Abstract
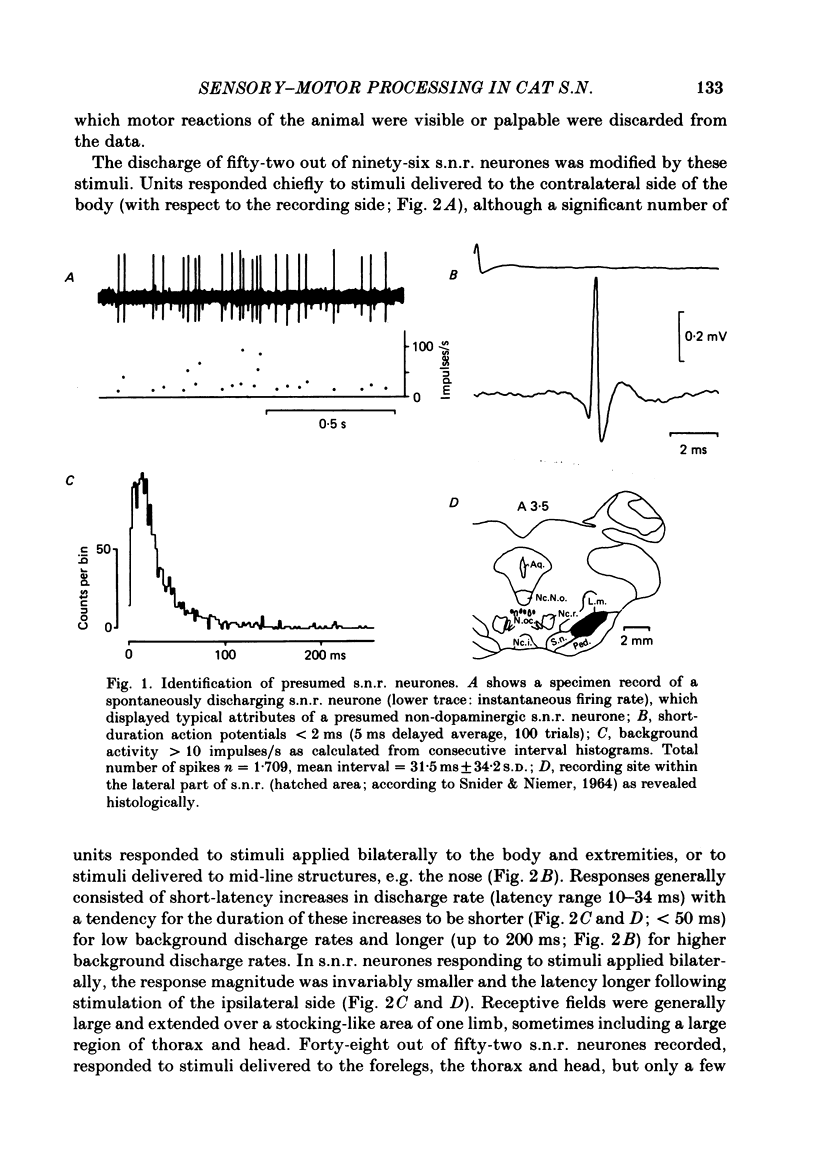
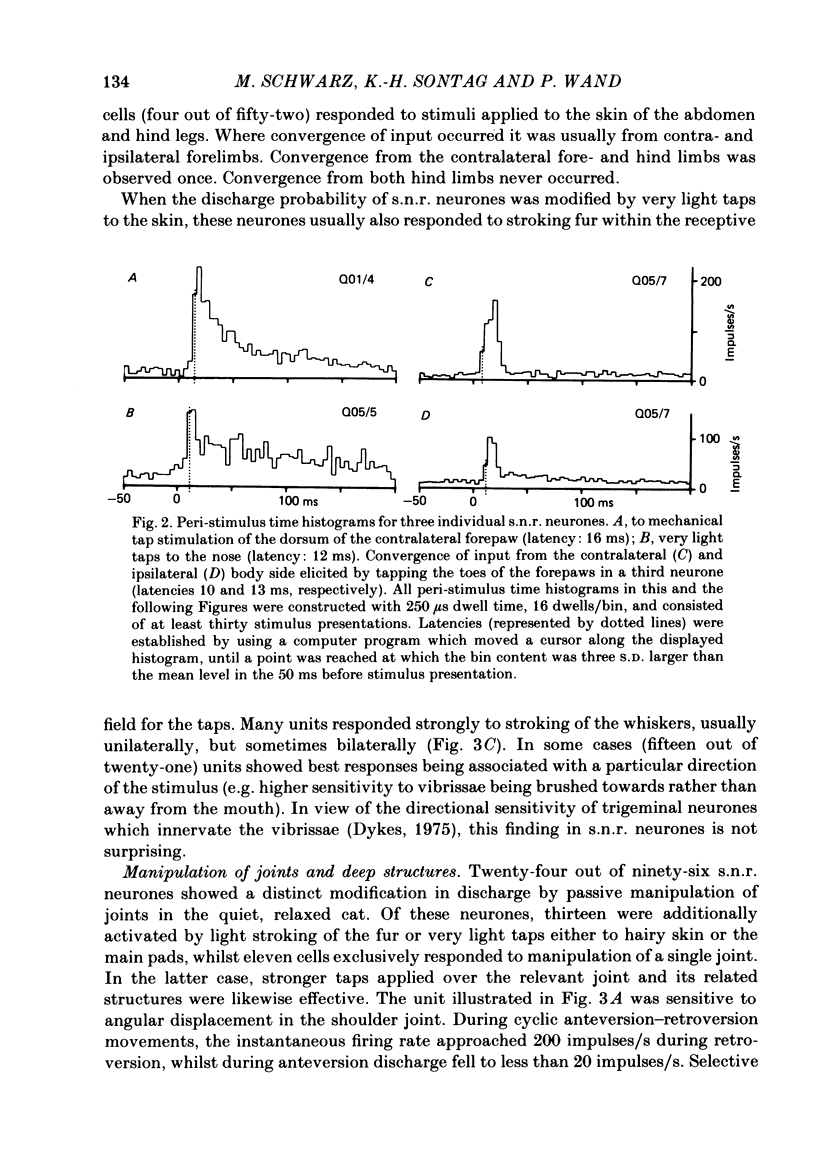
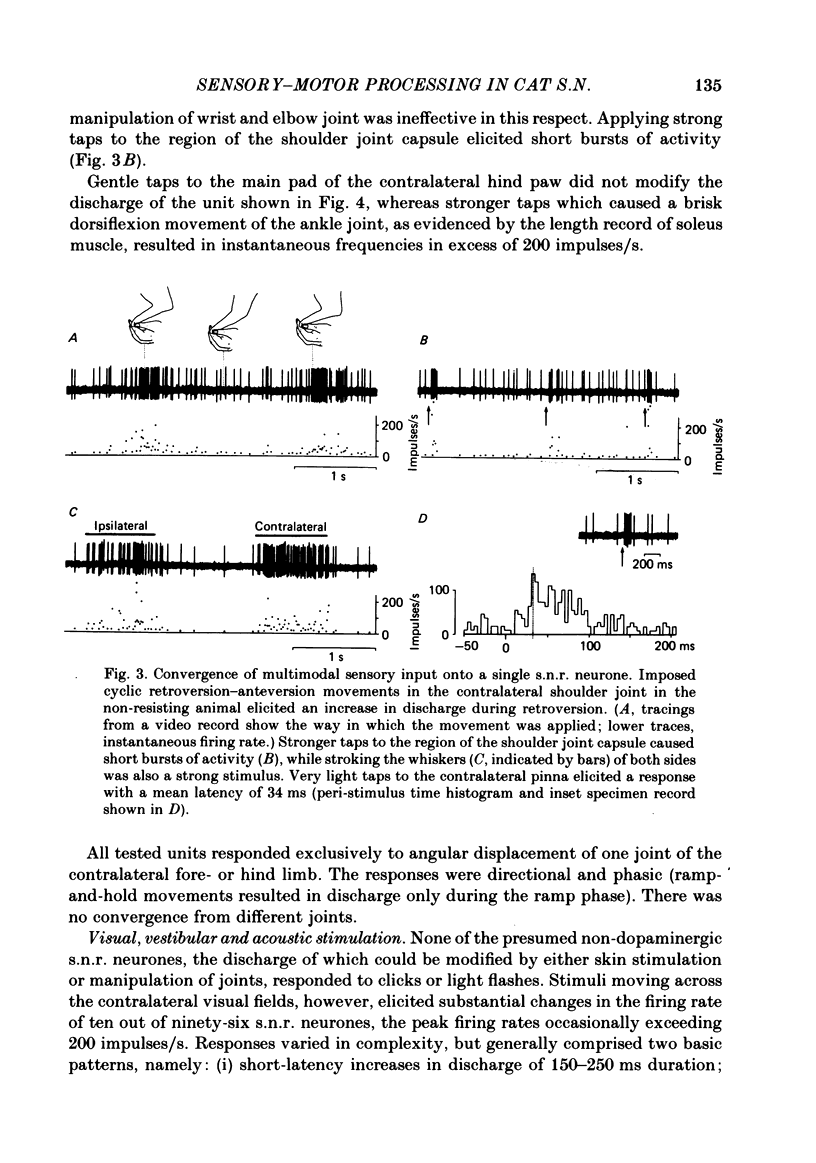
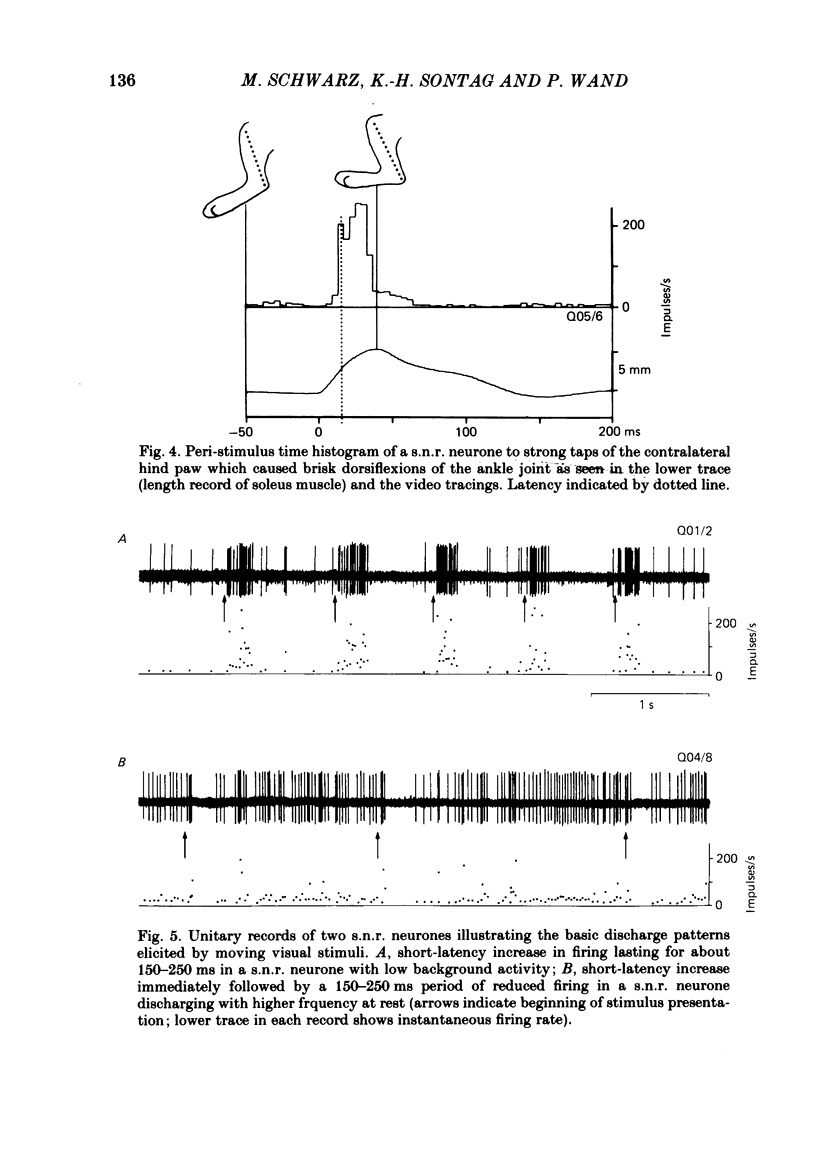
Extracellular recordings were made with chronically implanted micro-electrodes from 109 substantia nigra neurones in conscious cats. Ninety-six of 109 neurones met the criteria of presumed non-dopaminergic pars reticulata (s.n.r.) neurones. Background discharge, in animals in a state of relaxed wakefulness and in the absence of overt movements, was in the range of 11-37 impulses/s, mean 19.2 impulses/s. The discharges of fifty-two of ninety-six neurones tested were modified by innocuous mechanical skin stimulation. Neurones responded chiefly to stimuli delivered to the contralateral body side. Responses generally comprised net excitation and occurred with short latency (range 10-34 ms; mean 17.3 ms). Convergence from both forelimbs or the contralateral fore- and hind limbs was evident in a few cases. One-fourth (twenty-four out of ninety-six) of the s.n.r. neurones tested were sensitive to passive manipulation of limb joints in the quiet, conscious cat and responded exclusively to angular displacement of one contralateral joint. Responses were directional and phasic. None of the s.n.r. neurones tested responded to clicks and/or light flashes. However, stimuli moving across the contralateral visual field substantially modified the discharge rate of ten out of ninety-six s.n.r. neurones. Responses were directional and invariably associated with eye movements. Animals were also trained to walk on a treadmill and to perform certain self-generated limb movements. S.n.r. neurones with a receptive field on a limb regularly showed modulations in discharge during locomotion, phase-related to the step cycle, and also short-latency responses during disturbance of such movements. Ten out of ninety-six s.n.r. neurones discharged almost exclusively prior to and during self-generated movements of a single limb. Their most powerful modulations in