Abstract
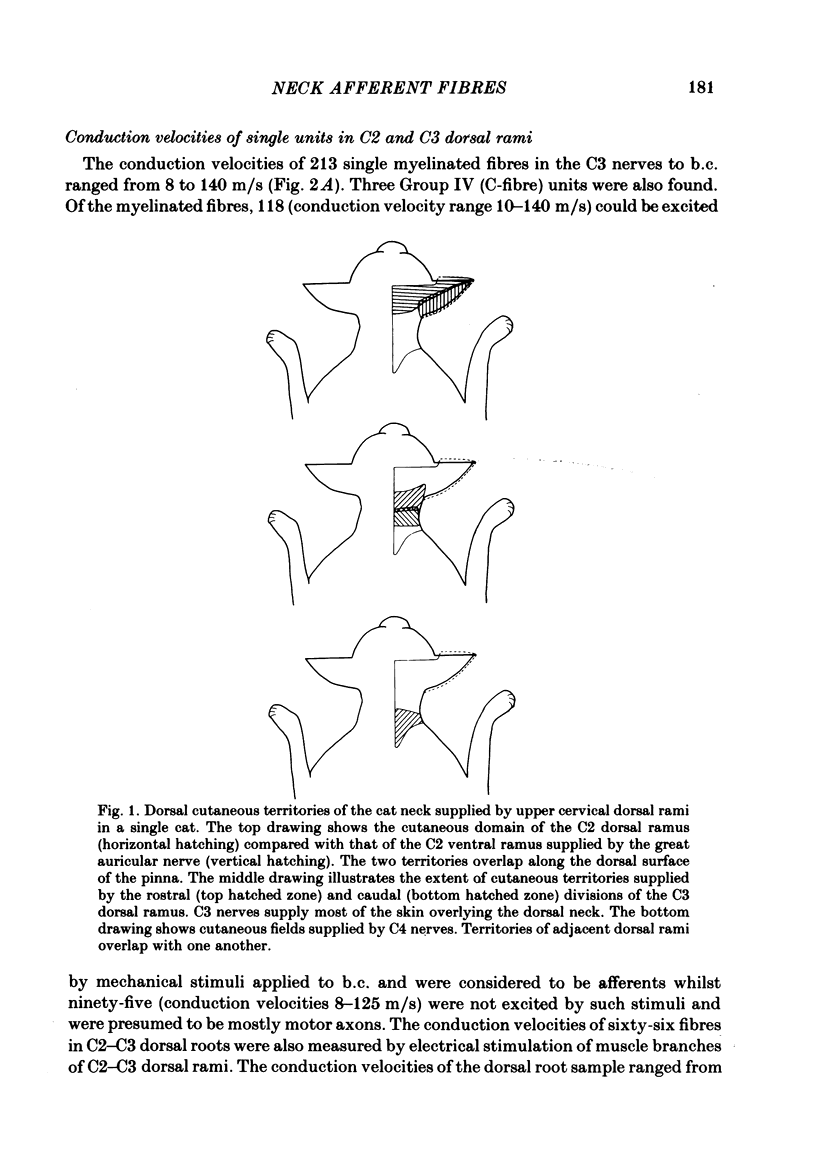
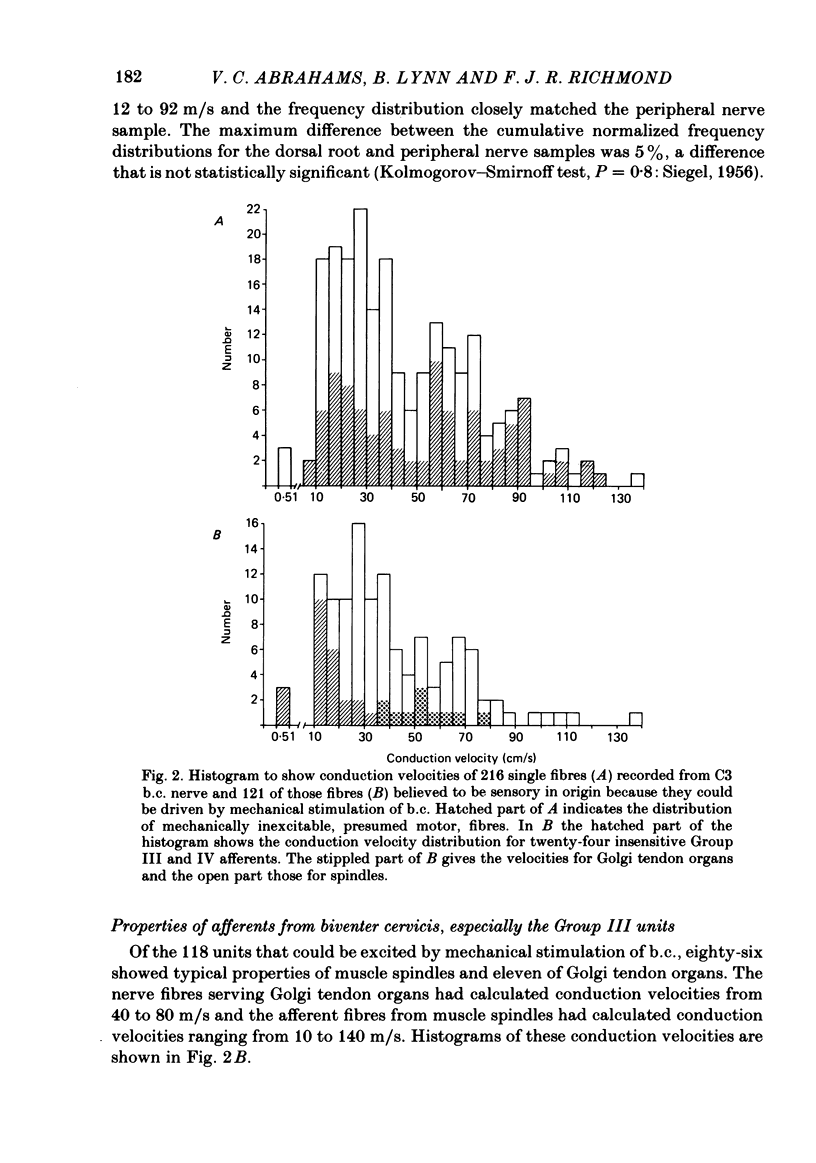
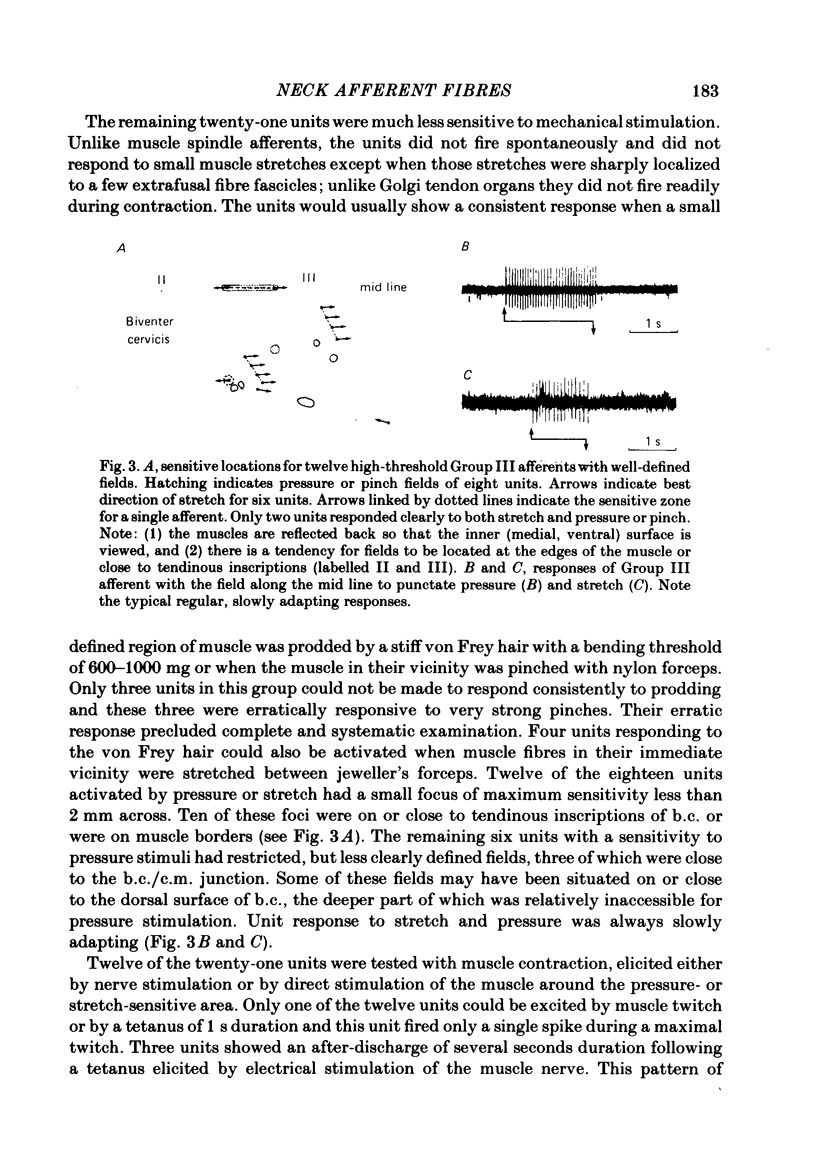
A study using both anatomical dissection and electrophysiological recording has been made to analyse sensory components of the cervical dorsal rami in the cat. Particular attention has been paid to the physiological properties of Group III muscle afferents. The dorsal rami from C2 to C4 are composed of many nerve bundles that contain afferents from both muscle and skin. Bundles containing only cutaneous and only muscle afferents were identified and found to follow a relatively consistent pattern of organization within the ramus. In nerve bundles serving the biventer cervicis muscle, a population of Group III afferent fibres was identified that responded to strong localized pressure or localized stretch. Their receptive fields were generally small and situated on the muscle borders or in tendinous inscriptions. Such units comprised 73% of those with conduction velocities less than 20 m/s. Group III afferent fibres from neck muscles fired readily to intramuscular 6% sodium chloride, but were mostly not sensitive to bradykinin injected locally or intra-arterially.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahams V. C., Turner C. J. The nature of afferents from the large dorsal neck muscles that project to the superior colliculus in the cat. J Physiol. 1981;319:393–401. doi: 10.1113/jphysiol.1981.sp013916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M. E. Segmental reflex inputs to motoneurons innervating dorsal neck musculature in the cat. Exp Brain Res. 1977 May 23;28(1-2):175–187. doi: 10.1007/BF00237095. [DOI] [PubMed] [Google Scholar]
