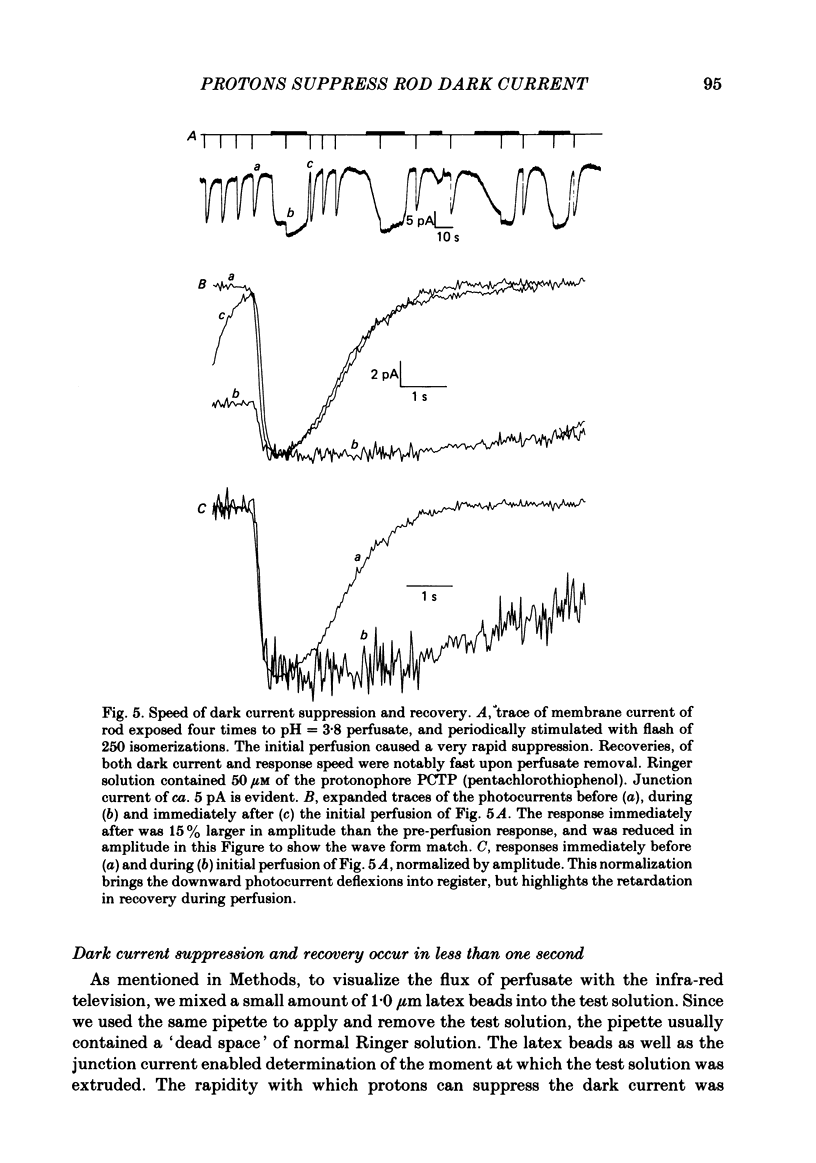
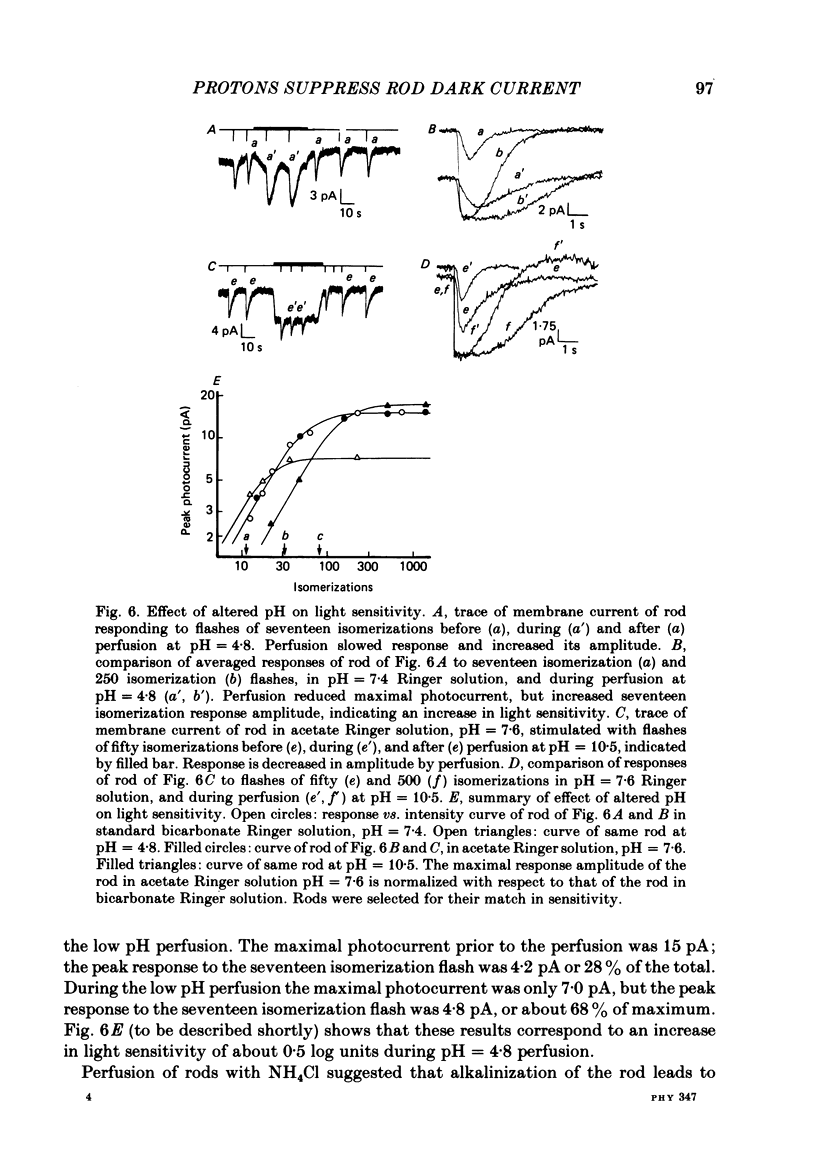
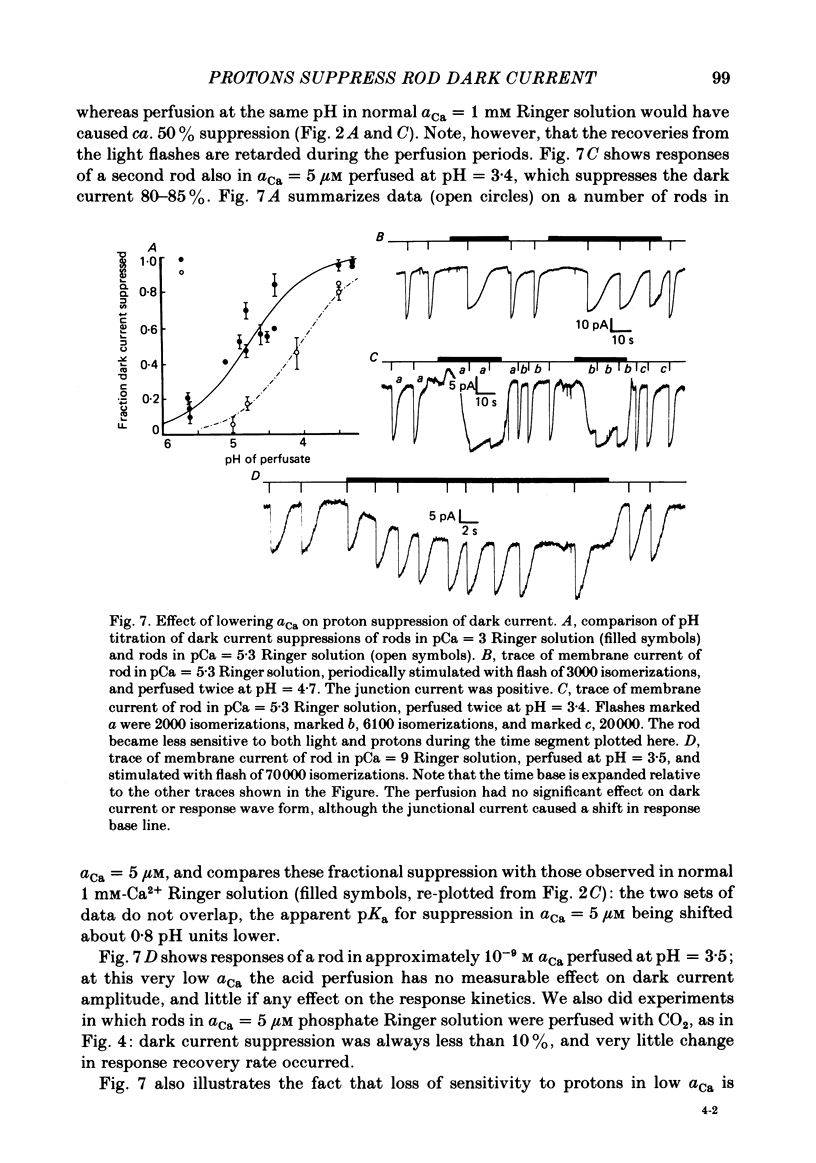
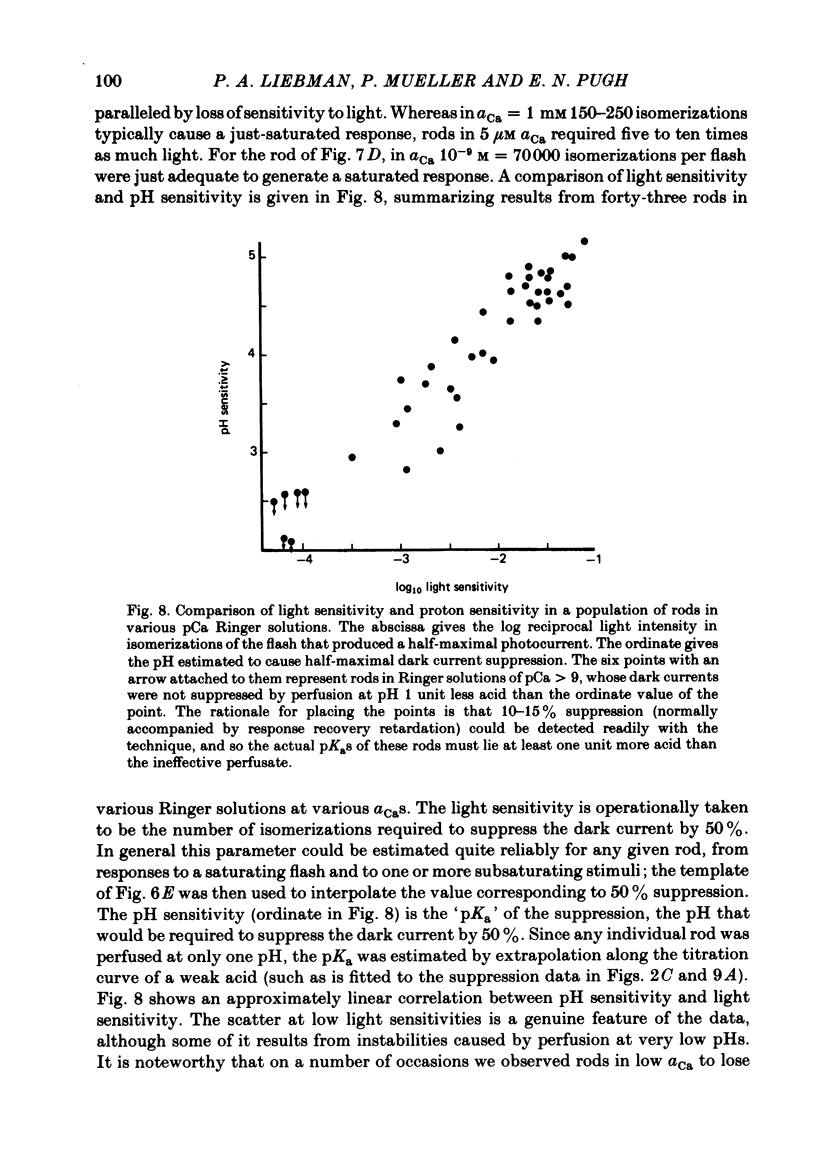
Abstract
Outer segments of rod photoreceptors with the attached ellipsoid region of the inner segment were isolated from Rana pipiens retinae, and their membrane photocurrents measured with the suction electrode technique in the 'ellipsoid-in' configuration. Under dark adapted conditions in standard Ringer solution, isolated rod outer segments with ellipsoids exhibited maximal photocurrents of 10-30 pA, and light sensitivities of 0.2-1.0 pA/isomerization. A local perfusion technique was employed to change rapidly the solution bathing the outer segment. Rods were tested for their sensitivity to protons by perfusion with Ringer solution of altered pH. The dark current was reversibly suppressed by low pH: in Ringer solution with Calcium activity aCa = 10(-3)M dark current suppression obeyed a hyperbolic saturation law with apparent dissociation constant, pKa = 4.8. The decay of dark current of rods following poisoning with ouabain was retarded by low pH perfusion, as it was by light. Protons thus act to suppress the outer segment Na+ conductance. Three experiments support the hypothesis that protons act interior to the plasma membrane in suppressing the dark current. (1) Perfusion of rods at constant pH with Ringer solution having increased CO2 suppressed the dark current. (2) Removal of perfusate containing 50 mM-NH4Cl causes transient dark current suppression. (3) Acetate, which acts as a neutral proton carrier, when added to Ringer solution, shifts the apparent pKa of dark current suppression to a higher pH. Dark current suppression by protons and recovery occurred with a time constant of ca. 1 s. Low pH perfusion retarded the recovery of the dark current from a saturating flash, slowed the light response in its linear range, and increased light sensitivity. Perfusion at pH = 10.5 caused a slight increase