firing rate occurred, whenever an animal tried to overcome an external impediment or to resist an imposed movement. These observations on s.n.r. neurones, taken together with previous findings on nigral influences on spinal motor circuitry, indicate that the s.n.r. represents an output station of the basal ganglia which is involved in the subconscious processing of convergent multimodal sensory information and which participates in setting appropriate gains and biasses of spinal motor neuronal systems to adequately deal with changing motor requirements.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALBE-FESSARD D., ROCHA-MIRANDA C., OSWALDO-CRUZ E. [Activity evoked in the caudate nucleus of the cat in response to various types of afferent stimulation. II. Microphysiological study]. Electroencephalogr Clin Neurophysiol. 1960 Aug;12:649–661. doi: 10.1016/0013-4694(60)90108-5. [DOI] [PubMed] [Google Scholar]
- Afifi A. K., Bahuth N., Jabbur S. J. The nigrotectal tract. An experimental study of its site of origin. Acta Anat (Basel) 1970;77(1):67–77. doi: 10.1159/000143529. [DOI] [PubMed] [Google Scholar]
- Barasi S. Responses of substantia nigra neurones to noxious stimulation. Brain Res. 1979 Jul 27;171(1):121–130. doi: 10.1016/0006-8993(79)90737-6. [DOI] [PubMed] [Google Scholar]
- Bunney B. S., Walters J. R., Roth R. H., Aghajanian G. K. Dopaminergic neurons: effect of antipsychotic drugs and amphetamine on single cell activity. J Pharmacol Exp Ther. 1973 Jun;185(3):560–571. [PubMed] [Google Scholar]
- Burgess P. R., Clark F. J. Dorsal column projection of fibres from the cat knee joint. J Physiol. 1969 Aug;203(2):301–315. doi: 10.1113/jphysiol.1969.sp008865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodo L. A., Antelman S. M., Caggiula A. R., Lineberry C. G. Sensory stimuli alter the discharge rate of dopamine (DA) neurons: evidence for two functional types of DA cells in the substantia nigra. Brain Res. 1980 May 12;189(2):544–549. doi: 10.1016/0006-8993(80)90366-2. [DOI] [PubMed] [Google Scholar]
