Abstract
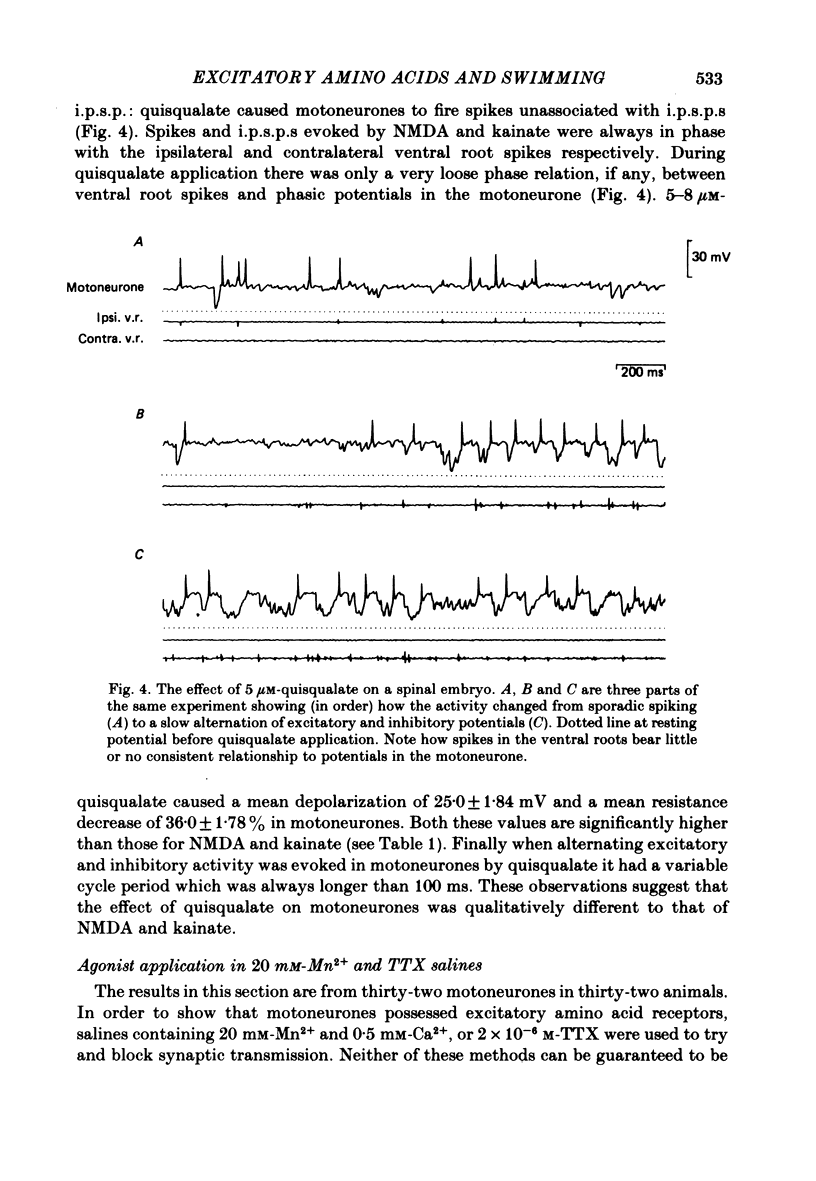
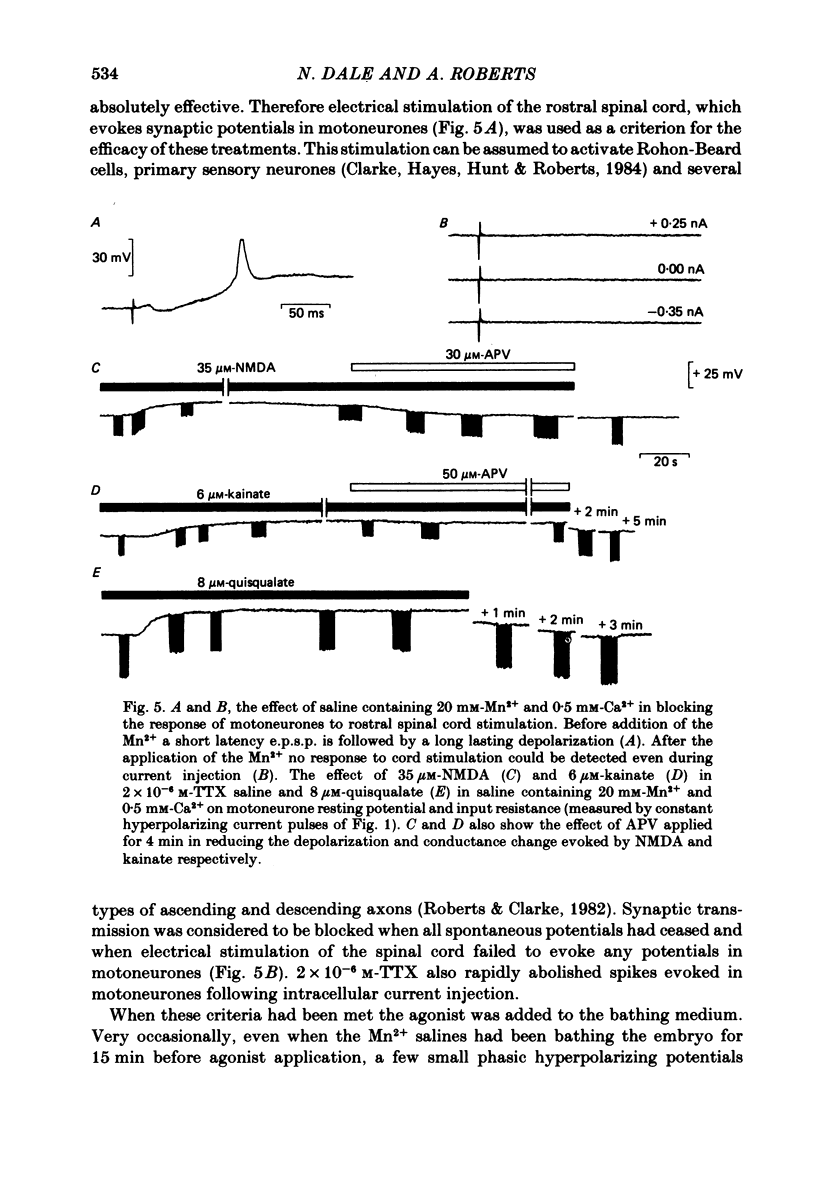
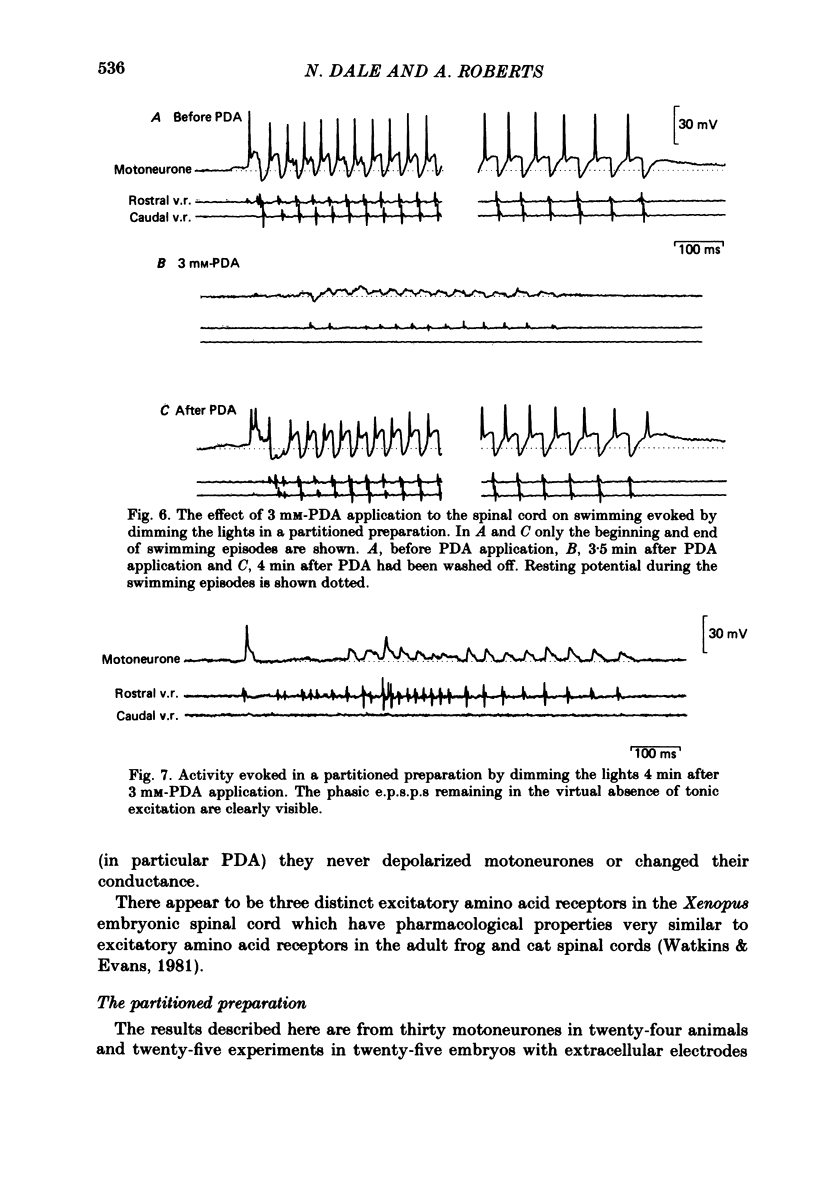
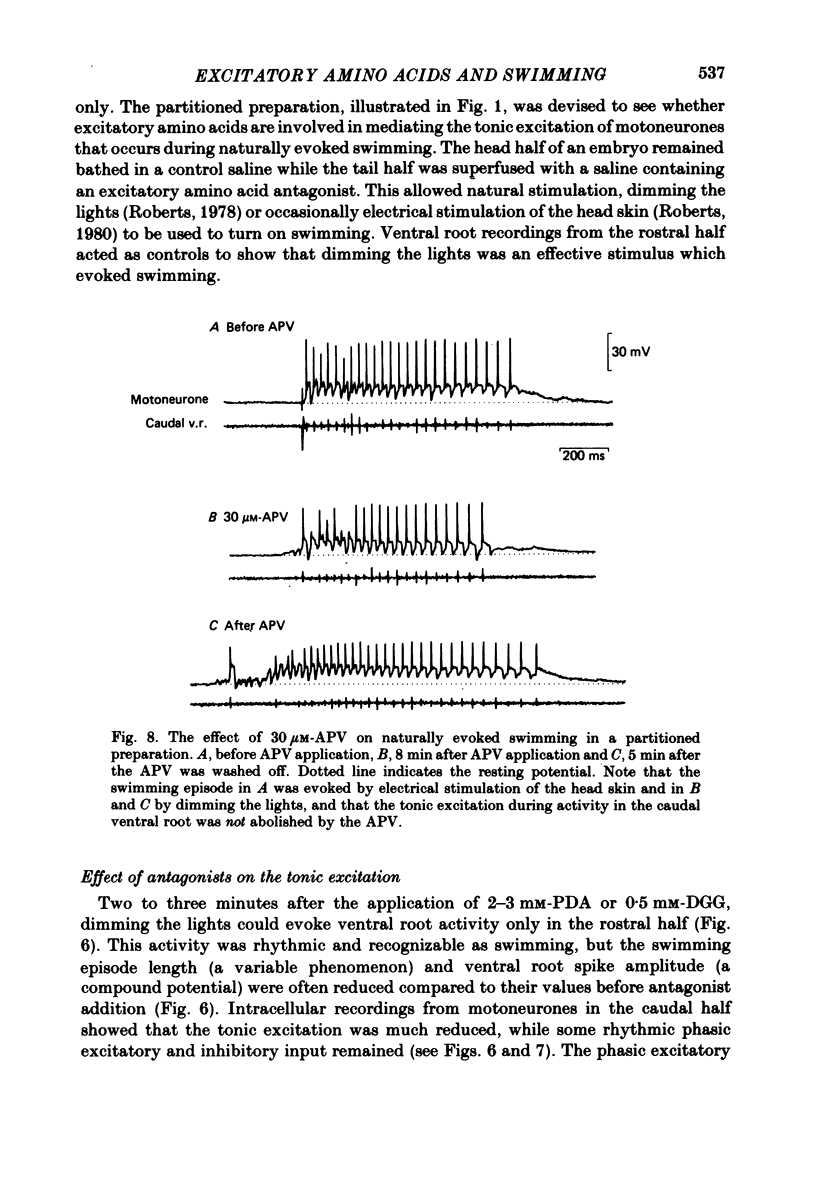
Bath application of N-methyl-D-aspartate (NMDA), kainate or quisqualate to Xenopus embryos depolarized spinal cord motoneurones and reduced their input resistance in both normal salines and salines containing 20 mM-Mn2+ and 0.5 mM-Ca2+, or 2 X 10(-6) M-tetrodotoxin. This suggests that motoneurones possess all three types of excitatory amino acid receptor. These receptors have similar specificities to excitatory amino acid antagonists as those occurring in adult frog and cat spinal cords. Application of 30-40 microM-NMDA or 5-6.5 microM-kainate to the medium bathing spinalized embryos can cause a