-cell subsets are preferentially activated with production of type 1 cytokines (e.g., interleukin 2 [IL-2], gamma interferon [IFN-γ], and lymphotoxin) leading to activation of macrophages and destruction of intracellular parasites. A delayed-type hypersensivity (DTH) to leishmanial antigens is elicited in resistant mice. In the second case, as observed in mouse strains susceptible to L. major infection, Th2 CD4+ T-cell subsets are preferentially activated, leading to production of type 2 cytokines (e.g., IL-4 and IL-5). Down-modulation of macrophage activation occurs, allowing parasite multiplication into the parasitophorous vacuole and aggravation of the disease. The Leishmania-specific cell-mediated immune response is depressed, leading to negative DTH.
There is evidence that CD8+ T cells may also play an important role in the mechanisms for cure of and resistance to Leishmania infection, either by production of IFN-γ and activation of macrophages (7, 16, 20, 23, 30), by a cytolytic effect of cytotoxic T lymphocytes (CTL) upon parasitized macrophages (9, 10), or by a combination of both effects. CD8+ T cells have been associated with protection against Leishmania reinfection in murine models (23); however, the induction of these T-cell subsets in humans seems to be also related to the healing process (12, 31).
The vast majority of patients infected with L. braziliensis present a single or a few number of skin ulcers characterizing a clinical form named cutaneous leishmaniasis (CL). These patients usually have partial immune resistance against the infection, leading to localized lesions, scarceness of parasites, and a tendency to spontaneous healing or good response to antimonial therapy. Only 3 to 5% of patients infected with L. braziliensis can have a severe disease, the mucosal leishmaniasis (ML), also called “espundia,” which produces a destructive secondary mucosal lesion in the nose and mouth. The extreme scarceness of parasites within these lesions does not correlate with the severity of disease and resistance to antimonial therapy usually observed.
Compared to L. major, the contribution of the experimental mouse model for a better understanding of the T-cell-mediated immune responses against L. braziliensis has been very poor, because mice are naturally resistant to this parasite species. Thus, most of the knowledge on the immune responses in CL and ML caused by L. braziliensis has originated from studies of patients (5, 11, 12, 17, 27, 28). Therefore, human tegumentary leishmaniasis does not present a clearly polarized Th1 or Th2 immune response as observed in the mouse-L. major experimental model (11). In fact, T-cell-mediated immune responses and hypersensitivity to leishmanial antigens are present in mild cutaneous disease as well as in severe mucosal disease. However, the magnitude of the T-cell responses tends to be greater in patients with ML than in patients with CL (5, 6, 8, 28), suggesting that patients with ML present an exacerbated hypersensitivity to parasite antigens, which may have a detrimental effect on aggravation of lesions. On the other hand, well-modulated T-cell-mediated responses as usually observed in CL may have a beneficial effect, leading to mild lesions and susceptibility to therapy (5, 6, 19, 22, 27, 28).
We have been interested in defining profiles of the cell-mediated immune responses in CL, during active disease, and soon after clinical cure through the analysis of Leishmania-reactive CD4+ and CD8+ T cells as well as cytokine production in peripheral blood mononuclear cell (PBMC) cultures stimulated by L. braziliensis antigens (11, 12). The intent was to detect possible beneficial parameters of the immune responses associated with clinical cure. Now, we have extended these observations to patients with ML during active disease and after cure. We also did a long-term evaluation of patients who had recovered from CL and ML in order to detect profiles of the T-cell-mediated immune responses associated with a sustained protection against leishmaniasis.
MATERIALS AND METHODS
Patients.
Forty-seven ATL patients suffering from CL (n = 28) and from ML (n = 19) were studied. The group was composed of 26 men and 21 women. The mean age ± standard deviation (SD) was 35.6 ± 15 years and 57 ± 11.4 years, respectively, for patients with CL and ML. All of them had acquired the disease in areas of endemic L. braziliensis infection. The following criteria were used for diagnosis: (i) type of lesion and epidemiological data compatible with ATL; (ii) positive Montenegro skin test (MST) or a DTH to leishmanial antigens; (iii) detection of Leishmania-specific serum antibodies; and (iv) detection of Leishmania parasites in lesion by microscopic examination of histological sections from biopsy samples or by culture in NNN (24) modified medium. Patients were treated with pentavalent antimonial (N-methyl-glucamine), at a dose of 15 to 20 mg of Sb5+/kg of body weight/day, given intramuscularly, for 20 to 30 days).
Patients were evaluated (i) before therapy, during the active disease (20 with CL and 11 with ML), (ii) during the first week after the end of therapy (end-T) (14 with CL and 8 with ML), (iii) 6 months after the end of therapy (6m-T) (7 with CL and 5 with ML), and (iv) at long term follow-up (1 to 17 years) posttherapy (long-term) (13 with CL and 12 with ML). Twenty-three patients (14 with CL and 9 with ML) were sequentially studied during the active disease and after therapy (end-T and/or 6m-T), while 9 of them were long-term followed up (5 with CL and 4 with ML). Blood was drawn after informed consent was obtained from each subject. All procedures were approved by the Ethical Committee of the Fundação Oswaldo Cruz, Ministério da Saúde, Rio de Janeiro, Brazil.
