Abstract
Chick lumbosacral motoneurones were caused to innervate foreign muscles by surgically rotating or shifting the limb bud about the anterior-posterior axis in stage 17-18 embryos. The activation pattern of such wrongly projecting motoneurones was assessed at stages 35-38 by recording electromyographic activity from muscles in an isolated spinal cord/hind limb preparation. Muscle activity was classed as flexor- or extensor-like according to the characteristics of the patterned sequence of bursts elicited by a single shock to the thoracic cord. Wrongly projecting motoneurones did not have their activation pattern altered to one appropriate for the muscle innervated; therefore in some cases a particular muscle was activated with a pattern similar to its original one, and in other cases in an opposite manner. Mixed flexor-extensor-like activation of a single muscle was, however, rare. The identity of motoneurones projecting to a muscle was determined by their cord location following retrograde labelling with horseradish peroxidase. This allowed us to conclude that motoneurones could develop their normal pattern of activation even when projecting to foreign muscles. It is concluded that the cord circuits (presumably composed of local interneurones responsible for the activation of motoneurones in the isolated cord preparation are not altered by retrograde influences from the muscle. Wrongly projecting motoneurones, which were maintained throughout the normal cell death period, were activated during spontaneous embryonic movements, and in many cases were found to have a behaviourally inappropriate activation pattern. These observations are discussed in relation to proposed mechanisms by which developmental errors in connectivity are corrected.