sustained patterned motor output similar to that of swimming evoked by natural stimulation of intact animals. At these concentrations NMDA and kainate depolarized motoneurones by 19.0 +/- 1.80 (mean +/- S.E. of mean) and 18.0 +/- 2.00 mV respectively and decreased their input resistance by 23.0 +/- 2.82% and 24.0 +/- 3.46%. These changes are similar to those associated with the tonic excitation which motoneurones receive during naturally evoked swimming. Bath application of 5-8 microM-quisqualate to spinal embryos can also cause a sustained motor output. However, this was different to that evoked by NMDA and kainate and was inappropriate for swimming. When applied to intact animals during swimming both 2-3 mM-cis-2,3-piperidine dicarboxylic acid (PDA) and 0.5 mM-gamma-D-glutamylglycine (DGG) selectively blocked the tonic excitation of motoneurones and in doing so abolished the motor output of the spinal cord. 50-200 microM-2-amino-5-phosphonovaleric acid reduced the tonic excitation but to a lesser extent than either PDA or DGG. The tonic excitation of motoneurones which occurs during swimming therefore appears to be mediated via an endogenous excitatory amino acid transmitter which acts on NMDA and kainate receptors.
Full text
PDF

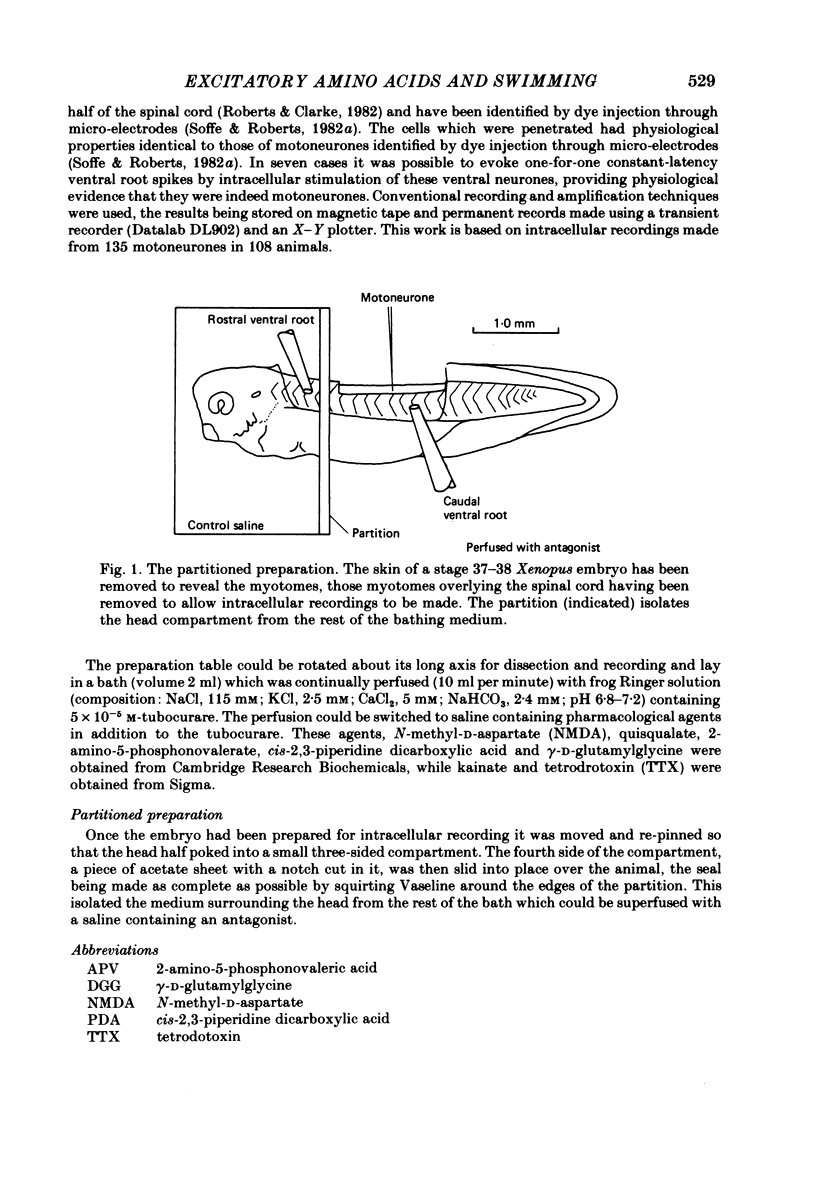
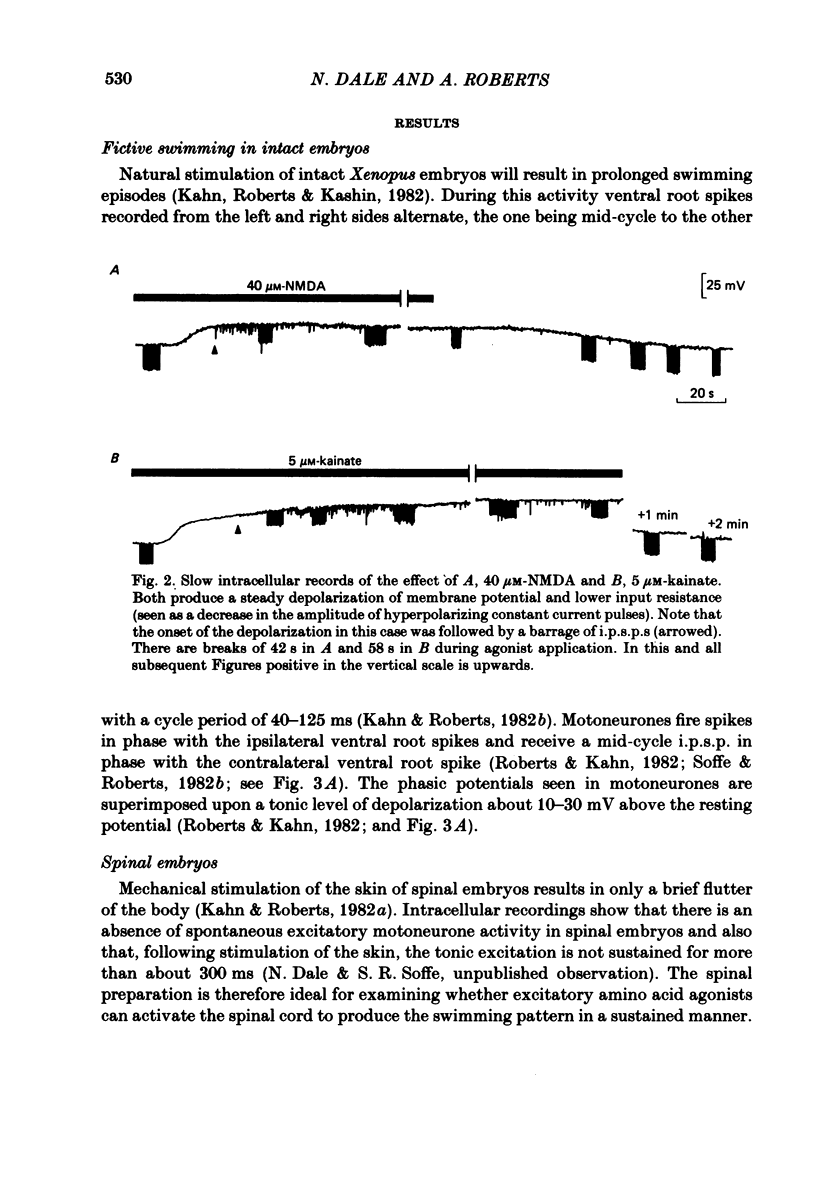
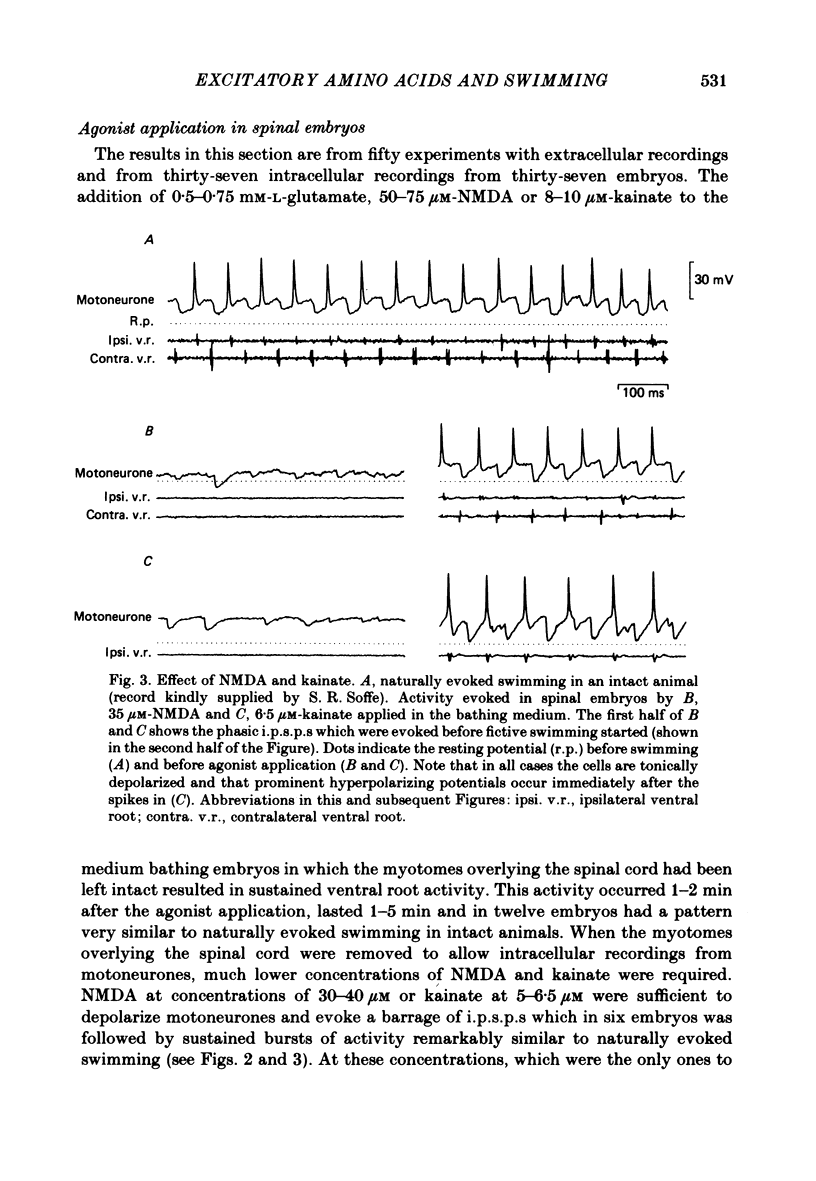
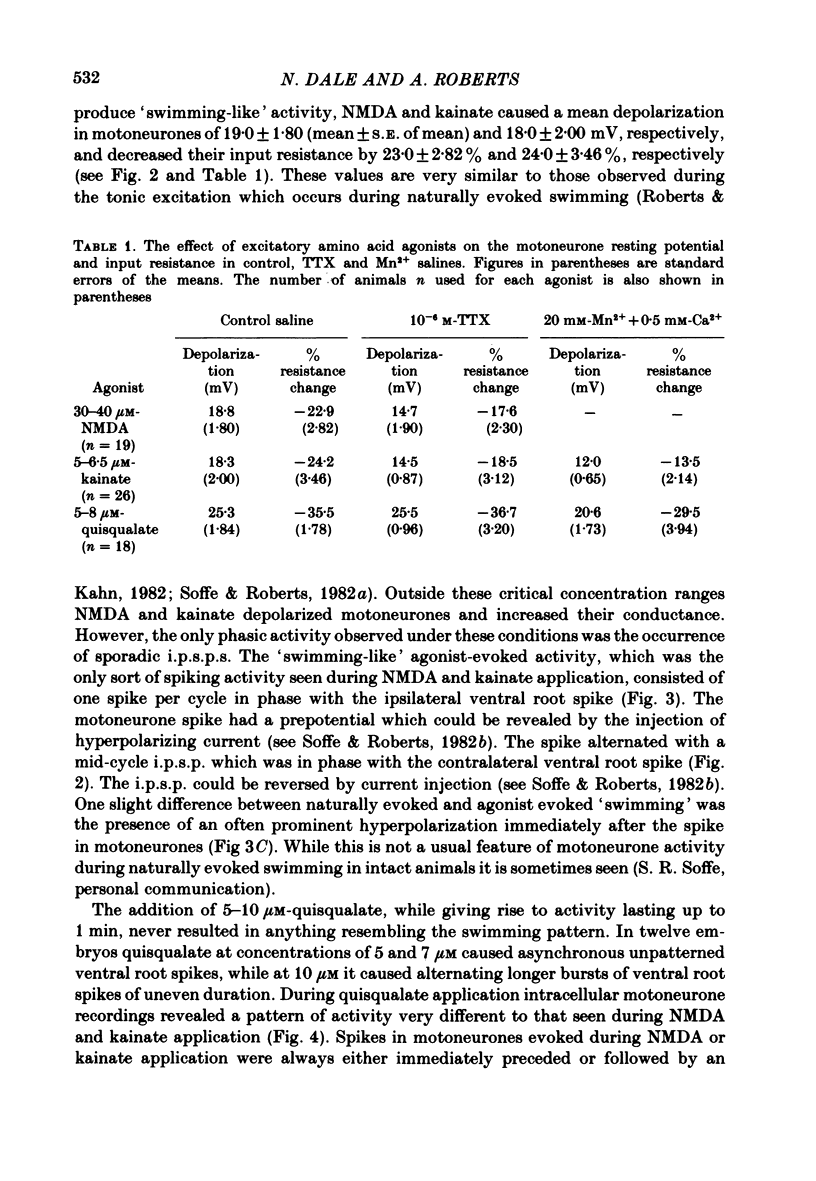





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ault B., Evans R. H., Francis A. A., Oakes D. J., Watkins J. C. Selective depression of excitatory amino acid induced depolarizations by magnesium ions in isolated spinal cord preparations. J Physiol. 