Abstract
Human pythiosis is an emerging, fatal, infectious disease caused by Pythium insidiosum and occurs in both tropical and subtropical countries. Thalassemic patients, farmers, and aquatic-habitat residents are predisposed to this disease. Delayed treatment due to the long time required for isolation and identification of the causative organism, as well as the difficulty in obtaining internal organ specimens, results in high morbidity and mortality. To facilitate rapid diagnosis, an in-house enzyme-linked immunosorbent assay (ELISA) for the detection of immunoglobulin G antibodies against P. insidiosum was developed and evaluated for the diagnosis and monitoring of human pythiosis. Sixteen sera were collected from seven culture-proven human pythiosis cases. A total of 142 sera from thalassemic patients, from patients with other infectious diseases, and from healthy blood donors served as controls. All sera were tested in duplicate. By choosing a suitable cutoff point to maximize sensitivity and specificity, sera from pythiosis cases were all determined to be positive, whereas sera from control groups were all determined to be negative. ELISA signals from serial samples of sera taken from treated patients showed gradually declining levels of antibodies to P. insidiosum. The ELISA test was highly sensitive (100%) and specific (100%) and was useful for early diagnosis and for monitoring the treatment for pythiosis.
Pythium insidiosum is classified in the phylum Oomycota of the kingdom Chromista (2). It is the only Pythium species that causes an infectious disease (pythiosis) in humans and animals (9), mostly in tropical and subtropical countries (6). Infection has been proposed to occur by invasion of asexual, biflagellate zoospores into injured host tissue after consecutive sequences of attachment, encystment, and germination (7).
In 1902, the etiologic agent of pythiosis was first isolated from a horse. It was initially named Hyphomyces destruens but was renamed Pythium insidiosum in 1987 (1, 9, 11). Since 1971, the disease has been recognized in a variety of animals, including cats, dogs, and cattle (8, 11, 14). In 1985, the first two human pythiosis cases were reported from Thailand (3), and other reports followed afterward (3, 6, 14-18). Three forms of human pythiosis have been observed, and they have been classified as (i) cutaneous or subcutaneous pythiosis affecting the periorbital area, face, or limbs as a granulomatous, ulcerating, abscess-like or cellulitic lesion; (ii) ophthalmic pythiosis affecting eyes as corneal ulcers or keratitis; or (iii) systemic pythiosis affecting vascular tissue and resulting in arterial occlusions or aneurysms leading to gangrene or vascular rupture, respectively (3, 6, 14, 17). Hemoglobinopathy, rice-field work, and aquatic habitats are considered to be risk factors (3, 6, 18).
The morbidity and mortality levels associated with pythiosis are very high (6). In the systemic form, limb amputation and fatal arterial leakage are common outcomes. Early diagnosis and proper treatments such as chemotherapy, surgery, and immunotherapy are needed to achieve a better prognosis (8, 18). Unfortunately, there are neither clinical nor histological pathognomonic features for this disease. Definitive laboratory diagnosis can be made by culture and zoospore induction. However, special expertise and considerable time are required for these laboratory procedures. Furthermore, obtaining arterial tissue specimens for culture may be fatally injurious to patients. A serodiagnostic test by immunodiffusion (ID) for the detection of specific antibodies has been reported to be convenient for diagnosing and monitoring the disease, but the test shows poor sensitivity (4, 8, 10, 12). To increase the detection sensitivity, Mendoza et al. (8) used an enzyme-linked immunosorbent assay (ELISA) instead of an ID test. The present study aimed to develop and evaluate an in-house ELISA test for the early diagnosis and monitoring of human pythiosis in comparison to both the culture identification and ID tests.
MATERIALS AND METHODS
Serum collection.
