Abstract
Forty-one 8- to 12-week-old wasted pigs were selected from several conventional farms with histories of postweaning multisystemic wasting syndrome (PMWS) and classified into two groups according to their porcine circovirus type 2 (PCV2) infection status, as determined by in situ hybridization (ISH). Twenty-four pigs tested positive for PCV2 (PCV2-positive group), while 17 pigs tested negative for PCV2 (PCV2-negative group). In addition, eight uninfected healthy pigs from an experimental farm were used as controls. Heparinized blood samples were taken to obtain peripheral blood mononuclear cells. The CD4+, CD8+, CD4+ CD8+ (double-positive [DP]), and immunoglobulin M-positive (IgM+) cell subsets were analyzed by flow cytometry with appropriate monoclonal antibodies. Histopathological studies were done to evaluate the apparent degrees of lymphocyte depletion in different lymphoid organs (superficial inguinal and mesenteric lymph nodes, Peyer's patches, tonsils, and spleen) and to determine the viral load of the PCV2 genome by using an ISH technique. Animals of the PCV2-positive group showed a significant downshift of the CD8+ and DP cell subsets compared to the other groups (P < 0.05). Moreover, in PCV2-positive pigs, the amount of PCV2 genome in lymphoid tissues was related to the degree of cell depletion in those tissues (P < 0.05) as well as to the relative decrease in IgM+ and CD8+ cells in peripheral blood. These data support the notion that PCV2-positive pigs might have an impaired immune response.
Since its first description in 1996 by Clark and Harding (Abstr. Proc. West Can. Assoc. Swine Pract., abstr. 21 to 25, 1996), postweaning multisystemic wasting syndrome (PMWS) has been reported worldwide (2, 4, 9, 11, 13, 22; S. C. Kyriakis, S. Kennedy, K. Saoulidis, S. Lekkas, C. C. Miliotis, G. C. Balkamos, and P. A. Papoutsis, Abstr. 16th Int. Pig Vet. Soc. Congr., p. 633, 2000). This syndrome, which affects weaned and fattening pigs (8), is characterized by progressive weight loss or unthriftiness, dyspnea, enlarged lymph nodes, and, less frequently, pallor, jaundice, and diarrhea (5, 8). Viral detection techniques and nucleotide sequence analyses have shown an association between porcine circovirus type 2 (PCV2) and the presence of a characteristic histopathologic pattern of PMWS (7, 8, 12).
Nowadays, PCV2 is included with Chicken anemia virus, Beak and feather disease virus, and PCV1 in the family Circoviridae. Members of this family are small, nonenveloped viruses characterized by circular, single-stranded-DNA genomes (6, 16, 24). Infections caused by circoviruses have been associated with damage to lymphoid tissues resulting in extensive lymphocyte depletion (18, 25). For instance, chicken anemia virus infects the hemocytoblasts in the bone marrow and precursor T lymphocytes in the thymus (25), and beak and feather disease virus infects macrophages and causes atrophy of lymphoid tissue in the thymus and bursa of Fabricius (25). Particularly, PMWS-affected pigs show histiocytic infiltration and lymphocyte depletion of both follicle centers and parafollicular zones, symptoms associated with the presence of PCV2 (18). Consequently, members of this viral family have been supposed to be immunosuppressive (1, 10, 14).
The effects of PCV2 on the pig immune system are not yet fully known, but it has been reported that the main target cells for PCV2 replication are the monocyte/macrophage lineage cells as well as other antigen-presenting cells such as follicular dendritic cells (18). In addition, PCV2 antigen in the nuclei of some lymphocyte subsets has been described (23). Recent studies have suggested that PCV2 infects dividing cells, macrophages, and B lymphocytes, inducing apoptosis of the B cells that leads to the depletion of the lymphoid organs (23). Moreover, studies of lymphocyte subsets showed that pigs suffering from PMWS had lower proportions of CD4+ and immunoglobulin M-positive (IgM+) cells in blood than healthy, uninfected pigs did (20). Taken together, these facts have led some to suggest that PCV2 infection might cause immunosuppression (20, 23). However, little is known regarding the immunological response of pigs suffering from PMWS and the mechanism by which PCV2 infection might result in the development of PMWS.
The aim of this study was to evaluate by means of flow cytometry the changes in lymphocyte subsets of peripheral blood of naturally PCV2-infected pigs suffering from PMWS as well as to correlate these changes with histopathological findings for lymphoid organs such as the superficial inguinal and mesenteric lymph nodes, Peyer's patches, tonsils, and spleen.
MATERIALS AND METHODS
Animals and study design.