- Chorover S. L., DeLuca A. M. A sweet new multiple electrode for chronic single unit recording in moving animals. Physiol Behav. 1972 Oct;9(4):671–674. doi: 10.1016/0031-9384(72)90030-3. [DOI] [PubMed] [Google Scholar]
- Cools A. R., Jaspers R., Kolasiewicz W., Sontag K. H., Wolfarth S. Substantia nigra as a station that not only transmits, but also transforms, incoming signals for its behavioural expression: striatal dopamine and GABA-mediated responses of pars reticulata neurons. Behav Brain Res. 1983 Jan;7(1):39–49. doi: 10.1016/0166-4328(83)90003-7. [DOI] [PubMed] [Google Scholar]
- Danner H., Pfister C. Sieben Neurontypen in der Substantia nigra der Ratte. Eine Golgi-rapid-Imprägnationsstudie. J Hirnforsch. 1982;23(5):553–556. [PubMed] [Google Scholar]
- Denny-Brown D., Yanagisawa N. The role of the basal ganglia in the initiation of movement. Res Publ Assoc Res Nerv Ment Dis. 1976;55:115–149. [PubMed] [Google Scholar]
- DiChiara G., Olianas M., Del Fiacco M., Spano P. F., Tagliamonte A. Intranigral kainic acid is evidence that nigral non-dopaminergic neurones control posture. Nature. 1977 Aug 25;268(5622):743–745. doi: 10.1038/268743a0. [DOI] [PubMed] [Google Scholar]
- Dilly P. N., Wall P. D., Webster K. E. Cells of origin of the spinothalamic tract in the cat and rat. Exp Neurol. 1968 Aug;21(4):550–562. doi: 10.1016/0014-4886(68)90072-1. [DOI] [PubMed] [Google Scholar]
- Dray A. The physiology and pharmacology of mammalian basal ganglia. Prog Neurobiol. 1980;14(4):221–335. doi: 10.1016/0301-0082(80)90017-9. [DOI] [PubMed] [Google Scholar]
- Dykes R. W. Afferent fibers from mystacial vibrissae of cats and seals. J Neurophysiol. 1975 May;38(3):650–662. doi: 10.1152/jn.1975.38.3.650. [DOI] [PubMed] [Google Scholar]
- Féger J., Jacquemin J., Ohye C. Peripheral excitatory input to substantia nigra. Exp Neurol. 1978 May 1;59(3):351–360. doi: 10.1016/0014-4886(78)90227-3. [DOI] [PubMed] [Google Scholar]