A total of 15 sera from seven culture-proven human pythiosis cases (five systemic, one ophthalmic, and one subcutaneous) were collected and kept at −20°C until use. Another 142 sera were collected for use in three control groups. The first group included 120 sera randomly collected from healthy blood donors who came to the Blood Bank Division, Ramathibodi Hospital. The second group included nine healthy thalassemic patients who showed no clinical evidence for pythiosis. The third group included sera from 2 patients with a highly positive antinuclear antibody (ANA) titer and sera from 11 patients with other infections (2 anti-human immunodeficiency virus positive, 2 anti-hepatitis B virus positive, 2 anti-hepatitis C virus positive, 2 toxoplasmotic, 1 cryptococcotic, 1 leptospirotic, and 1 zygomycotic).
Antigen preparation for ID and ELISA.
Antigen preparation was modified from the original methods of the ID test (12) and ELISA (8). Briefly, P. insidiosum strain RAMA-I from a systemic pythiosis patient was subcultured on Sabouraud dextrose agar and incubated at 35°C for 3 days. A small block of mycelium was transferred onto potato dextrose agar and incubated at 35°C for 24 h. Small hyphal blocks from the resulting colonies were transferred into 500 ml of Sabouraud dextrose broth and shaken at 100 rpm in a shaker-incubator at 35°C for 5 days. Merthiolate was added to a final concentration of 0.02% (wt/vol), and the broth culture was left at 4°C overnight before it was filtered by using filter paper (No. 1; Whatman, Maidstone, England). The broth was concentrated 20-fold by dialysis by using a membrane with a molecular weight cutoff point of 12,000 to 14,000 (Spectrum, Rancho-Dominguez, Calif.) against polyvinylpyrrolidone. This concentrated culture filtrate antigen (CCFA) was used in the ID test (4, 10, 12). The filtered mycelia were prepared for ELISA coating antigen by freezing them at −70°C for 3 days and grinding them in a mortar in the presence of liquid nitrogen (8). The broken hyphae were resuspended in 5 ml of sterile distilled water and kept at 4°C for 24 h. The suspension was refrigerated at 4°C and then centrifuged at 6,500 rpm for 10 min. The resulting supernatant was referred to as the soluble antigen from broken hyphae (SABH) and was collected and dialyzed by using a membrane molecular weight cutoff point of 8,000 to 10,000 (Membrane Filtration Products, San Antonio, Tex.) in phosphate-buffered saline (pH 7.2; PBS) at 4°C, with a change of PBS every 8 h for three times. The protein concentration was measured by spectrophotometer (Biochrom, Cambridge, England). Phenylmethylsulfonyl fluoride at 0.1 M and 0.1% (wt/vol) sodium azide were added as preservatives. CCFA and SABH were stored at 4°C until use.
ID test.
The ID test was modified from the method of Pracharktam et al. (12). Briefly, agar gel diffusion was carried out on a slide coated with 5 ml of 2% water agar (grade A in distilled water; Becton Dickinson). The CCFA and serum to be tested were each added to 4-mm-diameter wells separated by 4 mm. The slides were incubated in a moist chamber at room temperature for 24 h. The appearance of a precipitation line after examination by the naked eye was considered a positive test result.
ELISA test.
A 96-well U-shaped polystyrene plate (Nunc, Roskilde, Denmark) was coated with 75 μl of a 2.5-μg/ml concentration of SABH diluted in Tris-buffered saline (pH 7.2)/well and incubated at 4°C overnight. The coated plate was washed four times with PBS containing 0.05% Tween 20 (PBS-T) and blocked with 240 μl of 0.5% (wt/vol) bovine serum albumin in TBS/well at 37°C for 1 h. All tested sera (including controls) were diluted in duplicate at 1:800 with PBS-T. Diluted (50 μl) sera were added into each well, incubated at 37°C for 1 h, and then washed four times. After being washed, 50 μl of horseradish peroxidase-conjugated rabbit anti-human immunoglobulin G (Dako, Glostrup, Denmark) diluted at 1:40,000 with PBS-T solution was added to each well, and the mixtures were incubated at 37°C for 1 h. The plate was washed, and the color was developed by the addition of 100 μl of freshly prepared chromogen solution (1 ml of tetramethyl benzidine dihydrochloride [5 g/liter] and 10 ml of a hydrogen peroxide [0.1 g/liter] in acetate-buffered [25 mmol/liter] solution) to each well, followed by incubation in a dark chamber at room temperature for 15 min. The substrate-enzyme reaction was stopped by the addition of 100 μl of 0.5 N sulfuric acid. The optical density (OD) was measured with an ELISA Reader (Behring Diagnostic) at wavelengths of 450 and 650 nm. To standardize the ELISA results at each batch of testing, known positive and negative sera were pooled, divided into aliquots, and kept at −20°C. The OD values of all sera were divided by the OD value of the negative control sera for each test batch and defined as the ELISA values (EVs).