in dark current, sped up the recovery of the rod from a saturating flash, accelerated the linear response and decreased the light sensitivity. Lowering aCa of the Ringer solution caused the proton sensitivity of the dark current to drop. At aCa = 5 X 10(-6) M the apparent pKa of dark current suppression was shifted about 0.8 pH units to pH = 4.0. Cells at aCa = 10(-9) M were insensitive to pH = 3.5, which completely suppressed the dark current at normal aCa. Lowered aCa decreased light sensitivity. Both proton sensitivity and light sensitivity of dark current suppression were estimated for each member of a population of rods in various aCaS:proton sensitivity and light sensitivity were found to be linearly correlated over a range of 3 log units.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF
















Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bader C. R., Macleish P. R., Schwartz E. A. A voltage-clamp study of the light response in solitary rods of the tiger salamander. J Physiol. 1979 Nov;296:1–26. doi: 10.1113/jphysiol.1979.sp012988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian B. L., Fain G. L. The effects of low calcium and background light on the sensitivity of toad rods. J Physiol. 1982 Sep;330:307–329. doi: 10.1113/jphysiol.1982.sp014343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D. Local effects of bleaching in retinal rods of the toad. J Physiol. 1982 Jul;328:49–71. doi: 10.1113/jphysiol.1982.sp014252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. Responses of retinal rods to single photons. J Physiol. 1979 Mar;288:613–634. [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. The membrane current of single rod outer segments. J Physiol. 1979 Mar;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Matthews G., Yau K. W. Two components of electrical dark noise in toad retinal rod outer segments. J Physiol. 1980 Dec;309:591–621. doi: 10.1113/jphysiol.1980.sp013529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron W. F., De Weer P. Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. J Gen Physiol. 1976 Jan;67(1):91–112. doi: 10.1085/jgp.67.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Coles J. A., Pinto L. H. Effects of injections of calcium and EGTA into the outer segments of retinal rods of Bufo marinus. J Physiol. 1977 Aug;269(3):707–722. doi: 10.1113/jphysiol.1977.sp011924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caretta A., Cavaggioni A. Fast ionic flux activated by cyclic GMP in the membrane of cattle rod outer segments. Eur J Biochem. 1983 Apr 15;132(1):1–8. doi: 10.1111/j.1432-1033.1983.tb07317.x. [DOI] [PubMed] [Google Scholar]
- Fleischman D., Denisevich M. Guanylate cyclase of isolated bovine retinal rod axonemes. Biochemistry. 1979 Nov 13;18(23):5060–5066. doi: 10.1021/bi00590a006. [DOI] [PubMed] [Google Scholar]
- Fung B. K., Hurley J. B., Stryer L. Flow of information in the light-triggered cyclic nucleotide cascade of vision. Proc Natl Acad Sci U S A. 1981 Jan;78(1):152–156. doi: 10.1073/pnas.78.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gedney C., Ostroy S. E. Hydrogen ion effects of the vertebrate photoreceptor. The pK's of ionizable groups affecting cell permeability. Arch Biochem Biophys. 1978 May;188(1):105–113. doi: 10.1016/0003-9861(78)90362-4. [DOI] [PubMed] [Google Scholar]
- George J. S., Hagins W. A. Control of Ca2+ in rod outer segment disks by light and cyclic GMP. Nature. 1983 May 26;303(5915):344–348. doi: 10.1038/303344a0. [DOI] [PubMed] [Google Scholar]
- Gold G. H., Korenbrot J. I. Light-induced calcium release by intact retinal rods. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5557–5561. doi: 10.1073/pnas.77.9.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger J. L., Winkler M. M., Shen S. S., Steinhardt R. A. Intracellular pH controls protein synthesis rate in the sea urchine egg and early embryo. Dev Biol. 1979 Feb;68(2):396–406. doi: 10.1016/0012-1606(79)90213-6. [DOI] [PubMed] [Google Scholar]