Full text
PDF




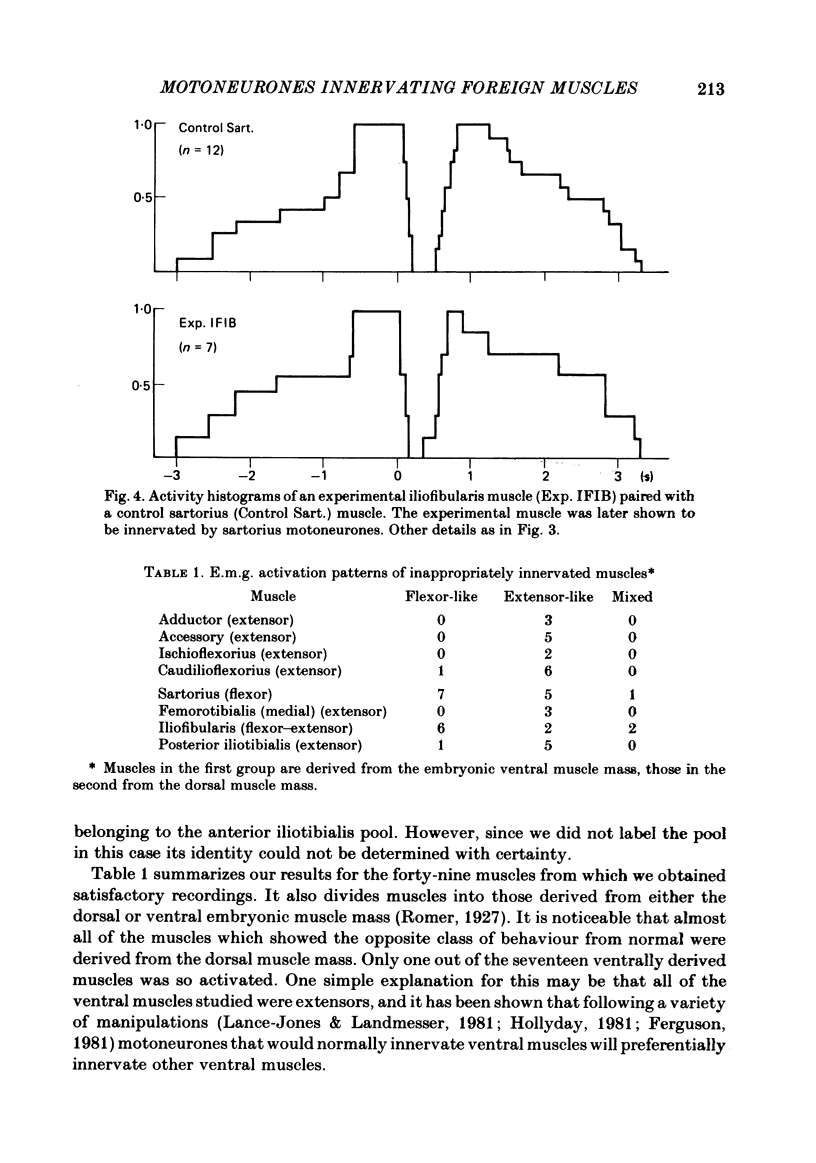

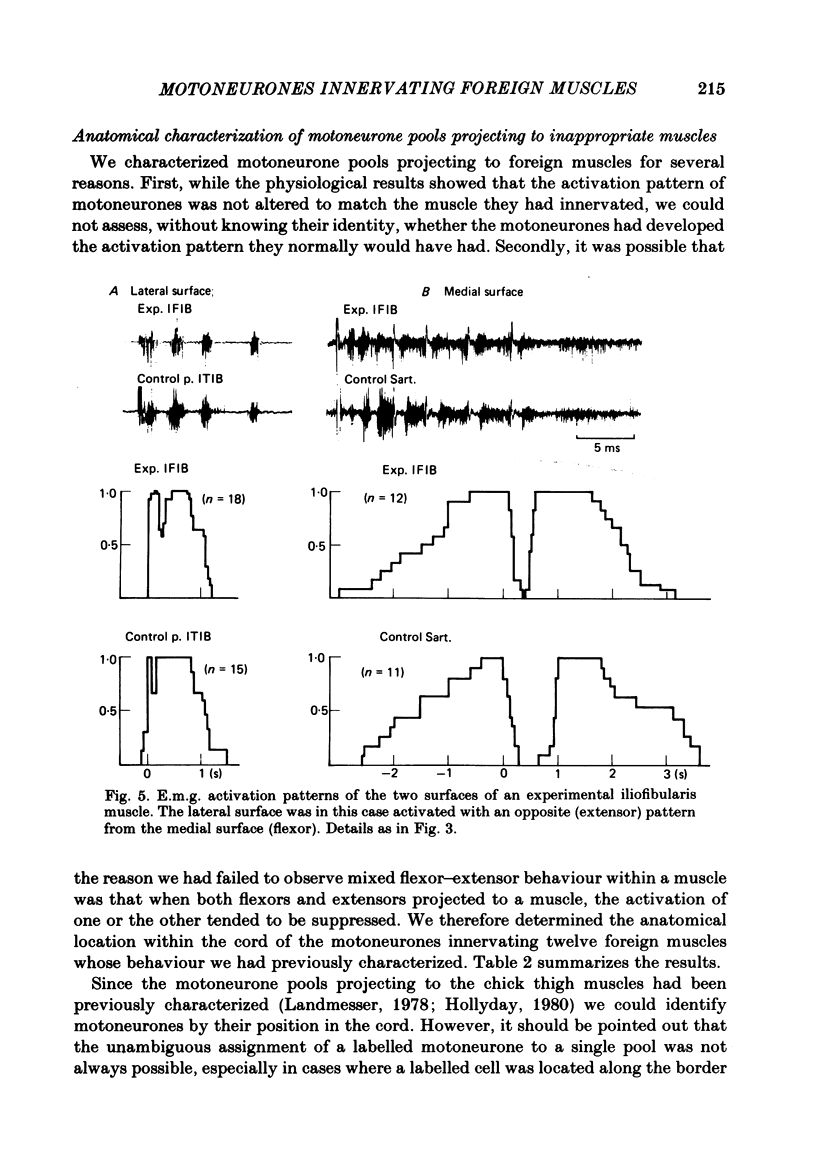


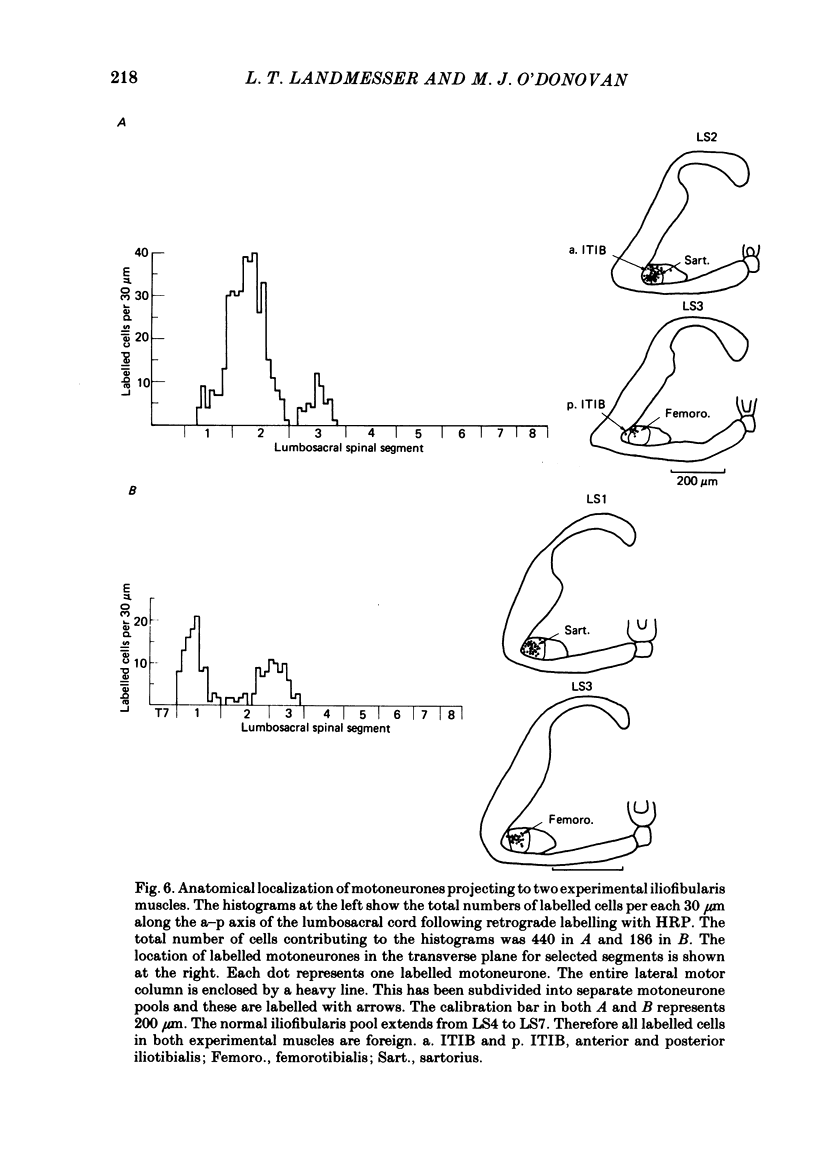
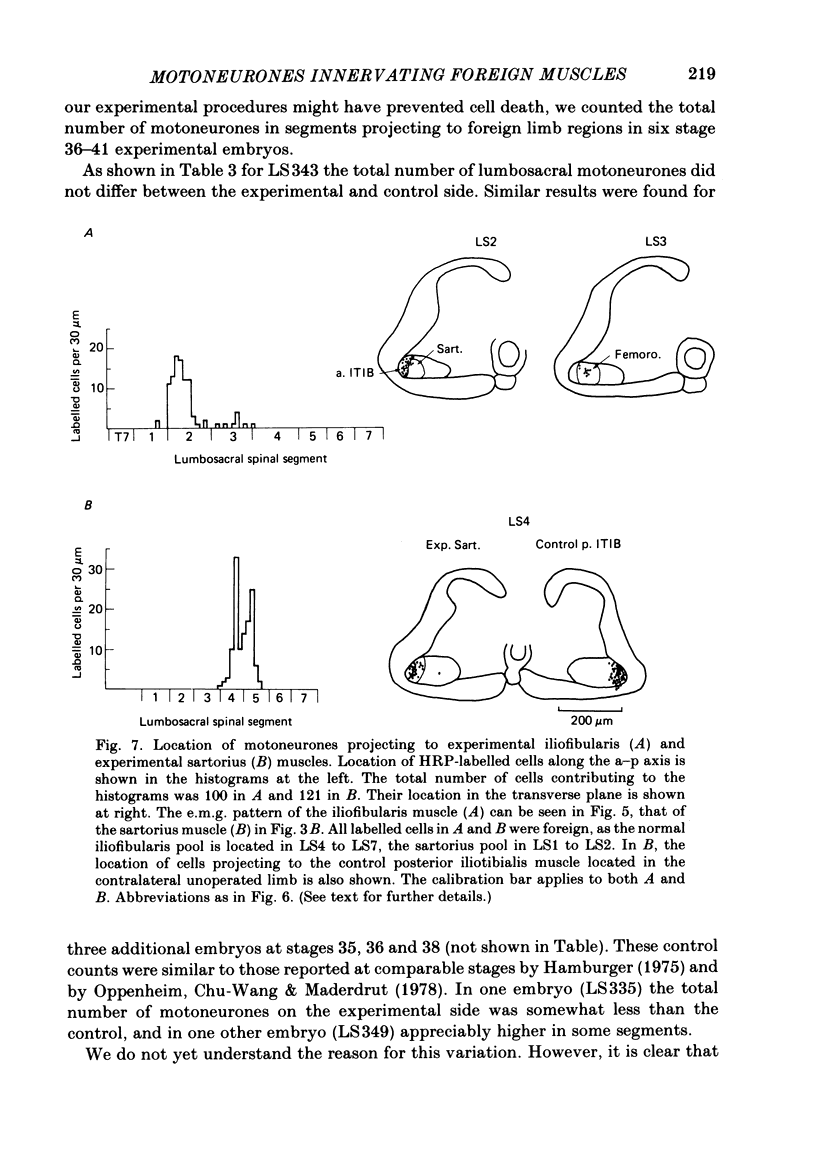




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bekoff A. Ontogeny of leg motor output in the chick embryo: a neural analysis. Brain Res. 1976 Apr 23;106(2):271–291. doi: 10.1016/0006-8993(76)91025-8. [DOI] [PubMed] [Google Scholar]
- Cohen A. H. Functional recovery following cross-reinnervation of antagonistic forelimb muscles in rats. Acta Physiol Scand. 1978 Jul;103(3):331–333. doi: 10.1111/j.1748-1716.1978.tb06220.x. [DOI] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., MAGNI F. Monosynaptic excitatory action on motoneurones regenerated to antagonistic muscles. J Physiol. 1960 Nov;154:68–88. doi: 10.1113/jphysiol.1960.sp006565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., ECCLES R. M., SHEALY C. N., WILLIS W. D. Experiments utilizing monosynaptic excitatory action on motoneurons for testing hypotheses relating to specificity of neuronal connections. J Neurophysiol. 1962 Jul;25:559–580. doi: 10.1152/jn.1962.25.4.559. [DOI] [PubMed] [Google Scholar]
- Frank E., Westerfield M. The formation of appropriate central and peripheral connexions by foreign sensory neurones of the bullfrog. J Physiol. 1982 Mar;324:495–505. doi: 10.1113/jphysiol.1982.sp014126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S., Zangger P. On the central generation of locomotion in the low spinal cat. Exp Brain Res. 1979 Jan 15;34(2):241–261. doi: 10.1007/BF00235671. [DOI] [PubMed] [Google Scholar]