RESULTS
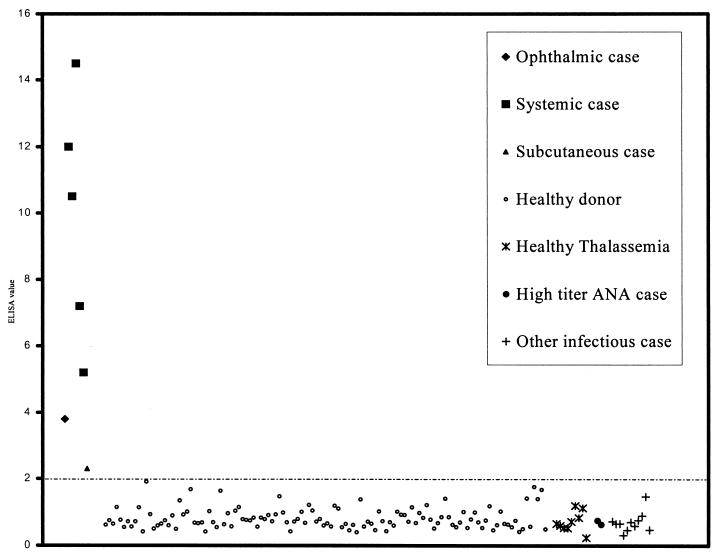
The in-house ELISA test was evaluated for diagnosis of human pythiosis by comparison to successful cultures derived from patients defined as the “gold standard.” The sensitivity and specificity for various cutoff points (Table 1) were based on results from culture positive cases and the control group. An ELISA signal of EV > 2.0 was chosen as the cutoff point for the ELISA test because at this value all culture-proven pythiosis sera were positive whereas all control sera were negative, resulting in 100% sensitivity, specificity, positive predictive, and negative predictive values (Fig. 1 and Table 1). The EV of the initial serum of each systemic case was >5.0, except for two samples, one an ophthalmic case and the other a subcutaneous case, for which the EVs were 3.8 and 2.3, respectively.
TABLE 1.
Comparison of four ELISA values for best cutoff point of in-house ELISA test
| Cutoff point | ELISA value | Sensitivity (%) | Specificity (%) | PPVa (%) | NPVb (%) |
|---|---|---|---|---|---|
| Mean + 2 SDc | 1.41 | 100.00 | 95.07 | 50.00 | 100.00 |
| Mean + 3 SD | 1.72 | 100.00 | 98.59 | 77.78 | 100.00 |
| Chosen-I | 2.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| Chosen-II | 3.00 | 85.71 | 100.00 | 100.00 | 99.30 |
PPV, positive predictive value.
NPV, negative predictive value.
Mean, that is, the mean ELISA value of the control sera. SD, standard deviation.
FIG. 1.
EVs of all pythiosis-proven and control sera. The cutoff value (EV > 2) is indicated by the dashed line.