- Boyd I. A., Kalu K. U. Scaling factor relating conduction velocity and diameter for myelinated afferent nerve fibres in the cat hind limb. J Physiol. 1979 Apr;289:277–297. doi: 10.1113/jphysiol.1979.sp012737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink E. E., Jinnai K., Wilson V. J. Pattern of segmental monosynaptic input to cat dorsal neck motoneurons. J Neurophysiol. 1981 Sep;46(3):496–505. doi: 10.1152/jn.1981.46.3.496. [DOI] [PubMed] [Google Scholar]
- DUN F. T. The delay and blockage of sensory impulses in the dorsal root ganglion. J Physiol. 1955 Feb 28;127(2):252–264. doi: 10.1113/jphysiol.1955.sp005254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway P. H., Murphy P. R., Tripathi A. Closely coupled excitation of gamma-motoneurones by group III Muscle afferents with low mechanical threshold in the cat. J Physiol. 1982 Oct;331:481–498. doi: 10.1113/jphysiol.1982.sp014385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT C. C., KUFFLER S. W. Stretch receptor discharges during muscle contraction. J Physiol. 1951 Apr;113(2-3):298–315. doi: 10.1113/jphysiol.1951.sp004573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumazawa T., Mizumura K. Thin-fibre receptors responding to mechanical, chemical, and thermal stimulation in the skeletal muscle of the dog. J Physiol. 