Abstract
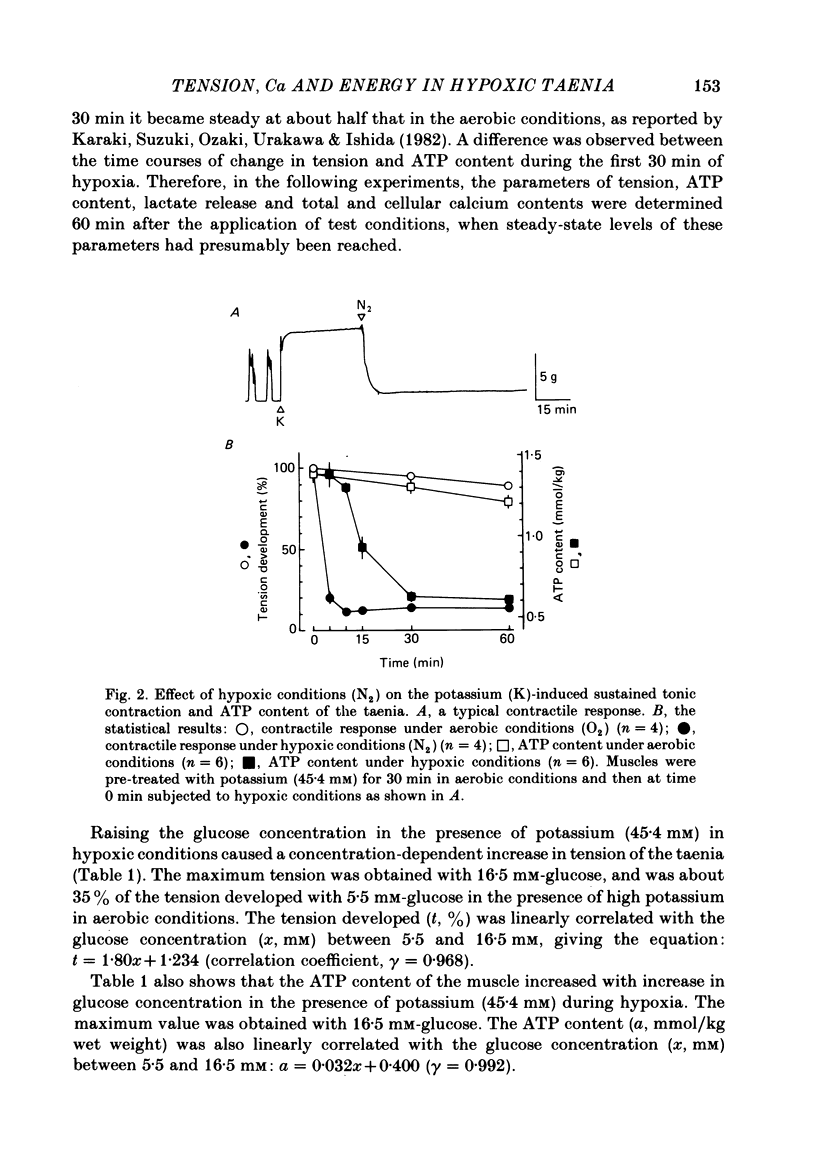
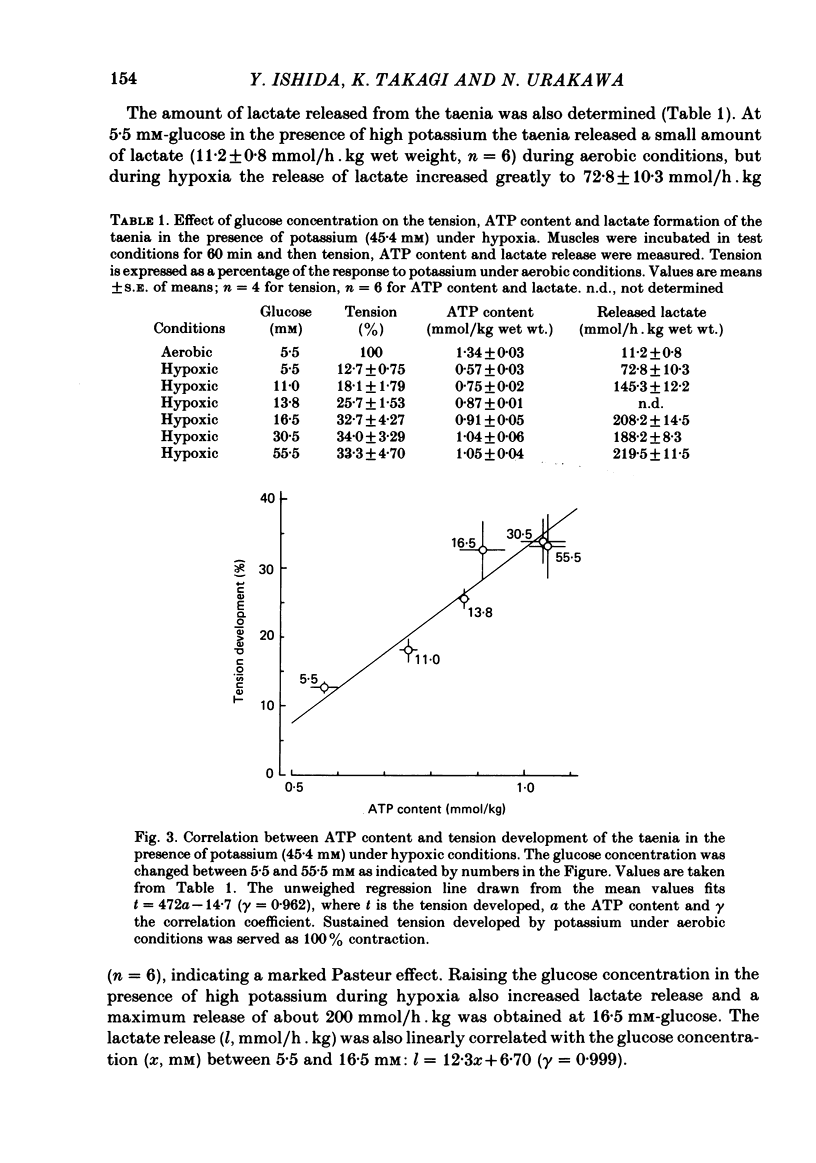
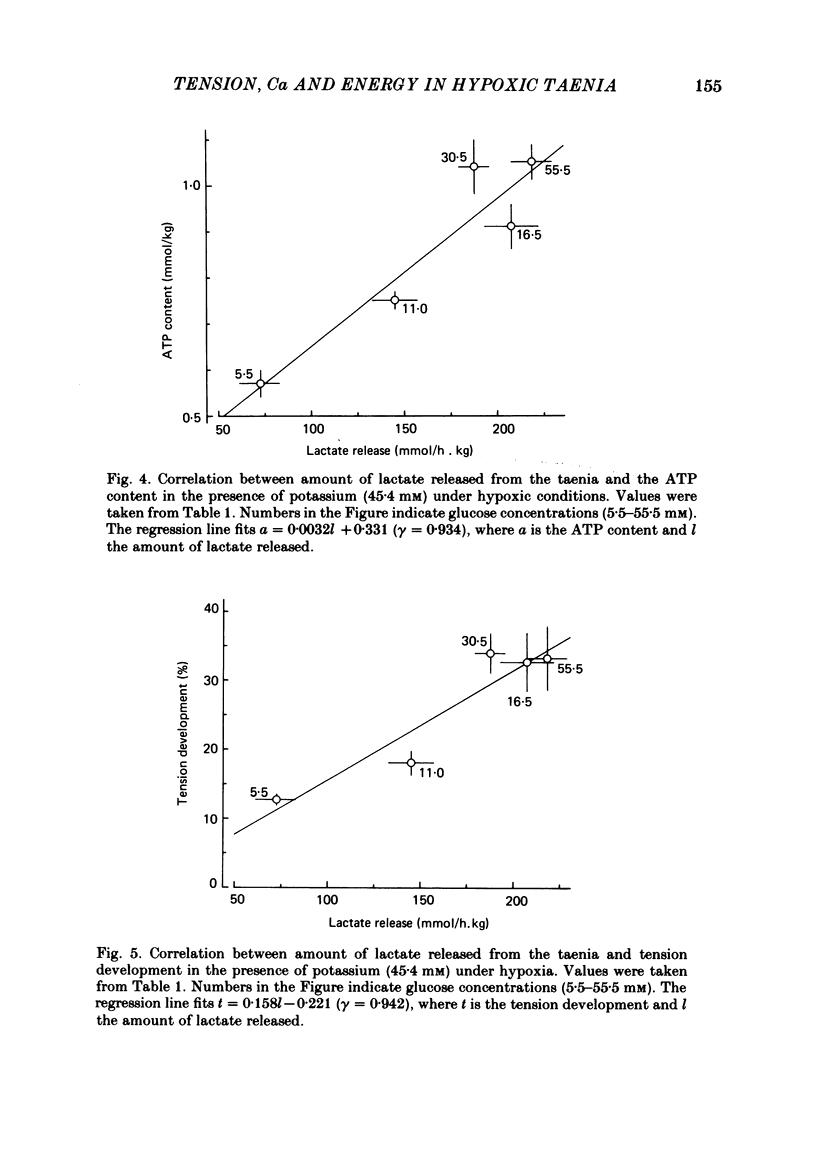
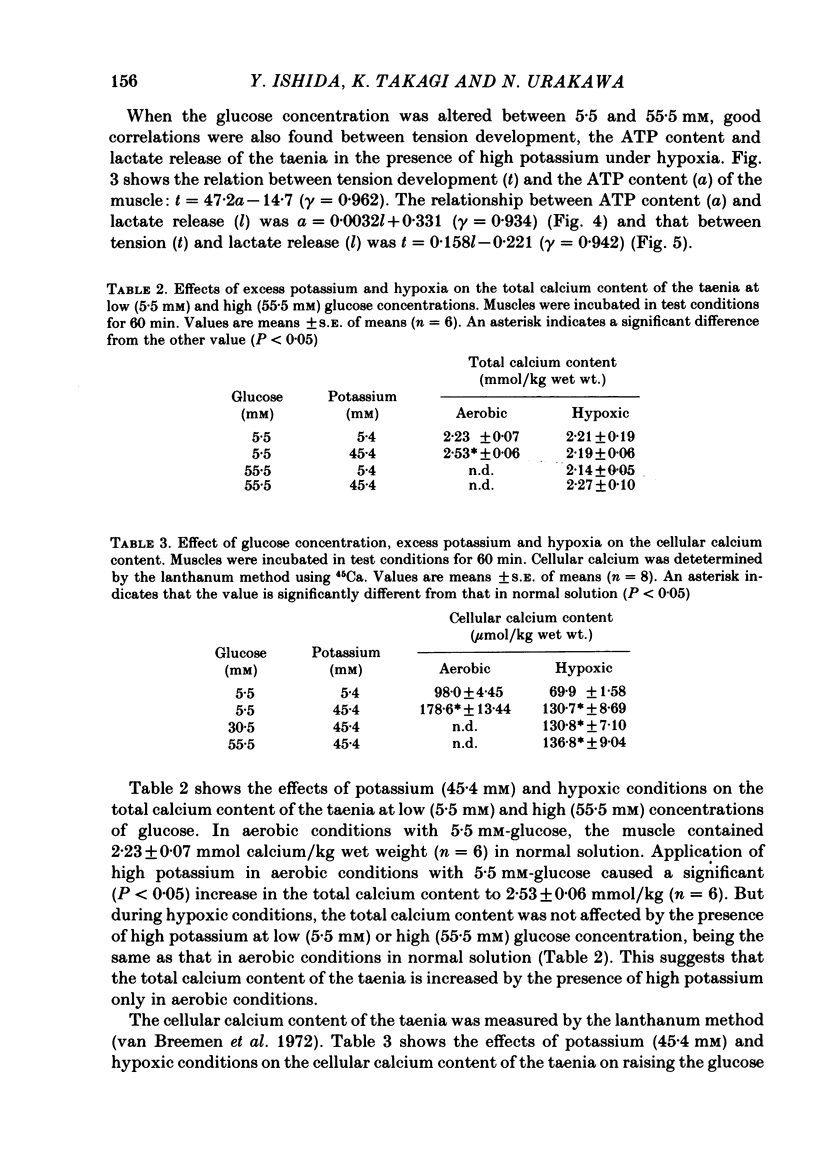
When potassium (45.4 mM) was applied to isolated taenia of guinea-pig caecum, the muscle developed a rapid phasic and sustained tonic tension during aerobic conditions bubbled with 95% O2:5% CO2. Under hypoxic conditions bubbled with 95% N2:5% CO2, the taenia lost its ability to respond to high potassium with sustained tonic contraction, although it still showed rapid phasic contraction. Raising the glucose concentration from 5.5 to 55.5 mM in the presence of high potassium during hypoxia caused development of a sustained contraction which was 50% that of the muscle in aerobic conditions. In the presence of high potassium, the ATP content of the taenia decreased in hypoxia, but increased with increasing glucose concentration. When the taenia was exposed to hypoxic conditions, the amount of lactate released from the muscle increased. Raising the glucose concentration caused a further increase in lactate release in the presence of high potassium under hypoxia. Good correlations (gamma greater than 0.9) were observed between tension development, the ATP content and lactate release of the taenia in the presence of high potassium under hypoxia when the glucose concentration was varied between 5.5 and 55.5 mM. The total calcium content was increased by the