- Garcia-Munoz M., Nicolaou M. N., Tulloch I. F., Wright A. K., Arguthnott G. W. Feedback loop or output pathway in striato-nigral fibres? Nature. 1977 Jan 27;265(5592):363–365. doi: 10.1038/265363a0. [DOI] [PubMed] [Google Scholar]
- Goslow G. E., Jr, Reinking R. M., Stuart D. G. The cat step cycle: hind limb joint angles and muscle lengths during unrestrained locomotion. J Morphol. 1973 Sep;141(1):1–41. doi: 10.1002/jmor.1051410102. [DOI] [PubMed] [Google Scholar]
- Guyenet P. G., Aghajanian G. K. Antidromic identification of dopaminergic and other output neurons of the rat substantia nigra. Brain Res. 1978 Jul 7;150(1):69–84. doi: 10.1016/0006-8993(78)90654-6. [DOI] [PubMed] [Google Scholar]
- Hallett M., Khoshbin S. A physiological mechanism of bradykinesia. Brain. 1980 Jun;103(2):301–314. doi: 10.1093/brain/103.2.301. [DOI] [PubMed] [Google Scholar]
- Harper J. A., Labuszewski T., Lidsky T. I. Substantia nigra unit responses to trigeminal sensory stimulation. Exp Neurol. 1979 Aug;65(2):462–470. doi: 10.1016/0014-4886(79)90112-2. [DOI] [PubMed] [Google Scholar]
- Hommer D. W., Bunney B. S. Effect of sensory stimuli on the activity of dopaminergic neurons: involvement of non-dopaminergic nigral neurons and striato-nigral pathways. Life Sci. 1980 Aug 4;27(5):377–386. doi: 10.1016/0024-3205(80)90185-x. [DOI] [PubMed] [Google Scholar]
- Hultborn H., Lindström S., Wigström H. On the function of recurrent inhibition in the spinal cord. Exp Brain Res. 1979 Oct;37(2):399–403. doi: 10.1007/BF00237722. [DOI] [PubMed] [Google Scholar]
- Juraska J. M., Wilson C. J., Groves P. M. The substantia nigra of the rat: a Golgi study. J Comp Neurol. 1977 Apr 15;172(4):585–600. doi: 10.1002/cne.901720403. [DOI] [PubMed] [Google Scholar]
- Kilpatrick I. C., Starr M. S. Involvement of dopamine in circling responses to muscimol depends on intranigral site of injection. Eur J Pharmacol. 1981 Feb 19;69(4):407–419. doi: 10.1016/0014-2999(81)90444-1. [DOI] [PubMed] [Google Scholar]