1980 Oct;307:413–428. doi: 10.1113/jphysiol.1980.sp013443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURTIS D. R., WATKINS J. C. Acidic amino acids with strong excitatory actions on mammalian neurones. J Physiol. 1963 Apr;166:1–14. doi: 10.1113/jphysiol.1963.sp007087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke J. D., Hayes B. P., Hunt S. P., Roberts A. Sensory physiology, anatomy and immunohistochemistry of Rohon-Beard neurones in embryos of Xenopus laevis. J Physiol. 1984 Mar;348:511–525. doi: 10.1113/jphysiol.1984.sp015122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forssberg H., Grillner S. The locomotion of the acute spinal cat injected with clonidine i.v. Brain Res. 1973 Feb 14;50(1):184–186. doi: 10.1016/0006-8993(73)90606-9. [DOI] [PubMed] [Google Scholar]
- Grillner S., McClellan A., Sigvardt K., Wallén P., Wilén M. Activation of NMDA-receptors elicits "fictive locomotion" in lamprey spinal cord in vitro. Acta Physiol Scand. 1981 Dec;113(4):549–551. doi: 10.1111/j.1748-1716.1981.tb06937.x. [DOI] [PubMed] [Google Scholar]
- Kahn J. A., Roberts A. Experiments on the central pattern generator for swimming in amphibian embryos. Philos Trans R Soc Lond B Biol Sci. 1982 Jan 27;296(1081):229–243. doi: 10.1098/rstb.1982.0004. [DOI] [PubMed] [Google Scholar]
- Kahn J. A., Roberts A., Kashin S. M. The neuromuscular basis of swimming movements in embryos of the amphibian Xenopus laevis. J Exp Biol. 1982 Aug;99:175–184. doi: 10.1242/jeb.99.1.175. [DOI] [PubMed] [Google Scholar]