presence of high potassium under aerobic conditions and the increase was abolished when the muscle was exposed to hypoxic conditions. Under hypoxia the total calcium content was not increased by raising the glucose concentration in the presence of high potassium. The cellular calcium content of the taenia, determined by the lanthanum method, was increased in the presence of high potassium under aerobic and hypoxic conditions, but the content was smaller in hypoxic conditions than in aerobic conditions. Under hypoxic conditions, raising the glucose concentration in the presence of high potassium did not affect the cellular calcium content. These results suggest that under hypoxic conditions the potassium-induced sustained contraction of the taenia is increased by raising the glucose concentration owing to increased ATP production through the glycolytic pathway, but not through mechanisms increasing the intracellular Ca concentration.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAUER H., GOODFORD P. J., HUETER J. THE CALCIUM CONTENT AND 45-CALCIUM UPTAKE OF THE SMOOTH MUSCLE OF THE GUINEA-PIG TAENIA COLI. J Physiol. 1965 Jan;176:163–179. doi: 10.1113/jphysiol.1965.sp007542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORN G. V., BULBRING E. The effect of 2:4-dinitrophenol (DNP) on the smooth muscle of the guinea-pig's taenia coli. J Physiol. 1955 Mar 28;127(3):626–635. doi: 10.1113/jphysiol.1955.sp005283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULBRING E. Measurements of oxygen consumption in smooth muscle. J Physiol. 1953 Oct;122(1):111–134. doi: 10.1113/jphysiol.1953.sp004983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979 Jul;59(3):606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Bose D., Bose R. Mechanics of guinea pig taenia coli smooth muscle during anoxia and rigor. Am J Physiol. 1975 Aug;229(2):324–328. doi: 10.1152/ajplegacy.1975.229.2.324. [DOI] [PubMed] [Google Scholar]
- CHUJYO N., HOLLAND W. C. Potassium-induced contracture and calcium exchange in the guinea pig's taenia coli. Am J Physiol. 1963 Jul;205:94–100. doi: 10.1152/ajplegacy.1963.205.1.94. [DOI] [PubMed] [Google Scholar]
- Casteels R., Van Breemen C. Active and passive Ca2+ fluxes across cell membranes of the guinea-pig taenia coli. Pflugers Arch. 1975 Sep 9;359(3):197–207. doi: 10.1007/BF00587379. [DOI] [PubMed] [Google Scholar]
- Ganeshanandan S. S., Karaki H., Ikeda M., Urakawa N. Mechanical response of guinea pig taenia coli in high-K-Na-deficient medium under anoxia. Jpn J Pharmacol. 1969 Jun;19(2):329–330. doi: 10.1254/jjp.19.329. [DOI] [PubMed] [Google Scholar]
- Ishida Y., Shibata S. Relaxing and metabolic inhibitory action of X537A (Lasalocid) on the taenia of the guinea-pig caecum. J Physiol. 