- Grimm L. M. An evaluation of myotypic respecification in axolotls. J Exp Zool. 1971 Dec;178(4):479–496. doi: 10.1002/jez.1401780406. [DOI] [PubMed] [Google Scholar]
- HAMBURGER V., BALABAN M. Observations and experiments on spontaneous rhythmical behavior in the chick embryo. Dev Biol. 1963 Mar;6:533–545. doi: 10.1016/0012-1606(63)90140-4. [DOI] [PubMed] [Google Scholar]
- Hamburger V. Cell death in the development of the lateral motor column of the chick embryo. J Comp Neurol. 1975 Apr 15;160(4):535–546. doi: 10.1002/cne.901600408. [DOI] [PubMed] [Google Scholar]
- Hollyday M., Mendell L. Area specific reflexes from normal and supernumerary hindlimbs of Xenopus laevis. J Comp Neurol. 1975 Jul 15;162(2):205–220. doi: 10.1002/cne.901620204. [DOI] [PubMed] [Google Scholar]
- Hollyday M. Organization of motor pools in the chick lumbar lateral motor column. J Comp Neurol. 1980 Nov 1;194(1):143–170. doi: 10.1002/cne.901940108. [DOI] [PubMed] [Google Scholar]
- Hollyday M. Rules of motor innervation in chick embryos with supernumerary limbs. J Comp Neurol. 1981 Nov 1;202(3):439–465. doi: 10.1002/cne.902020312. [DOI] [PubMed] [Google Scholar]
- Jacobson R. D., Hollyday M. A behavioral and electromyographic study of walking in the chick. J Neurophysiol. 1982 Jul;48(1):238–256. doi: 10.1152/jn.1982.48.1.238. [DOI] [PubMed] [Google Scholar]
- Kleinebeckel D. Movements of supernumerary hindlimbs after innervation by single lumbar spinal nerves of Xenopus laevis. Experientia. 1979 Apr 15;35(4):506–508. doi: 10.1007/BF01922734. [DOI] [PubMed] [Google Scholar]