Forty-one 8- to 12-week-old pigs were randomly selected from those that were submitted to the Veterinary Faculty of the Universitat Autònoma of Barcelona because of their evident chronic wasting and unhealthy status. To be included in the study, the pigs had to be from conventional farms with historical records of PMWS and had to present clear signs of wasting and labored breathing. Eight healthy PCV2-uninfected pigs obtained from an experimental herd were also included to provide standards for the immune parameters. Before the pigs were euthanatized, blood samples were aseptically taken from the jugular veins, and after the animals were killed, they were necropsied and histopathological analyses were performed.
Postmortem and histopathological studies.
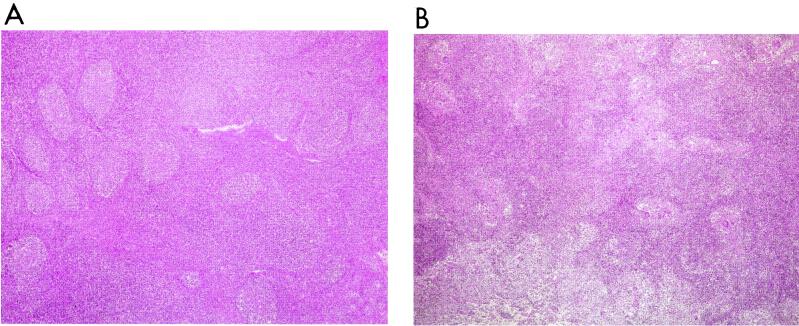
All pigs examined were euthanatized by means of a sodium pentobarbital overdose, and detailed necropsies were performed. Samples of the superficial inguinal and mesenteric lymph nodes, Peyer's patches, tonsils, and spleen were taken and fixed by immersion in 10% neutral-buffered formalin, dehydrated, embedded in paraffin wax, and sectioned at 4-μm intervals. The samples were then stained with hematoxylin and eosin (HE) in order to assess the degree of apparent lymphocellular depletion. The different lymphoid tissues were classified by a ranked scoring from no depletion (score 0) to severe depletion (score 3) (Fig. 1). The pattern of distribution of B- and T-cell-dependent areas of the examined tissues was evaluated as described previously (3). These evaluations were always done by the same pathologist, and samples were masked to ensure a blind study.
FIG. 1.
(A) Histological pattern of a superficial inguinal lymph node of a healthy pig. Follicular and interfollicular areas are clearly differentiated. (B) Moderate lymphocyte depletion (score 2) in a superficial inguinal lymph node of a pig with PMWS. Follicles are still perceptible, but cellular depletion is evident in all the lymphoid parenchyma. (C) Severe lymphocyte depletion (score 3) in a superficial inguinal lymph node of a pig with PMWS. No follicles or medulla-like tissues are evident in the lymphoid tissue. HE staining was used for all panels. Magnification, ×40.
ISH technique to detect PCV2 infection.
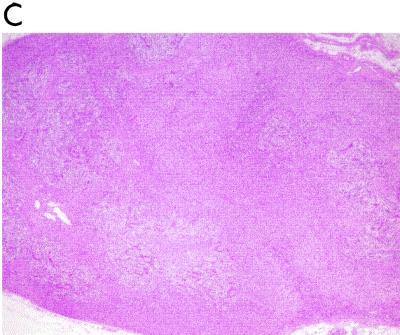
The in situ hybridization (ISH) technique was performed according to a previously described protocol (17). Briefly, sections were hybridized to a PCV2-specific, single-stranded, 40-nucleotide DNA probe (5′-CAGTAAATACGACCAGGACTACAATATCCGTG-TAACCATG-3′) that was complementary to nucleotides 1,085 to 1,124 of open reading frame 2 of the PCV2 genome. The DNA probe was end labeled with digoxigenin, and the prehybridization heating was carried out for 5 min at 105°C followed by the hybridization process at 37°C for 60 min. Positive control samples were obtained from pigs from Spain that had been previously diagnosed with PMWS (22), and negative control samples were lymph node tissues from pigs that tested negative for PCV2 and that came from an experimental farm with no record of PMWS. The apparent levels of viral genome stained by ISH in the different lymphoid tissues were evaluated as described previously (15). Accordingly, three categories were considered: high (Fig. 2A), low (Fig. 2B), and absent.
FIG. 2.
High (A) and low (B) levels of PCV2 nucleic acid in tissue from the tonsils of a pig with PMWS. Results were obtained by PCV2 ISH. All panels show fast green counterstaining. Magnification, ×400.
IHC technique to detect PRRSV infection.