MST.
A volume of 0.1 ml of Leishmania promastigote antigens (leishmanin, kindly provided by Wilson Mayrink, Federal University of Minas Gerais, Belo Horizonte, Brazil) containing 40 μg of total protein per ml was injected intradermally. After 48 h, the presence of an enduration with a diameter of ≥5 mm was considered a positive result. MST was performed for all patients during active disease. Nine patients (five with CL and four with ML) were also subjected to the test at the long-term evaluation.
Lymphocyte proliferative response (LPR) assays.
PBMC were separated by centrifugation over a gradient of Ficoll-Hypaque (Histopaque 1077; Sigma Chemical Company, St. Louis, Mo.). Mononuclear cells were resuspended in RPMI supplemented with 10% heat-activated human AB Rh+ serum, 10 mM HEPES, 1.5 μM l-glutamine, 0.04 mM 2-mercaptoethanol, 200 IU of penicillin per ml, and 200 μg of streptomycin (Sigma) per ml and were adjusted to 3 × 106 cells/ml. The cells were distributed (3 × 105 per well) in triplicate into 96-well, round-bottom plates (Nunc A/S, Roskilde, Denmark) in a final volume of 200 μl. The cultures were incubated for 5 days at 37°C in a humidified atmosphere of 5% CO2 in air, in the presence of the equivalent of 106 disrupted promastigotes of L. braziliensis (MHOM/BR75/M2903) per well as antigens or concanavalin A (lectin from Canavalia ensiformis [4 μg per well]; Sigma) as mitogen or medium alone. Sixteen hours before harvesting, 1 μCi of [3H] thymidine (Amersham International, Amersham, United Kingdom), with a specific activity of 5 Ci/mmol, was added to all wells. Cells were harvested onto fiber filters by using a cell harvester (Skatron Instruments AS, Flow Laboratories, Rockville, Md.), and radioactivity uptake was measured in a scintilation beta counter (TRI-CARB Liquid Scintilator Analyzer; Packard Instrument Company, Downer Gloves, Ill.). Results were expressed as stimulation indices (SI), defined as the mean counts in wells containing antigens or mitogen divided by the background (mean counts in nonstimulated wells). Indices equal to or greater than 2.5 were considered positive.
Phenotypic characterization of L. braziliensis-reactive T cells.
In parallel, leishmanial antigen-reactive T cells were obtained in cultures after in vitro stimulation of PBMC (3 × 106 per well) in 24-well flat-bottom plates (Nunc) in the presence of 5 × 106 disrupted L. braziliensis promastigotes (final volume of 2 ml per well) under the conditions described above. After 5 days in culture, the L. braziliensis-reactive T cells were harvested, washed, and then separated by centrifugation over a discontinuous Percoll gradient (Sigma). For phenotypic analysis, the L. braziliensis-reactive blast T cells were adjusted to 106 cells per 200 μl in a fixative solution and incubated for 30 min at 4°C in the presence of 5 μl of monoclonal antibodies for CD3+ (CD3-RD1; Coulter Corporation, Hialeah, Fla.), CD4+ (T4-FITC; Coulter), and CD8+ (T8-RD1; Coulter). After incubation, the L. braziliensis-reactive T cells were washed three times prior to analysis by flow cytometry (EPICS 751 device; Coulter). For flow cytometry analysis, the blast cell populations were defined by forward- and side-scatter gating.
The supernatant of each culture was collected on day 3 to test IL-4 and IL-5 concentrations and on day 5 to test IFN-γ concentration. The supernatants were stored at −20°C until use.
Cytokine measurement.
Cytokines were measured in supernatants of leishmanial in vitro-stimulated PBMC cultures by enzyme-linked immunosorbent assay (ELISA). All samples were tested in duplicate and compared to standard curves to determine the cytokine concentration. Results were expressed in picograms per milliliter.
An ELISA was used for measuring IFN-γ. Human recombinant IFN-γ antibody was used for capture (NIB4S mouse anti-human IFN-γ monoclonal antibody), and biotinylated IFN-γ antibody was used for detection (4S.B3 mouse anti-human IFN-γ monoclonal antibody); both were purchased from Pharmingen (San Diego, Calif.). The standard curve was obtained using a recombinant human IFN-γ. The minimum reproducible level detected was 62.5 pg/ml.
IL-4 and IL-5 were assessed by using ELISA kits. Quantikine kits (R&D Systems, Minneapolis, Minn.) for human IL-4 immunoassay (minimum IL-4 level detected, 31.2 pg/ml) and for human IL-5 immunoassay (minimum IL-5 level detected, 7.8 pg/ml) were used.