- Lamb A. H. Evidence that some developing limb motoneurons die for reasons other than peripheral competition. Dev Biol. 1979 Jul;71(1):8–21. doi: 10.1016/0012-1606(79)90078-2. [DOI] [PubMed] [Google Scholar]
- Lamb A. H. The projection patterns of the ventral horn to the hind limb during development. Dev Biol. 1976 Nov;54(1):82–99. doi: 10.1016/0012-1606(76)90288-8. [DOI] [PubMed] [Google Scholar]
- Lance-Jones C., Landmesser L. Pathway selection by embryonic chick motoneurons in an experimentally altered environment. Proc R Soc Lond B Biol Sci. 1981 Dec 9;214(1194):19–52. doi: 10.1098/rspb.1981.0080. [DOI] [PubMed] [Google Scholar]
- Landmesser L. T., O'Donovan M. J. Activation patterns of embryonic chick hind limb muscles recorded in ovo and in an isolated spinal cord preparation. J Physiol. 1984 Feb;347:189–204. doi: 10.1113/jphysiol.1984.sp015061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landmesser L. The distribution of motoneurones supplying chick hind limb muscles. J Physiol. 1978 Nov;284:371–389. doi: 10.1113/jphysiol.1978.sp012545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan I. S. Differentiation of muscle fiber types in the chicken hindlimb. Dev Biol. 1983 May;97(1):222–228. doi: 10.1016/0012-1606(83)90079-9. [DOI] [PubMed] [Google Scholar]
- Mendell L. M., Scott J. G. The effect of peripheral nerve cross-union on connections of single Ia fibers to motoneurons. Exp Brain Res. 1975 Mar 27;22(3):221–234. doi: 10.1007/BF00234765. [DOI] [PubMed] [Google Scholar]