In order to detect porcine reproductive and respiratory syndrome virus (PRRSV) infection, an immunohistochemical (IHC) technique was carried out on lung tissue sections by using an avidin-biotin-peroxidase method described previously (21). Briefly, tissue sections were placed on silane [3-(triethoxysilyl)-propylamine]-coated slides. Endogenous peroxidase activity was inhibited by immersing the tissue sections in a 3% solution of hydrogen peroxide in methanol for 30 min. Antigen retrieval was done by enzymatic treatment (protease type XIV) in Tris-buffered saline (TBS; pH 7.4) for 10 min. Blocking was carried out for 1 h with 10% normal goat serum in TBS. Tissue sections were mounted on slides and covered with the monoclonal antibody (MAb) anti-PRRS SDOW17 (dilution, 1:1,000 in TBS), and the slides were incubated overnight at 4°C. Then, the secondary antibody (biotinylated goat anti-mouse linking antibody) and peroxidase-conjugated avidin were applied (at dilutions of 1:200 and 1:100, respectively) for 1 h at room temperature. Sections were incubated in diaminobenzidine-hydrogen peroxide solution for 8 min, counterstained with Harris's hematoxylin, dehydrated, covered with a coverslip, and examined microscopically. Negative control procedures included the omission of the primary antiserum.
Serological techniques to detect PPV.
A commercially available enzyme-linked immunosorbent assay (Ingezim PPV; Ingenasa, Madrid, Spain) was used according to the manufacturer's recommendation.
Isolation of PBMC.
Ten milliliters of blood was collected from each pig, with heparin used as an anticoagulant. Blood samples were processed to separate peripheral blood mononuclear cells (PBMC) by using gradient density centrifugation with Histopaque 1.077 (Sigma, Barcelona, Spain) at 500 × g for 30 min. Once the PBMC were recovered from the plasma-Histopaque interphase, they were washed three times with phosphate-buffered saline (PBS) and resuspended in RPMI 1640 supplemented with 10% fetal calf serum (Life technologies, Barcelona, Spain). Cells were quantified with a hemocytometer, and a trypan blue-dye exclusion test was performed to assess viability.
Flow cytometry.
Phenotypic analysis of PBMC subsets was done by flow cytometry with the following MAbs: anti-CD4 (b38c6, IgG1; Labor, Reutlingen, Germany), anti-CD8 (295/33-25, IgG2a; Labor), and anti-IgM (k52-1c, IgG1; LabGen, Barcelona, Spain). For secondary antibodies, goat F(ab′)2 anti-mouse IgG2a R-phycoerythrin-conjugated antibody and goat F(ab′)2 anti-mouse IgG1 fluorescein-conjugated antibody (Southern Biotechnology, Birmingham, Ala.) were used. Irrelevant isotype-matched antibodies were used as background controls. Optimal dilutions of MAbs were standardized in previous experiments (data not shown). Mononuclear cells were dispensed at 106 cells/ml, washed with PBS supplemented with 0.01% sodium azide and 1% fetal calf serum (flow cytometry buffer [PBS-Flow]), and sedimented by centrifugation at 500 × g for 4 min. After the supernatants were discarded, the remaining cell pellets were resuspended with 50 μl of MAb against CD4 or CD8 (two-color cytofluorometry) and IgM (single-color) membrane antigens and incubated in ice for 30 min. Then, 1 ml of PBS-Flow was added, and the PBMC were washed three times. In the last wash, the supernatant fluid was removed and pelleted cells were incubated with 50 μl of the corresponding conjugate for 30 min in ice and in the dark. Finally, the PBMC were washed three more times and fixed in PBS-Flow with 0.5% formaldehyde before analysis with an EPICS XL-MLC cytometer (Coulter) to an excitation wavelength of 488 nm and with 580- and 630-nm filters.
Statistical analysis.
Statistics were calculated with Statsdirect and Epi-Info V.6.01. Comparison between groups regarding the proportion of cells in each subset was done by means of the Kruskal-Wallis test. Further pairwise comparisons were done by the Conover-Inman method. Additional statistical analyses (2-by-2 tables) were calculated by using the chi-square test, and regression analyses were carried out by the method of Draper and Smith (4a) with the Epi-Info program. A P value of ≤0.05 was considered statistically significant.
RESULTS
ISH technique for PCV2 infection and IHC technique for PRRSV infection.
Regarding the results of the ISH, 24 wasted pigs were positive for PCV2 (PCV2-positive group) and 17 wasted pigs were negative (PCV2-negative group). All healthy animals were also negative for PCV2. The IHC analysis showed that six pigs were positive for PRRSV. Four of them were coinfected with PCV2, and the other two animals were PCV2-negative wasted pigs. The eight healthy pigs were also negative for PRRSV infection. The sex distributions for the different groups were 12 males and 12 females for the PCV2-positive group, 11 males and 6 females for the PCV2-negative group, and 4 males and 4 females for the healthy group.
PPV status.
Eight out of 24 PCV2-positive pigs were seropositive for PPV, while 9 out of 17 PCV2-negative pigs were positive (insignificant differences). None of the healthy control pigs was seropositive.
Flow cytometry.