- Kornhuber H. H. Motor functions of cerebellum and basal ganglia: the cerebellocortical saccadic (ballistic) clock, the cerebellonuclear hold regulator, and the basal ganglia ramp (voluntary speed smooth movement) generator. Kybernetik. 1971 Apr;8(4):157–162. doi: 10.1007/BF00290561. [DOI] [PubMed] [Google Scholar]
- Kultas-Ilinsky K., Ilinsky I. A., Massopust L. C., Young P. A., Smith K. R. Nigrothalamic pathway in the cat demonstrated by autoradiography and electron microscopy. Exp Brain Res. 1978 Nov 15;33(3-4):481–492. doi: 10.1007/BF00235569. [DOI] [PubMed] [Google Scholar]
- Maeda H., Mogenson G. J. Effects of peripheral stimulation on the activity of neurons in the ventral tegmental area, substantia nigra and midbrain reticular formation of rats. Brain Res Bull. 1982 Jan;8(1):7–14. doi: 10.1016/0361-9230(82)90021-1. [DOI] [PubMed] [Google Scholar]
- Nieoullon A., Cheramy A., Glowinski J. Nigral and striatal dopamine release under sensory stimuli. Nature. 1977 Sep 22;269(5626):340–342. doi: 10.1038/269340a0. [DOI] [PubMed] [Google Scholar]
- Prochazka A., Stephens J. A., Wand P. Muscle spindle discharge in normal and obstructed movements. J Physiol. 1979 Feb;287:57–66. doi: 10.1113/jphysiol.1979.sp012645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazka A., Wand P. Tendon organ discharge during voluntary movements in cats. J Physiol. 1980 Jun;303:385–390. doi: 10.1113/jphysiol.1980.sp013293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinvik E., Grofová I., Ottersen O. P. Demonstration of nigrotectal and nigroreticular projections in the cat by axonal transport of proteins. Brain Res. 1976 Aug 13;112(2):388–394. doi: 10.1016/0006-8993(76)90293-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STERN J., WARD A. A., Jr Supraspinal and drug modulation of the alpha motor system. Arch Neurol. 1962 May;6:404–413. doi: 10.1001/archneur.1962.00450230066008. [DOI] [PubMed] [Google Scholar]
- Steinfels G. F., Heym J., Jacobs B. L. Single unit activity of dopaminergic neurons in freely moving cuts. Life Sci. 1981 Oct 5;29(14):1435–1442. doi: 10.1016/0024-3205(81)90007-2. [DOI] [PubMed] [Google Scholar]