Five healthy uninfected individuals were included as controls. Their MST and lymphocyte proliferative responses induced by L. braziliensis antigens were negative. Phenotypic characterization of L. braziliensis-reactive T cells was not done because those lymphocytes did not proliferate in response to L. braziliensis antigens.
Statistical analysis.
Statistical analysis was performed by the Mann-Whitney U test and the Kruskal-Wallis test by using the software GraphPad Instat (GraphPad Software; San Diego, Calif.).
RESULTS
Patients.
The periods of illness (mean ± SD) were 2.4 ± 1.7 months for CL and 11.8 ± 11.2 years for ML. All patients displayed active leishmaniasis when they were first examined at the Care Unit. All patients with CL showed completely healed lesions at the end of therapy. Although patients with ML still displayed discreet inflammatory signs on their mucous membranes at the end of therapy, they were considered cured after 6 months. Relapses were not observed among patients with CL or ML during the period of study. The MST (mean ± SD) performed during active disease was higher in patients with ML (42.8 ± 28 mm) than in patients with CL (24 ± 18.7 mm) (P = 0.01).
LPR of PBMC stimulated in vitro with L. braziliensis antigens.
The LPR induced by L. braziliensis antigens were positive in all patients with CL tested during the active disease (SI = 30.2 ± 5.8; n = 18). This mean SI as well as those observed at end-T (SI = 14 ± 4.4, n = 13), 6m-T (SI = 22.6 ± 5.3; n = 6), and long-term (SI = 15.2 ± 4.6; n = 13) were not significantly different, although the index during the active disease was higher than those at end-T, 6m-T, and long-term. Regarding ML during active disease, 10 out of 11 patients presented positive LPR to L. braziliensis antigens. The SI then observed (55.1 ± 24.4) was not significantly different from those observed at end-T (SI = 37.8 ± 11.3; n = 8), 6m-T (SI = 48.4 ± 16; n = 5), and long-term (SI = 37.3 ± 22.4; n = 12). No significant differences were observed among patients with CL and ML during the period of study, although a tendency to higher LPR indices was observed in ML patients.
Phenotypic characterization of L. braziliensis-reactive T cells.
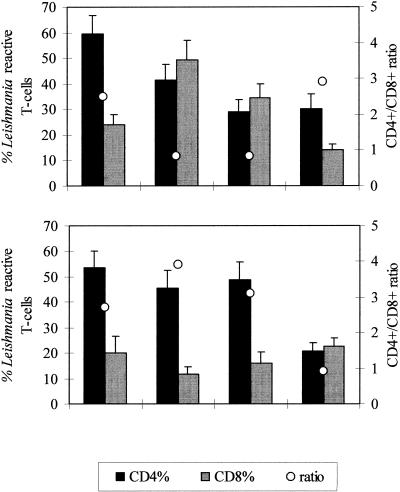
Patients were studied during the active disease (9 with CL and 9 with ML), at end-T (9 with CL and 8 with ML), 6m-T (7 with CL and 4 with ML), and long-term (13 with CL and 12 with ML). Figure 1 shows the percentages (means ± standard errors of the means of CD4+ and CD8+ L. braziliensis-reactive T cells after 5 days in culture as observed during the period of study (active disease, end-T, 6m-T, and long-term).
FIG. 1.
Percentages of Leishmania-reactive CD4+ and CD8+ proliferating T cells in patients with CL (top panel) or ML (bottom panel) during the active disease, at end-T, at 6m-T, and 1 to 17 years after the end of therapy (long-term). Results are expressed as means ± standard errors of the means. Averages of the CD4+/CD8+ ratio are also represented.
During the active disease patients with CL (Fig. 1) showed higher percentages of CD4+ (59.4% ± 7.2%) than CD8+ (23.8 ± 4.1%) L. braziliensis-reactive T cells (P = 0.0003). At end-T, when the lesions were healed, a shift of the CD4+ and CD8+ proportions was observed. Thus, an increase in the mean percentage of CD8+ cells (49.3% ± 7.7%) (P = 0.02) and similar or even lower proportions of CD4+ (41.5% ± 6%) were observed. Hence, a reduction in the CD4+/CD8+ ratio (active disease, 2.5; end-T, 0.8) occurred. A similar pattern was maintained at 6m-T (CD4+, 29.1% ± 4.5%; CD8+, 34.5% ± 5.3%; ratio, 0.8). However, patients with CL evaluated long term after therapy showed again a clear preferential induction of CD4+ cells (30.1% ± 5.7%) over that of CD8+ T cells (14.3% ± 1.8%) (P = 0.02). A significant decline in the proportion of CD8+ cells was observed at this time point, compared to that found at end-T or 6m-T (P < 0.01). The proportion of CD4+ cells also decreased significantly during the period of study (P < 0.01).