- Oppenheim R. W., Chu-Wang I. W., Maderdrut J. L. Cell death of motoneurons in the chick embryo spinal cord. III. The differentiation of motoneurons prior to their induced degeneration following limb-bud removal. J Comp Neurol. 1978 Jan 1;177(1):87–111. doi: 10.1002/cne.901770107. [DOI] [PubMed] [Google Scholar]
- Pettigrew A. G., Lindeman R., Bennett M. R. Development of the segmental innervation of the chick forelimb. J Embryol Exp Morphol. 1979 Jan;49:115–137. [PubMed] [Google Scholar]
- Pittman R., Oppenheim R. W. Cell death of motoneurons in the chick embryo spinal cord. IV. Evidence that a functional neuromuscular interaction is involved in the regulation of naturally occurring cell death and the stabilization of synapses. J Comp Neurol. 1979 Sep 15;187(2):425–446. doi: 10.1002/cne.901870210. [DOI] [PubMed] [Google Scholar]
- Purves D., Lichtman J. W. Elimination of synapses in the developing nervous system. Science. 1980 Oct 10;210(4466):153–157. doi: 10.1126/science.7414326. [DOI] [PubMed] [Google Scholar]
- Summerbell D., Stirling R. V. The innervation of dorsoventrally reversed chick wings: evidence that motor axons do not actively seek out their appropriate targets. J Embryol Exp Morphol. 1981 Feb;61:233–247. [PubMed] [Google Scholar]
- Székely G., Czéh G. Muscle activities of partially innervated limbs during locomotion in ambystoma. Acta Physiol Acad Sci Hung. 1971;40(3):269–286. [PubMed] [Google Scholar]
- Tsukahara N., Fujito Y. Physiological evidence of formation of new synapses from cerebrum in the red nucleus neurons following cross-union of forelimb nerves. Brain Res. 1976 Apr 16;106(1):184–188. doi: 10.1016/0006-8993(76)90085-8. [DOI] [PubMed] [Google Scholar]