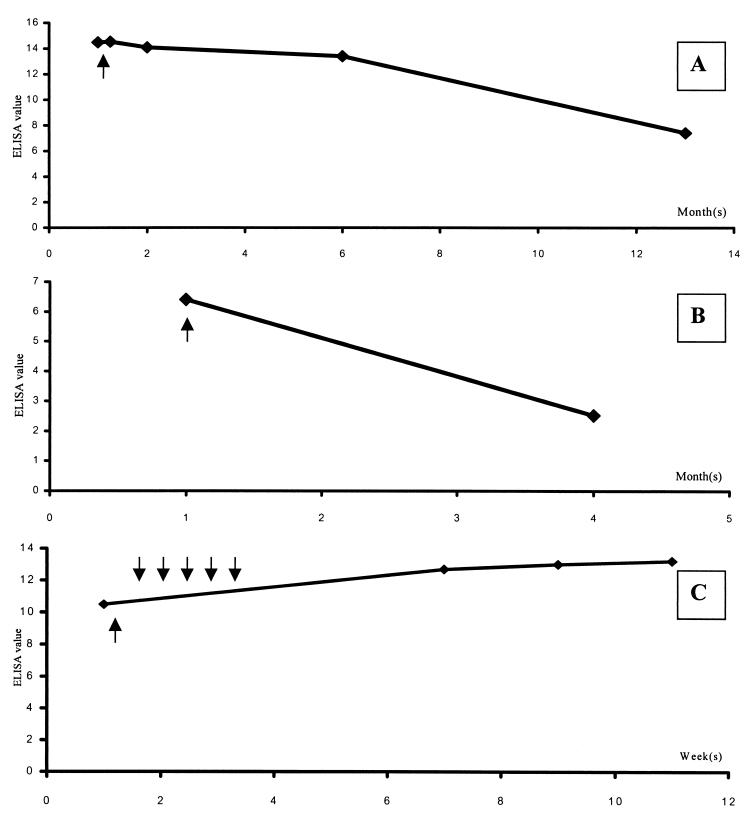
By using the ELISA for monitoring the clinical course of human pythiosis, serial serum samples from three systemic cases (S1 to S3) were tested (Fig. 2). Both the S1 (Fig. 2A) and the S2 (Fig. 2B) cases gave gradually decreasing EVs associated with clinical improvement after surgical treatment by adequate amputation. The patients recovered and were discharged from the hospital. No clinical recurrence was observed in follow-ups at ca. 1 year for S1 and 3 months for S2. Immunotherapy with a vaccine prepared from hyphae and exoantigen of Pythium sp. (17) was given to the S3 case because of inadequate amputation surgery. After vaccination, the EV gradually increased, but the clinical condition improved (Fig. 2C), and no symptoms of disease recurrence were detected during the period of follow-up.
FIG. 2.
EVs of the S1 (A), S2 (B), and S3 (C) systemic case serial serum samples. Arrows under the lines indicate the time of amputation. The arrows above the line in panel C represent the five vaccination doses administered over a 2-week interval.
For evaluation of the ID test, only one culture-proven pythiosis serum was positive by ID, whereas all of the control sera were found to be negative. Therefore, the ID test gave sensitivity, specificity, positive predictive, and negative predictive values of 14, 100, 100, and 96%, respectively.
DISCUSSION
Application of serodiagnostic tests such as ID and ELISA can facilitate the diagnosis of human pythiosis. The present study confirmed that ID was highly specific but had poor sensitivity (4, 8, 10, 12), whereas the ELISA had both high sensitivity and specificity as shown by another study (8). The ELISA test is a convenient method for the diagnosis of pythiosis, especially in suspected systemic or vascular cases, because only patient serum is required and it is not difficult to obtain vascular specimens. The chosen-I cutoff point was used in order to eliminate (i.e., the mean plus several standard deviations) false-positive results that naturally occur in the control population (Table 1). Using this cutoff point, both sensitivity and specificity were maximized to 100%. The high EVs of pythiosis cases resulted in a high sensitivity of the ELISA. While the cutoff was set at EV > 2.0, systemic cases had EVs of >5.0. The only exception was the ophthalmic pythiosis case, for which there was a lower antibody level due to the ophthalmic tissue being a poor site for immunological induction (5). Likewise, the single subcutaneous pythiosis case had a human immunodeficiency virus load of 418,000 copies/ml and a CD4 count of 52 cells/μl and was immunocompromised. Nevertheless, both of these cases were successfully diagnosed by the ELISA test, indicating its good sensitivity.