The relative proportions of CD4+, CD8+, CD4+ CD8+ (double-positive [DP]), and IgM+ cell subsets in PBMC were significantly different among the groups (Table 1). With regard to the CD4+ cell subset, differences were observed between wasted (PCV2-positive or -negative) and healthy animals. Thus, wasted pigs always had a lower proportion of the CD4+ cell subset than the healthy ones (P < 0.001). When the relative proportions of CD8+ or DP cells were compared among groups, a significant downshift of these cells was observed for the PCV2-positive group (P < 0.01).
TABLE 1.
Percentages of lymphocyte subsets of PBMC among the different pig groups
| Group (no. of pigs) | Percentage (mean ± SD) of indicated lymphocyte surface antigen marker in PBMCa
|
|||
|---|---|---|---|---|
| CD4+ | CD8+ | CD4+ CD8+ | IgM+ | |
| PCV2 positive (24) | 5.0 ± 4.3 A | 17.6 ± 14.3 A | 1.4 ± 1.6 A | 8.0 ± 6.4 A* |
| High PCV2 (17)b | 5.1 ± 4.4 A | 16.4 ± 13.7 A | 1.3 ± 1.2 A | 6.5 ± 6.5 C |
| Low PCV2 (7)b | 4.8 ± 4.5 A | 20.5 ± 16.4 B | 1.7 ± 1.2 A | 11.6 ± 5.1 A |
| PCV2 negative (17) | 6.7 ± 3.8 A | 40.8 ± 18.7 C | 3.2 ± 2.9 B | 14.3 ± 9.6 B |
| Healthy control (8) | 15.2 ± 4.1 B | 27.2 ± 7.8 C | 5.2 ± 1.7 B | 12.5 ± 5.3 AB* |
Values followed by the same letter (A, B, or C) are statistically similar, while different letters for values within the same lymphocyte subset indicate significant differences in the mean values (P ≤ 0.05). *, P = 0.09.
The PCV2-positive group was subcategorized with respect to the apparent level of PCV2 stained by the ISH technique.
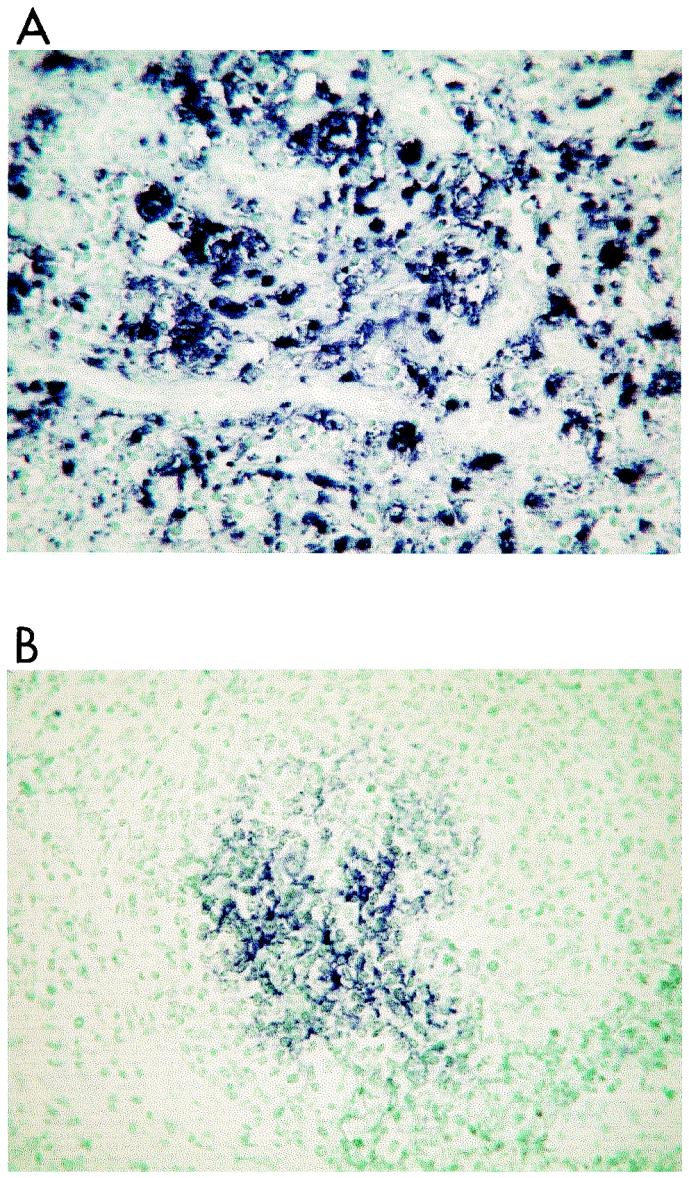
The results for the IgM+ cell subset were compared among groups. Results for the PCV2-positive animals were significantly different from those for the PCV2-negative animals (P < 0.05) but did not differ from those for the healthy animals, although the P value was close to the significance level (P = 0.09). However, when the actual distribution of individual results was considered, we found that the PCV2-positive group included a cluster of animals with an IgM+ cell proportion lower than 5% while the other 13 animals in this group had IgM+ cell proportions ranging from 6.5 to 22.2% (Fig. 3). Comparison of these results with the apparent level (high or low) of PCV2 genome in tissues showed that most of the pigs with proportions of IgM+ cells lower than 5% had high levels of PCV2 infection (P < 0.05). Thus, data for IgM+ cells were analyzed again, with PCV2-positive animals classified into two subgroups according to the ISH results. With this analysis, it was evident that pigs with higher levels of PCV2 had lower proportions of IgM+ cells in their blood (mean = 6.5%), results that were statistically different from the IgM+ cell proportions in the pigs in the healthy group (mean = 12.5%; P < 0.05). In contrast, pigs with low levels of PCV2 were similar to the healthy ones with respect to IgM+ cells (Table 1). In addition, PCV2-positive pigs with lower proportions of IgM+ cells (<5%) also had lower proportions of CD8+ cells (r = 0.43; P < 0.05). As for DP cells, PCV2-infected animals had lower proportions of those cells than animals in all other groups (P < 0.05).