Patients with ML (Fig. 1) evaluated during the active disease also showed higher percentages of CD4+ than CD8+ T cells (CD4+, 53.7% ± 6.5%; CD8+, 20.1% ± 6.5%; ratio, 2.7) (P = 0.003). These proportions were not significantly different from those at end-T (CD4+, 45.5% ± 7.1%; CD8+, 11.6% ± 2.9%; ratio, 3.9) and 6m-T (CD4+, 49% ± 6.9%; CD8+, 16% ± 4.3%; ratio, 3.1). However, when they were evaluated at long-term follow-up after therapy a significant decline in the percentage of CD4+ cells (P < 0.004) and an increase in the percentage of CD8+ lymphocytes (P = 0.03) were found. Thus, in patients with ML, the occurrence of similar proportions of L. braziliensis-reactive CD4+ and CD8+ T cells was delayed, only observed at long-term follow-up after therapy (CD4+, 20.9% ± 3.2%; CD8+, 22.6% ± 3.2%; ratio, 0.9), while in patients with CL this pattern was seen earlier at the end of therapy.
Five patients with CL and four with ML were studied sequentially during the whole period of study. The proportions of CD4+ and CD8+ T cells from each patient evaluated at the mentioned time points (during the active disease and at end-T, 6m-T, and long-term) were similar to the proportions observed in CL and ML groups, respectively.
Cytokine production in response to L. braziliensis antigens.
To determine the cytokine production induced by Leishmania stimulation, PBMC from patients with CL and ML were cultured in the presence of L. braziliensis antigens. The supernatants were collected at day 3 for IL-4 and IL-5 tests and at day 5 for IFN-γ tests. Patients were evaluated during active disease, at end-T, and long-term.
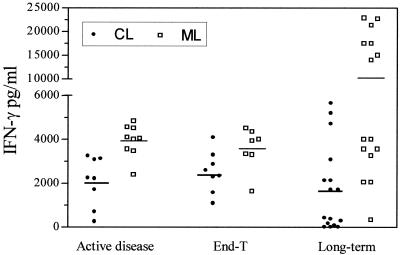
Patients with CL showed no significant differences of the mean levels of IFN-γ detected during the active disease (2,075 ± 392 pg/ml, n = 8), at end-T (2,521 ± 330 pg/ml, n = 8), and long-term (2,174 ± 654 pg/ml, n = 11) (Fig. 2).
FIG. 2.
IFN-γ production in supernatants of PBMCs stimulated in vitro with leishmanial antigens. Patients with CL or ML were evaluated during the active disease, at end-T, and 1 to 17 years after the end of therapy (long-term). Patients are represented by points, and means are indicated by lines.
Patients with ML showed similar mean levels of IFN-γ quantified during active disease (3,929± 247 pg/ml; n = 9) and at end-T (3,611 ± 321 pg/ml; n = 8). A tendency to increasing levels of IFN-γ was observed long-term (11,849 ± 2,509 pg/ml, n = 12), although not statistically significant (Fig. 2). However, Fig. 2 also shows that patients with ML long after therapy can clearly be subdivided into low IFN-γ producers and high IFN-γ producers according to the levels of this cytokine, if below or above the mean. Similar results, although not so clearly, can also be observed in patients with CL.
The mean levels of IFN-γ in the cell culture supernatants were higher in patients with ML than in patients with CL during the active disease (P = 0.0006) (Fig. 2). This pattern was maintained at end-T (P = 0.03) and long-term (P = 0.0005).
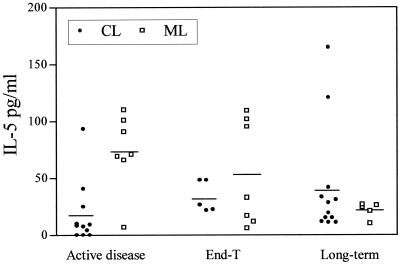
The levels of type 2 cytokines (IL-4 and IL-5) were not high. During the active disease the levels of IL-5 were significantly higher in patients with ML (73.4 ± 12.8 pg/ml, n = 7) than in patients with CL (18 ± 8.3 pg/ml, n = 11) (P = 0.01). Not significant differences between patients with CL and those with ML were observed at end-T (CL, 33.7 ± 6.1 pg/ml, n = 5; ML, 46.3 ± 17.5 pg/ml, n = 7) and long-term (CL, 41.9 ± 14.1 pg/ml, n = 12; ML, 21.6 ± 3.1 pg/ml, n = 5) (Fig. 3).
FIG. 3.
IL-5 production in supernatants of PBMCs stimulated in vitro with leishmanial antigens. Patients with CL or ML were evaluated during the active disease, at end-T, and 1 to 17 years after the end of therapy (long-term). Patients are represented by points, and means are indicated by lines.