- Hagins W. A., Penn R. D., Yoshikami S. Dark current and photocurrent in retinal rods. Biophys J. 1970 May;10(5):380–412. doi: 10.1016/S0006-3495(70)86308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagins W. A., Yoshikami S. Proceedings: A role for Ca2+ in excitation of retinal rods and cones. Exp Eye Res. 1974 Mar;18(3):299–305. doi: 10.1016/0014-4835(74)90157-2. [DOI] [PubMed] [Google Scholar]
- Kilbride P., Ebrey T. G. Light-initiated changes of cyclic guanosine monophosphate levels in the frog retina measured with quick-freezing techniques. J Gen Physiol. 1979 Sep;74(3):415–426. doi: 10.1085/jgp.74.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok-Keung Fung B., Stryer L. Photolyzed rhodopsin catalyzes the exchange of GTP for bound GDP in retinal rod outer segments. Proc Natl Acad Sci U S A. 1980 May;77(5):2500–2504. doi: 10.1073/pnas.77.5.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman P. A., Evanczuk A. T. Real time assay of rod disk membrane cGMP phosphodiesterase and its controller enzymes. Methods Enzymol. 1982;81:532–542. doi: 10.1016/s0076-6879(82)81074-4. [DOI] [PubMed] [Google Scholar]
- Liebman P. A., Pugh E. N., Jr ATP mediates rapid reversal of cyclic GMP phosphodiesterase activation in visual receptor membranes. Nature. 1980 Oct 23;287(5784):734–736. doi: 10.1038/287734a0. [DOI] [PubMed] [Google Scholar]
- Miller W. H. Physiological evidence that light-mediated decrease in cyclic GMP is an intermediary process in retinal rod transduction. J Gen Physiol. 1982 Jul;80(1):103–123. doi: 10.1085/jgp.80.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller P., Pugh E. N., Jr Protons block the dark current of isolated retinal rods. Proc Natl Acad Sci U S A. 1983 Apr;80(7):1892–1896. doi: 10.1073/pnas.80.7.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol G. D., Miller W. H. Cyclic GMP injected into retinal rod outer segments increases latency and amplitude of response to illumination. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5217–5220. doi: 10.1073/pnas.75.10.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannbacker R. G. Control of guanylate cyclase activity in the rod outer segment. Science. 1973 Dec 14;182(4117):1138–1140. doi: 10.1126/science.182.4117.1138. [DOI] [PubMed] [Google Scholar]
- Penn R. D., Hagins W. A. Kinetics of the photocurrent of retinal rods. Biophys J. 1972 Aug;12(8):1073–1094. doi: 10.1016/S0006-3495(72)86145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto L. H., Ostroy S. E. Ionizable groups and conductances of the rod photoreceptor membrane. J Gen Physiol. 1978 Mar;71(3):329–345. doi: 10.1085/jgp.71.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racker E., Miyamoto H., Mogerman J., Simons J., O'Neal S. Cation transport in reconstituted systems. Ann N Y Acad Sci. 1980;358:64–72. doi: 10.1111/j.1749-6632.1980.tb15386.x. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. The effect of carbon dioxide on the intracellular pH and buffering power of snail neurones. J Physiol. 1976 Mar;255(3):715–735. doi: 10.1113/jphysiol.1976.sp011305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormington C. M., Cone R. A. Ionic blockage of the light-regulated sodium channels in isolated rod outer segments. J Gen Physiol. 1978 Jun;71(6):657–681. doi: 10.1085/jgp.71.6.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau K. W., McNaughton P. A., Hodgkin A. L. Effect of ions on the light-sensitive current in retinal rods. Nature. 1981 Aug 6;292(5823):502–505. doi: 10.1038/292502a0. [DOI] [PubMed] [Google Scholar]
- Yee R., Liebman P. A. Light-activated phosphodiesterase of the rod outer segment. Kinetics and parameters of activation and deactivation. J Biol Chem. 1978 Dec 25;253(24):8902–8909. [PubMed] [Google Scholar]
- Yoshikami S., George J. S., Hagins W. A. Light-induced calcium fluxes from outer segment layer of vertebrate retinas. Nature. 1980 Jul 24;286(5771):395–398. doi: 10.1038/286395a0. [DOI] [PubMed] [Google Scholar]