FIG. 3.
Distribution of individual results (percentage of cells for each animal) with respect to the different lymphocyte subsets. The large open circles comprise the clusters of pigs with the lowest relative proportions of peripheral blood lymphocyte subsets, pigs that thereby differ from the other pigs in the study. +ve, positive; −ve, negative.
Histopathological studies.
The evaluation of cellular depletion in the different lymphoid tissues showed significant differences between groups. The PCV2-positive group had the highest proportion of pigs with any degree of depletion in most lymphoid tissues (Table 2), and the pigs in this group had a more severe degree of depletion than the pigs in the PCV2-negative group did; although there were some PCV2-negative animals with lymphocyte depletion, that depletion was always much slighter than that of the PCV2-positive pigs. Moreover, PCV2-positive pigs that had moderate to severe depletions of lymphocytes in both T- and B-cell-dependent zones of a tissue also had similar degrees of depletion in all of the other examined tissues (P < 0.05). Furthermore, for PCV2-positive pigs with moderate (score 2) or severe (score 3) depletions in B-cell-dependent areas of a given organ, a similar score was determined for the T-cell-dependent areas of that organ (P < 0.05). On the other hand, the degree of cellular depletion in superficial inguinal lymph nodes or tonsils was inversely correlated to the IgM+ cell proportions in blood (P < 0.01) and was also related to the apparent level of PCV2 genome in lymphoid tissues (P < 0.05). Thus, the greater the amount of PCV2, the more severe the depletion, except in periarteriolar lymphoid sheaths (P = 0.07) (Table 3).
TABLE 2.
Distribution of pigs according to the histopathologic stage of cellular depletion in different lymphoid tissuesa
| Group (no. of pigsb) | No. of pigs showing lymphocyte depletion in indicated tissue
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Superficial inguinal LNc
|
Mesenteric LN
|
Peyer's patches
|
Tonsil
|
Spleen (PALSd) | |||||
| B zonee | T zonef | B zone | T zone | B zone | T zone | B zone | T zone | ||
| PCV2 negative (17) | 5 | 1 | 10 | 4 | 7 | 8 | 3 | 2 | 3 |
| PCV2 positive (24) | 19g | 17g | 19 | 17g | 18h | 19h | 17g | 14g | 13h |
Since healthy control pigs showed no lesions, results for them have been excluded from the table, and the results of the statistical analysis reflect a comparison, between the PCV2-negative and PCV2-positive wasted-pig groups. P values were obtained from 2-by-2 contingency tables.
Total number of pigs in each group, including those not showing lymphocyte depletion.
LN, lymph node.
PALS, periarteriolar lymphoid sheaths.
B zone, B-cell-dependent area.
T zone, T-cell-dependent area.
P < 0.001.
P < 0.05.
TABLE 3.
Comparison of the degrees of lymphocyte depletion of different lymphoid tissues with respect to the apparent amount of PCV2 DNA genome observed after ISH
| Level of PCV2 DNA (no. of pigsb) | No. of PCV2-positive pigs showing indicated degree of lymphocyte depletion in indicated tissuea
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Superficial inguinal LNc
|
Mesenteric LN
|
Peyer's patches
|
Tonsil
|
Spleen (PALSd)
|
||||||||||||||
| B zonee
|
T zonef
|
B zone
|
T zone
|
B zone
|
T zone
|
B zoneg
|
T zoneh
|
|||||||||||
| 1 | 2 or 3 | 1 | 2 or 3 | 1 | 2 or 3 | 1 | 2 or 3 | 1 | 2 or 3 | 1 | 2 or 3 | 1 | 2 or 3 | 1 | 2 or 3 | 1 | 2 or 3 | |
| Low (7) | 4 | 0 | 0 | 0 | 3 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 3 | 0 | 1 | 0 | 1 | 0 |
| High (17) | 0 | 15 | 8 | 9 | 0 | 17 | 1 | 16 | 0 | 16 | 1 | 16 | 1 | 13 | 4 | 9 | 5 | 7 |
Score of lymphocyte depletion degree: 1, slight; 2, moderate; 3, severe.