The IL-4 production was only measured in patients with ML. This cytokine was detected in four out of six patients (20.4, 24.6, 50.8, and 64.4 pg/ml) during the active disease, while no IL-4 production was observed at the end of therapy. Previous results from our group also showed an absence of IL-4 production in L. braziliensis-stimulated PBMC cultures from patients with CL at the end of therapy (11).
DISCUSSION
Hypersensitivity to L. braziliensis antigens has been considered extremely important in the pathophysiology of ATL (5, 6, 28). Mild disease (CL) is usually associated with apparently well-modulated T-cell-mediated immune responses, whereas severe cases (ML) tend to present higher T-cell responses. In this connection, the detection of immunological profiles developed by ATL patients, in terms of Leishmania-reactive T cells and specific cytokine production as detected in 5-day cultures stimulated by parasite antigens, constitutes an important approach for determining the mechanisms involved in the progression or control of the disease.
Previous results from our group have shown that leishmaniasis patients and normal subjects have similar numbers of CD4+ and CD8+ T cells in PBMC (12). This can be explained because the patients with CL and ML have very low frequencies of Leishmania responder T lymphocytes in the blood (about 1:50,000) (8). Thus, to better evaluate these specific T-cell populations, we decided to expand them in vitro by stimulation with Leishmania antigens.
We have already shown (11, 12) that in PBMC cultures from patients with CL during active disease there is a preferential induction of L. braziliensis-reactive CD4+ T cells and a mixed profile of cytokine production (type 1 and type 2). Resolution of CL lesions at the end of the antimonial therapy is, however, associated with induction of increased proportions of L. braziliensis-reactive-CD8+ T cells, decline of CD4+ L. braziliensis-reactive T cells, and a consequent equilibrium of CD4+ and CD8+ T-cell proportions or even a switch in the CD4+/CD8+ ratio (11, 12). These results were confirmed in the present report as well as in a recent publication from another group (31).
In terms of IFN-γ production, CD4+ T cells have been considered as its main source, although CD8+ and CD4− CD8− cells can also be involved (2, 19). Other authors have observed that patients with CL evaluated after therapy presented increased percentage of IFN-γ producing T cells, particularly among CD8+ lymphocytes (31). Preliminary results from our group have shown that IFN-γ can intracellularly be detected in CD4+ and CD8+ T cells (unpublished data). Similar levels of IFN-γ were presently detected during active disease and at the end of therapy, as has already been demonstrated. Higher (11, 12, 31) or even lower (27) levels of IFN-γ have been shown after cure, although not significantly, in both cases. Thus, the switch in the proportions of CD4+ and CD8+ T cells observed in CL patients after therapy apparently did not have any influence on the IFN-γ production.
On the other hand, previous results from our laboratory have demonstrated that IL-4 is associated with active disease, because this cytokine was detected before therapy but not recently after cure (11). Studies on patients infected by Leishmania aethiopica in Africa have shown similar results (22). All these results suggest that the healing process can be associated with a well-modulated type 1 response, although we have detected production of IL-5 (levels lower than 50 pg/ml) during that period. Interestingly, volunteers vaccinated with crude promastigote antigens from New World Leishmania species also showed L. braziliensis-specific T-cell responses similar to that observed in recently patients cured of CL (14), i.e., a higher proportion of CD8+ than CD4+ T cells and IFN-γ production in the absence of IL-4.
Taken together, these results point to a beneficial effect of increasing CD8+ T cells, decreasing CD4+ T cells, and the absence of IL-4 in the mechanisms for cure and probably protection against American CL, as observed early after therapy (Fig. 1) as well as after vaccination (14). However, we cannot exclude the possibility that NK cells may be present in the L. braziliensis-stimulated cultures (22).
We then decided to investigate the T-cell-mediated immune responses of patients suffering from mucosal lesions (ML). The intent was to get a better explanation for the severity of the disease and consequently to provide possibilities for interventions which could alter the usually severe prognosis.
Similar to findings for patients with CL, higher proportions of CD4+ than CD8+ L. braziliensis-reactive T cells were also found during active disease in patients with ML. However, a switch in the CD4+/CD8+ ratio was not observed soon after therapy (end-T). This can be related to the severity and exacerbated hypersensitivity of the mucosal disease leading to longer periods for resolution of lesions after therapy. In fact, a complete resolution of the mucosal lesions is usually observed about 6 months after therapy, whereas CL lesions are frequently healed even before the end of therapy (13, 24). Moreover, we observed that ML patients during active disease had significantly higher production of type 1 (IFN-γ) and type 2 (IL-4 and IL-5) cytokines compared to patients with CL. Previous studies have associated the induction of cytokines such as IFN-γ or tumor necrosis factor alpha, as well as the increased expression of mucosal IL-4 in mucosal lesions with the pathogenesis of the disease (5, 6, 13, 26). Thus, it is likely that the observed exacerbated hypersensitivity to leishmanial antigens in ML associated with high production of both cytokine types may have a detrimental effect, contributing to the severity of the disease (5, 6, 13, 27). Indeed, stronger intradermal leishmanin test and lymphoproliferative responses, as well as higher frequencies of L. braziliensis-reactive T cells and higher specific cytotoxic activity have been observed in patients with ML compared to patients with CL (3, 5, 6, 8, 28). These results suggest that not only an exacerbated functional activity of T cells but also a dysregulation of the cytokine network may contribute to the destruction of mucosal tissues.