- Kahn J. A., Roberts A. The central nervous origin of the swimming motor pattern in embryos of Xenopus laevis. J Exp Biol. 1982 Aug;99:185–196. doi: 10.1242/jeb.99.1.185. [DOI] [PubMed] [Google Scholar]
- Lennard P. R., Getting P. A., Hume R. I. Central pattern generator mediating swimming in Tritonia. II. Initiation, maintenance, and termination. J Neurophysiol. 1980 Jul;44(1):165–173. doi: 10.1152/jn.1980.44.1.165. [DOI] [PubMed] [Google Scholar]
- Lennard P. R., Stein P. S. Swimming movements elicited by electrical stimulation of turtle spinal cord. I. Low-spinal and intact preparations. J Neurophysiol. 1977 Jul;40(4):768–778. doi: 10.1152/jn.1977.40.4.768. [DOI] [PubMed] [Google Scholar]
- Roberts A., Clarke J. D. The neuroanatomy of an amphibian embryo spinal cord. Philos Trans R Soc Lond B Biol Sci. 1982 Jan 27;296(1081):195–212. doi: 10.1098/rstb.1982.0002. [DOI] [PubMed] [Google Scholar]
- Roberts A., Khan J. A. Intracellular recordings from spinal neurons during 'swimming' in paralysed amphibian embryos. Philos Trans R Soc Lond B Biol Sci. 1982 Jan 27;296(1081):213–228. doi: 10.1098/rstb.1982.0003. [DOI] [PubMed] [Google Scholar]
- Roberts A. Pineal eye and behaviour in Xenopus tadpoles. Nature. 1978 Jun 29;273(5665):774–775. doi: 10.1038/273774a0. [DOI] [PubMed] [Google Scholar]
- Shik M. L., Orlovsky G. N. Neurophysiology of locomotor automatism. Physiol Rev. 1976 Jul;56(3):465–501. doi: 10.1152/physrev.1976.56.3.465. [DOI] [PubMed] [Google Scholar]
- Soffe S. R., Roberts A. Activity of myotomal motoneurons during fictive swimming in frog embryos. J Neurophysiol. 1982 Dec;48(6):1274–1278. doi: 10.1152/jn.1982.48.6.1274. [DOI] [PubMed] [Google Scholar]
- Soffe S. R., Roberts A. Tonic and phasic synaptic input to spinal cord motoneurons during fictive locomotion in frog embryos. J Neurophysiol. 1982 Dec;48(6):1279–1288. doi: 10.1152/jn.1982.48.6.1279. [DOI] [PubMed] [Google Scholar]
- Spitzer N. C. The ionic basis of the resting potential and a slow depolarizing response in Rohon-Beard neurones of Xenopus tadpoles. J Physiol. 1976 Feb;255(1):105–135. doi: 10.1113/jphysiol.1976.sp011272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins J. C., Evans R. H. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]