P. insidiosum and other genera of the class Zygomycetes that are human pathogens have similar morphology, including right angle branching and broad nonseptate hyphae. Moreover, the infected victims occasionally present with similar clinical manifestations (13). As a result, diagnosis by morphology for these organisms is very difficult. The high specificity of the ELISA was shown by the absence of false positives when sera from zygomycotic cases (Basidiobolus ranarum and Conidiobolus coronatus) were tested, even though Western blot analysis showed a weak immuno-cross-reactive band for the 44-kDa antigen of P. insidiosum with C. coronatus-infected sera (8, 11). To further evaluate test specificity, sera from healthy thalassemic subjects and subjects with other infections were used as controls, and all were negative. One of the latter was a patient suggested by histopathological analysis to be suffering from mucormycosis (i.e., a disease caused by members of the zygomycete group). The patient had diabetes but neither thalassemia nor a history of farming exposure. Both of our serodiagnostic tests gave negative results. We therefore concluded that this case was not pythiosis, based on our highly specific and sensitive serodiagnostic tests.
To use the ELISA for monitoring purposes, the OD values had to be normalized and defined as EVs to minimize the OD fluctuations between batch tests. The EVs of the positive controls from different batches were almost the same, suggesting good reproducibility of the test. Concerning the three systemic cases for which ELISA was used as a monitoring tool, we found that two cases gave a decrease in EV associated with clinical improvement (Fig. 2A and B). The EVs dropped within 1 month after effective leg amputation. Another patient who received a Pythium vaccine gave gradually increasing EVs during 2 months of follow-up (Fig. 2C), although the clinical symptoms improved. This phenomenon, which was paradoxical compared to the first two cases, was probably due to a rising host humoral immune response to the vaccine that seemed to induce protective immunity. However, the exact mechanism of this apparent vaccine-induced host immunity requires further investigation. For such studies, our ELISA test could be used as a monitoring tool combined with clinical observation.
Thalassemic patients and farmers are predisposed to pythiosis. According to our data in Thailand (unpublished), ca. 90% of the systemic human cases had hemoglobinopathy and especially thalassemia. Most of these individuals were farmers. Since Thailand is an area where P. insidiosum is endemic and also a country with a high prevalence of thalassemia, seroprevalence ELISA screening method for pythiosis would be a useful tool for gathering epidemiological data on pythiosis.
Acknowledgments
This work was supported by a Ramathibodi Foundation grant from the Faculty of Medicine, Ramathibodi Hospital, Mahidol University.
We are grateful to Timothy William Flegel for reviewing the manuscript, Boonmee Sathapatayavongs for sharing data on human pythiosis and providing patient sera, and Pimpan Tadthong for the blood donor control sera. We also thank Malai Vorachit, Kalayanee Atamasirikul, Chavachol Setthaudom, Kanchana Sriwanichrak, Sureerut Pitinunt, and Kanong Angkananukul for helpful suggestions and material support.
REFERENCES
- 1.De Cock, A. W. A. M., L. Mendoza, A. A. Padhye, L. Ajello, and L. Kaufman. 1987. Pythium insidiosum sp. nov., the etiologic agent of pythiosis. J. Clin. Microbiol. 25:344-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guarro, J., J. Gene, and A. M. Stchigel. 1999. Developments in fungal taxonomy. Clin. Microbiol. Rev. 12:454-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Imwidthaya, P. 1994. Human pythiosis in Thailand. Postgrad. Med. J. 70:558-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imwidthaya, P., and S. Srimuang. 1989. Immunodiffusion test for diagnosis human pythiosis. Mycopathologia 106:109-112. [DOI] [PubMed] [Google Scholar]
- 5.Janeway, C. A., P. Travers, M. Walport, and J. D. Capra. 1999. Antigens in immunologically privileged sites do not induce immune attack but serve as targets, p. 525-527. In C. A. Janeway, P. Travers, M. Walport, and J. D. Capra (ed.), Immunobiology: the immune system in health and disease, 4th ed. Garland Publishing, New York, N.Y.