Long-term evaluation of patients after therapy would be an interesting approach to understand why some CL patients, despite the clinical cure, may relapse, presenting secondary mucosal lesions, whereas the vast majority of healed patients show a consistent cure and protection against reinfection. In this connection, patients with CL at 6m-T showed proportions of L. braziliensis-reactive CD4+ and CD8+ T cells similar to those observed recently after therapy, although a tendency for decreasing proportions of these T-cell subsets occurred (Fig. 1). However, in long-term follow-up of patients with CL, continuous decreasing of levels of CD8+ lymphocytes occurred, leading to another switch in the CD4+/CD8+ ratio. This positive CD4+/CD8+ ratio, as well as the production of IFN-γ and IL-5, was similar to that observed during active disease. IL-4, however, was not detected in any period after therapy. Thus, in patients with CL we cannot distinguish between T-cell responses induced during the active disease and at long-term follow-up after cure in terms of IFN-γ or IL-5 production and CD4+/CD8+ ratio, although the percentages of these T-cell subsets were significantly lower long after cure. We can speculate that decreasing proportions of L. braziliensis-reactive CD4+ and CD8+ T cells and the persistence of type 1 CD4+ T cells in the absence of IL-4 production could constitute an important step to avoid reactivation of the disease. Recently, it was demonstrated that patients who had recovered from L. major infection had preferential induction of Leishmania-reactive CD4+ T cells and IFN-γ production (1). These observations would be in agreement with our present results if they refer to patients studied one or more years after therapy.
The T-cell-mediated immune responses observed long-term after cure may represent a sustained immune response occurring one or more years after clinical cure, associated with protection against relapses or reinfections. The CD8+ T-cell populations that were expanded at the end of therapy could be mainly constituted by effector cells that would have a tendency to became apoptotic shortly after the pathogen elimination (18). The CD8+ T lymphocytes that remain in circulation could be reexpanded and differentiated into effector cells for controlling relapses or reinfection when restimulated by the parasite antigens (4, 21, 23, 25). The presence of L. braziliensis-specific CD4+ T cells should also be extremely important, because these cells may contribute to stabilize the remaining CD8+ T-cell populations in circulation (29, 32).
When clinical cure of mucosal lesions is apparently consolidated (long-term), a clear pattern of type 1 cytokine appears, associated with a decreased proportion of CD4+ L. braziliensis-reactive T cells and a CD4+/CD8+ ratio near 1. Compared to active disease, patients with ML evaluated at long-term follow-up after therapy showed increasing IFN-γ levels, significant decrease in IL-5, and no IL-4 production. Interestingly, two subgroups of IFN-γ producers were found: high and low responders (Fig. 2). Unfortunately, the statistical analysis on association among IFN-γ low or high responders and other immunologic parameters or clinical features did not show any significant difference, probably because of the small number of ML patients so far studied long-term after cure. What these results mean in terms of possibility for reactivation of mucosal lesions will probably be answered during the next 5 years of evaluation.
We detected at least two apparently beneficial immunological parameters in tegumentary leishmaniasis: (i) decreasing proportions of CD4+ L. braziliensis-reactive T cells in the absence of IL-4 production associated with cure of CL and ML and (ii) decreasing levels of IL-5, better detected in ML long-term after cure.
These CD4+/CD8+ and cytokine profiles observed in patients with CL and ML during active disease, recently after therapy, and at long-term follow-up after therapy can be useful for prognosis of the disease and also as immunological parameters associated with protection that could be helpful for studies on screening of antigen candidates for future vaccines.
Acknowledgments
This work was supported by the European Economic Community (grant TS3 CT 94.0319), the Conselho Nacional de Desenvolvimento Científico e Tecnológico (sponsorship of A.M.D.-C. and V.P.-R.), and the Fundação de Amparo à Pesquisa do Estado de Rio de Janeiro (FAPERJ) (grant E-26/170.295/2000).
We are grateful to C. Pirmez for critical review of the manuscript. We thank A. Bertho and M. Santiago for flow cytometry analysis and to R. Pellegrino and A. Oliveira for their secretarial assistance.