Total number of pigs, including those not showing lymphocyte depletion.
LN, lymph node.
PALS, periarteriolar lymphoid sheaths; P = 0.07.
B zone, B-cell-dependent area.
T zone, T-cell-dependent area.
P < 0.01; for other groups, P < 0.001, except as indicated.
P < 0.05.
DISCUSSION
In our study, flow cytometric analyses showed that PCV2-infected animals suffered a shift in the relative counts of lymphocyte subsets in PBMC. Some of these changes have been described in other reports (19, 20, 23), in which the reduction of T cells was associated with a decrease or shift in the CD4+ cell subset (19, 20). In those reports, only PCV2-positive or healthy pigs were examined. In our report, we included PCV2-positive and PCV2-negative wasted pigs. Thus, the statistical evaluation of our results showed that the CD4+ cell shift occurred in both wasted groups, regardless of whether or not the pigs were PCV2 infected. This might suggest that the changes observed for CD4+ cells are related to the wasted state, regardless of the PCV2 status. By contrast, when the CD8+ and DP cell subsets were analyzed, the relative decreases in these cells were detected only in PCV2-infected pigs. In consequence, these results might suggest that CD8+ and DP cells are specifically affected during the development of PMWS. Other studies of experimental PCV2 inoculation in conventional pigs showed that animals infected with PCV2 which did not develop clinical signs of PMWS had, in the initial stages of infection (approximately 21 days postinfection), a downshift of CD8+ cells that recovered later on (L. Darwich, J. Segalés, A. Resendes, C. Rosell, M. Domingo, M. Balasch, J. Plana, and E. Mateu, Abstr. 6th Int. Vet. Immunol. Symp., abstr. 146, 2001). Since the CD8+ cell subset is mainly responsible for the cytotoxic responses, it would be advisable to focus future studies on the general and virus-specific cytotoxic responses of PMWS-affected animals.
The fact that DP cells were also downshifted is difficult to explain and deserves further study. However, DP cells are mature lymphocytes with properties of memory and effector cells (26). If that shift in DP cells represents a true decrease, it can be inferred that memory responses could also be altered.
Changes in the proportion of IgM+ cells in PBMC of PCV2-infected pigs have been reported (19, 20, 23). It was suggested that this decrease could be due to the apoptotic effect that PCV2 infection might have on B cells (23). The results of our analysis of IgM+ cell levels showed two different clusters of animals within the PCV2-positive group: pigs with proportions of IgM+ cells of <5% and pigs with proportions of >5%. Though this 5% cutoff was obviously identified after the analysis, it clearly correlates with the amount of virus in the tissues and the severity of the lesions. Thus, this proportion of 5% of IgM+ cells might be a good candidate for a marker of severe progression of the PCV2 infection, particularly since PCV2-negative wasted animals were not clinically distinguishable, while alive, from PMWS-affected pigs.
The histopathological analysis showed that the intensity of depletion in B- or T-cell-dependent areas of the examined lymphoid tissues was related to the apparent amount of viral DNA in those tissues. Also, when severe cellular depletions were found in one lymphoid organ, it became more likely that similar lesions would be found in others. We think that these results indicate that lymphoid cell depletion occurs generally in all secondary or peripheral lymphoid organs affected by PMWS. On the other hand, present knowledge supports the theory that in the pathogenesis of PMWS, the first replication of PCV2 takes place in local lymphoid organs such as the tonsils and lymph nodes (18). This is thought to cause lymphocyte damage that leads to the typical histopathological pattern of PMWS—absence of follicles and lymphoid tissue atrophy (18, 19)—that we found in PCV2-infected pigs.
In relating the histopathological observations to the flow cytometry results, it is difficult to determine whether the downshift of lymphocyte subsets in the blood of PMWS-affected pigs represents a depletion or a relative change with respect to other cell types (T-γδ cells or monocytes) that were not examined. However, since the most severe depletions in lymphoid tissues were found in pigs with lower blood counts, we think that changes in the blood probably reflect general lymphocyte depletion. Unfortunately, we did not examine primary organs like the thymus or bone marrow, nor did we further characterize the phenotype of existing lymphocytes to ascertain whether they were naïve or effector or memory cells (LFA-3, CD45RO/RA, CD69, etc.). Thus, additional studies are needed to clarify whether these decreases are general or specific to a given type of lymphocyte subset.