- 6.Kaufman, L. 1998. Penicilliosis marneffei and pythiosis: emerging tropical disease. Mycopathologia 143:3-7. [DOI] [PubMed] [Google Scholar]
- 7.Mendoza, L., F. Hernandez, and L. Ajello. 1993. Life cycle of the human and animal oomycete pathogen Pythium insidiosum. J. Clin. Microbiol. 31:2967-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mendoza, L., L. Kaufman, W. Mandy, and R. Glass. 1997. Serodiagnosis of human and animal pythiosis using an enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 4:715-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mendoza, L., L. Kaufman, and P. Standard. 1987. Antigenic relationship between the animal and human pathogen Pythium insidiosum and nonpathogenic Pythium species. J. Clin. Microbiol. 25:2159-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mendoza, L., L. Kaufman, and P. G. Standard. 1986. Immunodiffusion test for diagnosis and monitoring pythiosis in horses. J. Clin. Microbiol. 23:813-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendoza, L., V. Nicholson, and J. F. Prescott. 1992. Immunoblot analysis of the humoral immune response to Pythium insidiosum in horses with pythiosis. J. Clin. Microbiol. 30:2980-2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pracharktam, R., P. Chongtrakool, B. Sathapatayavongs, P. Jayanetra, and L. Ajello. 1991. Immunodiffusion test for diagnosis and monitoring of human pythiosis insidiosi. J. Clin. Microbiol. 29:2661-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Richardson, M. D., and G. S. Shankland. 1995. Rhizopus, rhizomucor, absidia, and other agents of systemic and subcutaneous zygomycetes, p. 809-824. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 6th ed. American Society for Microbiology, Washington, D.C.
- 14.Sathapatayavongs, B., P. Leelachaikul, R. Pracharktam, V. Atichartrkarn, S. Sriphojanart, P. Trairatvorakul, S. Jirasiritham, S. Nontasut, C. Eyrvilaichit, and T. Flegel. 1989. Human pythiosis associated with thalassemia hemoglobinopathy syndrome. J. Infect. Dis. 159:274-280. [DOI] [PubMed] [Google Scholar]
- 15.Tanphaichitra, D. 1989. Tropical disease in the immunocompromised host. Rev. Infect. Dis. 11(Suppl. 7):S1629-S1643. [DOI] [PubMed] [Google Scholar]
- 16.Thianprasit, M., A. Chaiprasert, and P. Imwidthaya. 1996. Human pythiosis. Curr. Trop. Med. Mycol. 7:43-54. [PubMed] [Google Scholar]
- 17.Thitithanyanont, A., L. Mendoza, A. Chuansumrit, R. Pracharktam, J. Laothamatas, B. Sathapatayavongs, S. Lolekha, and L. Ajello. 1998. Use of immunotherapeutic vaccine to treat a life-threatening human arteritic infection caused by Pythium insidiosum. Clin. Infect. Dis. 27:1394-1400. [DOI] [PubMed]
- 18.Wanachiwanawin, W., M. Thianprasit, S. Fucharoen, A. Chaiprasert, N. Sudasna, N. Ayudhya, N. Sirithanaratkul, and A. Piankijagum. 1993. Fatal arteritis due to Pythium insidiosum infection in patients with thalassemia. Trans. Roy. Soc. Trop. Med. Hyg. 87:296-298. [DOI] [PubMed] [Google Scholar]