1977 Dec;273(1):179–194. doi: 10.1113/jphysiol.1977.sp012088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mense S. Nervous outflow from skeletal muscle following chemical noxious stimulation. J Physiol. 1977 May;267(1):75–88. doi: 10.1113/jphysiol.1977.sp011802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mense S. Reduction of the bradykinin-induced activation of feline group III and IV muscle receptors by acetylsalicylic acid. J Physiol. 1982 May;326:269–283. doi: 10.1113/jphysiol.1982.sp014191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAINTAL A. S. Functional analysis of group III afferent fibres of mammalian muscles. J Physiol. 1960 Jul;152:250–270. doi: 10.1113/jphysiol.1960.sp006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond F. J., Abrahams V. C. Morphology and distribution of muscle spindles in dorsal muscles of the cat neck. J Neurophysiol. 1975 Nov;38(6):1322–1339. doi: 10.1152/jn.1975.38.6.1322. [DOI] [PubMed] [Google Scholar]
- Richmond F. J., Abrahams V. C. Physiological properties of muscle spindles in dorsal neck muscles of the cat. J Neurophysiol. 1979 Mar;42(2):604–617. doi: 10.1152/jn.1979.42.2.604. [DOI] [PubMed] [Google Scholar]
- Richmond F. J., Anstee G. C., Sherwin E. A., Abrahams V. C. Motor and sensory fibres of neck muscle nerves in the cat. Can J Physiol Pharmacol. 1976 Jun;54(3):294–304. doi: 10.1139/y76-043. [DOI] [PubMed] [Google Scholar]
- Rymer W. Z., Houk J. C., Crago P. E. Mechanisms of the clasp-knife reflex studied in an animal model. Exp Brain Res. 1979 Sep;37(1):93–113. doi: 10.1007/BF01474257. [DOI] [PubMed] [Google Scholar]