The results of the present report reinforce the notion that in natural cases of PMWS, there is an evident change (absolute or relative) in the proportions of the different lymphocyte subsets. This change seems to be related to the levels of PCV2 in lymphoid tissues and to the extent of depletion in both B- and T-cell-dependent areas of these tissues. Taken together, these facts could suggest the existence of damage to the immune systems of PMWS-affected pigs. However, it remains unclear how PCV2 acts upon the immune systems of infected pigs.
Acknowledgments
This work has been performed thanks to funding from Project QLRT-PL-199900307 of the Fifth Framework Programme 1998-2002 of the European Commission, Project 2-FEDER-1997-1341 of the I+D National Plan (Spanish Ministry of Science and Technology), and a grant from Generalitat de Catalunya.
REFERENCES
- 1.Adair, B., F. McNeilly, C. D. McConnell, D. Todd, R. Nelson, and M. S. McNulty. 1991. Effects of chicken anemia agent on lymphokine production and lymphocyte transformation in experimentally infected chickens. Avian Dis. 35:783-792. [PubMed] [Google Scholar]
- 2.Allan, G. M., F. McNeilly, B. M. Meehan, S. Kennedy, D. P. Mackie, J. A. Ellis, E. G. Clark, E. Espuna, N. Saubi, P. Riera, A. Botner, and C. E. Charreyre. 1999. Isolation and characterisation of circoviruses from pigs with wasting syndromes in Spain, Denmark and Northern Ireland. Vet. Microbiol. 66:115-123. [DOI] [PubMed] [Google Scholar]
- 3.Chianini, F., N. Majó, J. Segalés, J. Domínguez, and M. Domingo. 2001. Immunohistological study of the immune system cells in paraffin-embedded tissues of conventional pigs. Vet. Immunol. Immunopathol. 82:245-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi, C., C. Chae, and E. G. Clark. 2000. Porcine postweaning multisystemic wasting syndrome in Korean pig: detection of porcine circovirus 2 infection by immunohistochemistry and polymerase chain reaction. J. Vet. Diagn. Investig. 12:151-153. [DOI] [PubMed] [Google Scholar]
- 4a.Draper, N. R., and H. Smith. 1998. Applied regression analysis, 3rd ed. John Wiley & Sons, Inc., New York, N.Y.
- 5.Ellis, J., L. Hassard, E. Clark, J. Harding, G. Allan, P. Willson, J. Strokappe, K. Martin, F. McNeilly, B. Meehan, D. Todd, and D. Haines. 1998. Isolation of circovirus from lesions of pigs with postweaning multisystemic wasting syndrome. Can. Vet. J. 39:44-51. [PMC free article] [PubMed] [Google Scholar]
- 6.Gelderblom, H., S. Kling, R. Lurz, I. Tischer, and V. V. Bulow. 1989. Morphological characterization of chicken anaemia agent (CAA). Arch. Virol. 109:115-120. [DOI] [PubMed] [Google Scholar]
- 7.Hamel, A. L., L. L. Lin, and G. P. S. Nayar. 1998. Nucleotide sequence of porcine circovirus associated with postweaning multisystemic wasting syndrome in pigs. J. Virol. 72:5262-5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harding, J. C. S., E. G. Clark, J. H. Strokappe, P. I. Willson, and J. A. Ellis. 1998. Postweaning multisystemic wasting syndrome: epidemiology and clinical presentation. Swine Health Prod. 6:249-254. [Google Scholar]
- 9.Kiupel, M., G. W. Stevenson, S. K. Mittal, E. G. Clark, and D. M. Haines. 1998. Circovirus-like viral associated disease in weaned pigs in Indiana. Vet. Pathol. 35:303-307. [DOI] [PubMed] [Google Scholar]
- 10.Latimer, K. S., P. M. Rakich, F. D. Niagro, B. W. Ritchie, W. L. Steffens, R. P. Campagnoli, D. A. Pesti, and P. D. Lukert. 1991. An updated review of psittacine beak and feather disease. J. Assoc. Avian Vet. 5:211-220. [Google Scholar]
- 11.Madec, F., E. Eveno, P. Morvan, L. Hamon, P. Blanchard, R. Cariolet, N. Amenna, H. Morvan, C. Truong, D. Mahe, E. Albina, and A. Jestin. 2000. Post-weaning multisystemic wasting syndrome (PMWS) in pigs in France: clinical observations from follow-up studies on affected farms. Livest. Prod. Sci. 63:223-233. [Google Scholar]