REFERENCES
- 1.Ajdary, S., M. H. Alimohammadian, M. B. Eslami, K. Kemp, and A. Kharazmi. 2000. Comparison of the immune profile of nonhealing cutaneous leishmaniasis patients with those with active lesions and those who have recovered from infection. Infect. Immun. 68 :1760-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bottrel, R. L. A., W. O. Dutra, F. A. Martins, B. Gontijo, E. Carvalho, M. Barral-Netto, A. Barral, R. P. Almeida, W. Mayrink, R. Locksley, and K. J. Gollob. 2001. Flow cytometry determination of cellular sources and frequencies of key-cytokine-producing lymphocytes directed against recombinant LACK and soluble Leishmania antigen in human cutaneous leishmaniasis. Infect. Immun. 69:3232-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brodskyn, C. I., A. Barral, V. Boaventura, E. Carvalho, and M. Barral-Netto. 1997. Parasite-driven in vitro human lymphocyte cytotoxicity against autologous infected macrophages from mucosal leishmaniasis. J. Immunol. 159:4467-4473. [PubMed] [Google Scholar]
- 4.Callan, M. F. C., N. Annels, N. Steven, L. Tan, J. Wilson, A. J. McMichael, and A. B. Rickinson. 1998. T cell selection during the evolution of CD8+ T cells memory in vivo. Eur. J. Immunol. 28:4382-4390. [DOI] [PubMed] [Google Scholar]
- 5.Carvalho, E. M., W. D. Johnson, E. Barreto, P. D. Marsden, J. L. M. Costa, S. Reed, and H. Rocha. 1985. Cell mediated immunity in American cutaneous and mucosal leishmaniasis. J. Immunol. 135:4144-4148. [PubMed] [Google Scholar]
- 6.Castés, M., M. Cabrera, D. Trujillo, and J. Convit. 1988. T-cell subpopulations, expression of interleukin-2 receptor, and production of interleukin-2 and gamma interferon in human American cutaneous leishmaniasis. J. Clin. Microbiol. 26:1207-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan, M.-Y. C. 1993. T cell response in murine Leishmania mexicana amazonensis infection: production of interferon-γ by CD8+ cells. Eur. J. Immunol. 23:1181-1184. [DOI] [PubMed] [Google Scholar]
- 8.Conceição-Silva, F., R. C. C. Dórea, C. Pirmez, A. Schubach, and S. G. Coutinho. 1990. Quantitative study of Leishmania braziliensis braziliensis reactive T cells in peripheral blood and in the lesions of patients with American mucocutaneous leishmaniasis. Clin. Exp. Immunol. 79:221-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conceição-Silva, F., B. L. Perlaza, J. A. Louis, and P. Romero. 1994. Leishmania major infection in mice primes for specific major histocompatibility complex class I-restricted CD8+ cytotoxic T cell responses. Eur. J. Immunol. 24:2813-2817. [DOI] [PubMed] [Google Scholar]
- 10.Coutinho, S. G., J. A. Louis, J. Mauel, and H. D. Engers. 1984. Induction by specific T lymphocytes of intracellular destruction of Leishmania major in infected murine macrophages. Parasite Immunol. 6:157-170. [DOI] [PubMed] [Google Scholar]
- 11.Coutinho, S. G., M. P. Oliveira, A. M. Da-Cruz, P. M. De Luca, S. C. F. Mendonça, A. L. Bertho, S. Lynn, and D. McMahon-Pratt. 1996. T-cell responsiveness of American cutaneous leishmaniasis patients to purified Leishmania pifanoi amastigote antigens and Leishmania braziliensis promastigote antigens: immunologic patterns associated with cure. Exp. Parasitol. 86:144-155. [DOI] [PubMed] [Google Scholar]
- 12.Da-Cruz, A. M., F. Conceição-Silva, A. L. Bertho, and S. G. Coutinho. 1994. Leishmania-reactive CD4+ and CD8+ T cells associated with cure of human cutaneous leishmaniasis. Infect. Immun. 62:2614-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Da-Cruz, A. M., M. P. Oliveira, P. M. De Luca, S. C. F. Mendonça, and S. G. Coutinho. 1996. Tumor necrosis factor-α in human American tegumentary leishmaniasis. Mem. Inst. Oswaldo Cruz 91:225-229. [DOI] [PubMed] [Google Scholar]
- 14.De Luca, P. M., W. Mayrink, C. R. Alves, S. G. Coutinho, M. P. Oliveira, A. L. Bertho, V. P. Toledo, C. A. Costa, O. Genaro, and S. C. F. Mendonça. 1999. Evaluation of the stability and immunogenicity of autoclaved and nonautoclaved preparations of a vaccine against American tegumentary leishmaniasis. Vaccine 17:1179-1185. [DOI] [PubMed] [Google Scholar]
- 15.Grimaldi, Jr., G., R. B. Tesh, and D. McMahon-Pratt. 1989. A review of the geographic distribution and epidemiology of leishmaniasis in the New World. Am. J. Trop. Med. Hyg. 41:687-725. [DOI] [PubMed] [Google Scholar]