1982 Dec;333:293–304. doi: 10.1113/jphysiol.1982.sp014454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaki H., Suzuki T., Ozaki H., Urakawa N., Ishida Y. Dissociation of K+-induced tension and cellular Ca2+ retention in vascular and intestinal smooth muscle in normoxia and hypoxia. Pflugers Arch. 1982 Aug;394(2):118–123. doi: 10.1007/BF00582912. [DOI] [PubMed] [Google Scholar]
- Karaki H., Suzuki T., Urakawa N., Ishida Y., Shibata S. High K+,Na+-deficient solution inhibits tension, O2 consumption, and ATP synthesis in smooth muscle. Jpn J Pharmacol. 1982 Aug;32(4):727–733. doi: 10.1254/jjp.32.727. [DOI] [PubMed] [Google Scholar]
- Kishimoto T., Ozaki H., Karaki H., Urakawa N., Ishida Y. The inhibitory effect of monensin on high K-induced contraction in guinea-pig taenia coli. Eur J Pharmacol. 1982 Oct 15;84(1-2):25–32. doi: 10.1016/0014-2999(82)90153-4. [DOI] [PubMed] [Google Scholar]
- Knull H. R., Bose D. Reversibility of mechanical and biochemical changes in smooth muscle due to anoxia and substrate depletion. Am J Physiol. 1975 Aug;229(2):329–333. doi: 10.1152/ajplegacy.1975.229.2.329. [DOI] [PubMed] [Google Scholar]
- Nasu T., Yui K., Nakagawa H., Ishida Y. Role of glycolysis in the tension development under anoxia in guinea pig taenia coli. Jpn J Pharmacol. 1982 Feb;32(1):65–71. doi: 10.1254/jjp.32.65. [DOI] [PubMed] [Google Scholar]
- PFAFFMAN M., URAKAWA N., HOLLAND W. C. ROLE OF METABOLISM IN K-INDUCED TENSION CHANGES IN GUINEA PIG TAENIA COLI. Am J Physiol. 1965 Jun;208:1203–1205. doi: 10.1152/ajplegacy.1965.208.6.1203. [DOI] [PubMed] [Google Scholar]