- 12.Meehan, B. M., F. McNeilly, D. Todd, S. Kennedy, V. A. Jewhurst, J. A. Ellis, L. E. Hassard, E. G. Clark, D. M. Haines, and G. M. Allan. 1998. Characterization of novel circovirus DNAs associated with wasting syndromes in pigs. J. Gen. Virol. 79:2171-2179. [DOI] [PubMed] [Google Scholar]
- 13.Onuki, A., K. Abe, K. Togashi, K. Kawashima, A. Taneichi, and H. Tsunemitsu. 1999. Detection of porcine circovirus from lesions of a pig with wasting disease in Japan. J. Vet. Med. Sci. 61:1119-1123. [DOI] [PubMed] [Google Scholar]
- 14.Otaki, Y., M. Tajima, K. Saito, and K. Nomura. 1988. Immune response of chicks inoculated with chicken anemia agent alone or in combination with Marek's disease virus or turkey herpesvirus. Jpn. J. Vet. Sci. 50:1040-1047. [DOI] [PubMed] [Google Scholar]
- 15.Quintana, J., J. Segalés, C. Rosell, M. Calsamiglia, G. M. Rodríguez-Arrioja, F. Chianini, J. M. Folch, J. Maldonado, M. Canal, J. Plana-Durán, and M. Domingo. 2001. Clinical and pathological observations on pigs with postweaning multisystemic wasting syndrome. Vet. Rec. 149:357-361. [DOI] [PubMed] [Google Scholar]
- 16.Ritchie, B. W., F. D. Niagro, P. D. Lukert, W. L. Steffens, and K. S. Latimer. 1989. Characterization of a new virus from cockatoos with psittacine beak and feather disease. Virology 171:83-88. [DOI] [PubMed] [Google Scholar]
- 17.Rosell, C., J. Segalés, and M. Domingo. 2000. Hepatitis and staging of hepatic damage in pigs naturally infected with porcine circovirus type 2. Vet. Pathol. 37:687-692. [DOI] [PubMed] [Google Scholar]
- 18.Rosell, C., J. Segalés, J. Plana-Durán, M. Balasch, G. M. Rodríguez-Arrioja, S. Kennedy, G. M. Allan, F. McNeilly, K. S. Latimer, and M. Domingo. 1999. Pathological, immunohistochemical, and in-situ hybridization studies of natural cases of postweaning multisystemic wasting syndrome (PMWS) in pigs. J. Comp. Pathol. 120:59-78. [DOI] [PubMed] [Google Scholar]
- 19.Sarli, G., L. Mandrioli, M. Laurenti, L. Sidoli, C. Cerati, G. Rolla, and P. S. Marcato. 2001. Immunohistochemical characterization of the lymph node reaction in pig post-weaning multisystemic wasting syndrome (PMWS). Vet. Immunol. Immunopathol. 83:53-67. [DOI] [PubMed] [Google Scholar]
- 20.Segalés, J., F. Alonso, C. Rosell, J. Pastor, F. Chianini, E. Campos, L. López-Fuertes, J. Quintana, G. Rodríguez-Arrioja, M. Calsamiglia, J. Pujols, J. Domínguez, and M. Domingo. 2001. Changes in peripheral blood leukocyte populations in pigs with natural postweaning multisystemic wasting syndrome (PMWS). Vet. Immunol. Immunopathol. 81:37-44. [DOI] [PubMed] [Google Scholar]
- 21.Segalés, J., M. Domingo, G. I. Solano, and C. Pijoan. 1999. Porcine reproductive and respiratory syndrome virus and Haemophilus parasuis antigen distribution in dually infected pigs. Vet. Microbiol. 64:287-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Segalés, J., M. Sitjar, M. Domingo, S. Dee, M. Del Pozo, R. Noval, C. Sacristan, A. De Las Heras, A. Ferro, and K. S. Latimer. 1997. First report of post-weaning multisystemic wasting syndrome in pigs in Spain. Vet. Rec. 141:600-601. [PubMed] [Google Scholar]
- 23.Shibahara, T., K. Sato, Y. Ishikawa, and K. Kadota. 2000. Porcine circovirus induces B lymphocyte depletion in pigs with wasting disease syndrome. J. Vet. Med. Sci. 62:1125-1131. [DOI] [PubMed] [Google Scholar]
- 24.Tischer, I., H. Gelderblom, W. Vettermann, and M. A. Koch. 1982. A very small porcine virus with circular single-stranded DNA. Nature 295:64-66. [DOI] [PubMed] [Google Scholar]
- 25.Todd, D. 2000. Circoviruses: immunosuppressive threats to avian species: a review. Avian Pathol. 29:373-394. [DOI] [PubMed] [Google Scholar]
- 26.Zuckermann, F. A., and R. J. Husmann. 1996. Functional and phenotypic analysis of porcine peripheral blood CD4/CD8 double-positive T cells. Immunology 87:500-512. [PMC free article] [PubMed] [Google Scholar]