- 16.Gurunathan, S., D. L. Sacks, D. R. Brown, S. L. Reiner, H. Charest, N. Glaichenhaus, and R. A. Seder. 1997. Vaccination with DNA encoding the immunodominant LACK parasite antigen confers protective immunity to mice infected with Leishmania major. J. Exp. Med. 186:1137-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haberer, J. E., A. M. Da-Cruz, L. Soong, M. P. Oliveira-Neto, L. Rivas, D. McMahon-Pratt, and S. G. Coutinho. 1998. Leishmania pifanoi amastigote antigen P-4: epitopes involved in T-cell responsiveness in human cutaneous leishmaniasis. Infect. Immun. 66:3100-3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamann, D., M. T. L. Roos, and R. A. W. van Lier. 1999. Faces and phases of human CD8+ T-cell development. Immunol. Today 20:177-180. [DOI] [PubMed] [Google Scholar]
- 19.Kemp, K., T. G. Theander, L. Hviid, A. Garfar, A. Kharazmi, and M. Kemp. 1999. Interferon-γ- and tumour necrosis factor-α-producing cells in humans who are immune to cutaneous leishmaniasis. Scand. J. Immunol. 49:655-659. [DOI] [PubMed] [Google Scholar]
- 20.Kima, P. E., N. H. Ruddle, and D. McMahon-Pratt. 1997. Presentation via the class I pathway by Leishmania amazonensis-infected macrophages of an endogenous leishmanial antigens to CD8+ T cells. J. Immunol. 159:1828-1834. [PubMed] [Google Scholar]
- 21.Lalvani, A., R. Brookes, S. Hambleton, W. J. Britton, A. V. S. Hill, and A. J. McMichael. 1997. Rapid effector function in CD8+ memory T cells. J. Exp. Med. 186:859-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maasho, K., F. Sanchez, E. Schurr, A. Hailu, and H. Akuffo. 1998. Indications of the protective role of natural killer cells in human cutaneous leishmaniasis in an area of endemicity. Infect. Immun. 66:2698-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Müller, I., P. Kropf, J. A. Louis, and G. Milon. 1994. Expansion of gamma interferon-producing CD8+ T cells following secondary infection of mice immune to Leishmania major. Infect. Immun. 62:2575-2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oliveira-Neto, M. P., M. S. Mattos, M. A. Perez, A. M. Da-Cruz, O. Fernandes, J. Moreira, S. C. Gonçalves-Costa, L. R. Brahim, C. R. Menezes, and C. Pirmez. 2000. American tegumentary leishmaniasis (ATL) in Rio de Janeiro state, Brazil: main clinical and epidemiologic characteristics. Int. J. Dermatol. 39:506-514. [DOI] [PubMed] [Google Scholar]
- 25.Opferman, J. T., B. T. Ober, and P. G. Ashton-Rickardt. 1999. Linear differentiation of cytotoxic effectors into memory T lymphocytes. Science 283:1745-1748. [DOI] [PubMed] [Google Scholar]
- 26.Pirmez, C., M. Yamamura, K. Uyemura, M. Paes-Oliveira, F. Conceição-Silva, and R. L. Modlin. 1993. Cytokine patterns in the pathogenesis of human leishmaniasis. J. Clin. Investig. 91:1390-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ribeiro-de-Jesus, A., R. P. Almeida, H. Lessa, O. Bacellar, and E. M. Carvalho. 1998. Cytokine profile and pathology in human leishmaniasis. Braz. J. Med. Biol. Res. 31:143-148. [DOI] [PubMed] [Google Scholar]
- 28.Saravia, N. G., L. Valderrama, M. Labrada, A. F. Holguin, C. Navas, G. Palma, and K. A. Weigle. 1989. The relationship of Leishmania braziliensis subspecies and immune response to disease expression in New World leishmaniasis. J. Infect. Dis. 159:725-735. [DOI] [PubMed] [Google Scholar]
- 29.Schoenberger, S. P., R. E. M. Toes, E. I. H. van der Voort, R. Offringa, and C. J. M. Melief. 1998. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature 393:480-483. [DOI] [PubMed] [Google Scholar]
- 30.Scott, P., and J. P. Farrell. 1998. Experimental cutaneous leishmaniasis: induction and regulation of T cells following infection of mice with Leishmania major. Chem. Immunol. 70:60-80. [DOI] [PubMed] [Google Scholar]
- 31.Toledo, V. P. C. P., W. Mayrink, K. J. Gollob, M. A. P. Oliveira, C. A. Costa, O. Genaro, J. A. Pinto, and L. C. C. Afonso. 2001. Immunochemotherapy in American cutaneous leishmaniasis: immunological aspects before and after treatment. Mem. Inst. Oswaldo Cruz 96:89-98. [DOI] [PubMed] [Google Scholar]
- 32.Varga, S. M., and R. M. Welsh. 1998. Stability of virus-specific CD4+ T cell frequencies from acute infection into long term memory. J. Immunol. 161:367-374. [PubMed] [Google Scholar]