- Saito Y., Sakai Y., Ikeda M., Urakawa N. Effect of histamine on oxygen consumption in guinea pig taenia coli. Jpn J Pharmacol. 1971 Oct;21(5):605–611. doi: 10.1254/jjp.21.605. [DOI] [PubMed] [Google Scholar]
- Saito Y., Sakai Y., Ikeda M., Urakawa N. Oxygen consumption during potassium-induced contracture in guinea pig taenia coli. Jpn J Pharmacol. 1968 Sep;18(3):321–331. doi: 10.1254/jjp.18.321. [DOI] [PubMed] [Google Scholar]
- URAKAWA N., HOLLAND W. C. CA45 UPTAKE AND TISSUE CALCIUM IN K-INDUCED PHASIC AND TONIC CONTRACTION IN TAENIA COLI. Am J Physiol. 1964 Oct;207:873–876. doi: 10.1152/ajplegacy.1964.207.4.873. [DOI] [PubMed] [Google Scholar]
- Urakawa N., Karaki H., Ikeda M. Effects of ouabain and metabolic inhibiting factors on Ca distribution during K-induced contracture in guinea pig taenia coli. Jpn J Pharmacol. 1970 Sep;20(3):360–366. doi: 10.1254/jjp.20.360. [DOI] [PubMed] [Google Scholar]
- Van Breemen C., Farinas B. R., Gerba P., McNaughton E. D. Excitation-contraction coupling in rabbit aorta studied by the lanthanum method for measuring cellular calcium influx. Circ Res. 1972 Jan;30(1):44–54. doi: 10.1161/01.res.30.1.44. [DOI] [PubMed] [Google Scholar]
- WEST T. C., HADDEN G., FARAH A. Effect of anoxia on response of the isolated intestine to various drugs and enzyme inhibitors. Am J Physiol. 1951 Feb;164(2):565–572. doi: 10.1152/ajplegacy.1951.164.2.565. [DOI] [PubMed] [Google Scholar]