- Steinfels G. F., Heym J., Strecker R. E., Jacobs B. L. Behavioral correlates of dopaminergic unit activity in freely moving cats. Brain Res. 1983 Jan 10;258(2):217–228. doi: 10.1016/0006-8993(83)91145-9. [DOI] [PubMed] [Google Scholar]
- Trulson M. E., Preussler D. W., Howell G. A. Activity of substantia nigra units across the sleep-waking cycle in freely moving cats. Neurosci Lett. 1981 Oct 23;26(2):183–188. doi: 10.1016/0304-3940(81)90346-3. [DOI] [PubMed] [Google Scholar]
- Tsai C. T., Nakamura S., Iwama K. Inhibition of neuronal activity of the substantia nigra by noxious stimuli and its modification by the caudate nucleus. Brain Res. 1980 Aug 18;195(2):299–311. doi: 10.1016/0006-8993(80)90066-9. [DOI] [PubMed] [Google Scholar]
- Tulloch I. F., Arbuthnott G. W. Electrophysiological evidence for an input from the anterior olfactory nucleus to substantia nigra. Exp Neurol. 1979 Oct;66(1):16–29. doi: 10.1016/0014-4886(79)90059-1. [DOI] [PubMed] [Google Scholar]
- Usunoff K. G., Hassler R., Romansky K., Usunova P., Wagner A. The nigrostriatal projection in the cat. Part 1. Silver impregnation study. J Neurol Sci. 1976 Jul;28(3):265–288. doi: 10.1016/0022-510x(76)90021-6. [DOI] [PubMed] [Google Scholar]
- Wagner A., Kalmring K. The dynamic and static sensibility of the la afferents during electrical stimulation of the substantia nigra. Brain Res. 1968 Aug 26;10(2):277–280. doi: 10.1016/0006-8993(68)90136-4. [DOI] [PubMed] [Google Scholar]
- Wand P., Schwarz M., Kolasiewicz W., Sontag K. H. Nigral output neurons are engaged in regulation of static fusimotor action onto flexors in cat. Pflugers Arch. 1981 Sep;391(3):255–257. doi: 10.1007/BF00596180. [DOI] [PubMed] [Google Scholar]
- York D. H. Potentiation of lumbo-sacral monosynaptic reflexes by the substantia nigra. Exp Neurol. 1972 Sep;36(3):437–448. doi: 10.1016/0014-4886(72)90004-0. [DOI] [PubMed] [Google Scholar]
- Yoshida M. The GABAergic systems and the role of basal ganglia in motor control. Adv Biochem Psychopharmacol. 1981;30:37–52. [PubMed] [Google Scholar]


