ABSTRACT
Silique dehiscence, despite being an essential physiological process for seed dispersal for dehiscent fruits, is disadvantageous for the agricultural industry. While crops have been selected against the expression of natural, spontaneous shattering to protect the seeds for harvest, fruit dehiscence in the field can be promoted through abiotic factors such as wind, drought, and hail that can be detrimental in reducing crop yield and profitability. In crops like canola, pennycress, and Camelina, this impact could be as high as 50%, creating economic losses for both the industry and the economy. Mitigating the effects of fruit dehiscence is crucial to prevent seed loss, economic loss, and the persistence of volunteer plants, which interfere with crop rotation and require increased weed control. Developing agronomic traits through genetic manipulation to enhance the strength of the fruiting body can prevent seed dispersal mechanisms from occurring and boost yield efficiency and preservation. Current research into this area has created mutant plants with indehiscent fruits by reducing allele function that determines the identity of the various anatomical layers of the fruit. Future genetic approaches may focus on strengthening siliques by enhancing secondary cell walls through either increased lignification or reducing cell wall‐degrading enzymes to achieve shatter tolerance. This review focuses on improving our knowledge within members of the Brassicaceae family to create a better understanding of silique/silicle dehiscence for researchers to establish a groundwork for broader applications across diverse crops. This knowledge will directly lead to improved agricultural productivity and ensure a stable food supply, addressing global challenges the world is facing.
Keywords: agriculture production, cell wall, lignification, oilseed crops, silique dehiscence, yield increases
1. Introduction
Silique dehiscence is necessary for seed dispersal at the end of a dehiscent plant's lifecycle. While this process is critical for seed dispersal, this trait is disadvantageous in cropping systems due to the need to retain the seeds in the siliques for harvesting. Therefore, cultivated crops have been naturally selected or bred against the spontaneous seed dispersal trait. Despite this, premature dispersal can still occur because of abiotic factors, such as wind, drought, and hail that can be detrimental to crop yield. Mitigating the effects of fruit dehiscence is important because seed loss, in addition to economic losses, can lead to volunteer seeds persisting in the field, giving rise to plants that interfere with crop rotation and herbicide selection (Price et al. 1996). These volunteer plants have been shown to demand increased weed control measures and even have phytotoxic effects on the subsequent generations (Price et al. 1996; Gan et al. 2008; The Canola Council of Canada 2024). Preventing silique and silicle dehiscence by developing novel agronomic traits in siliques can have several benefits, ranging from increased yield efficiencies, yield preservation, and reduction of volunteer plants (Price et al. 1996; Gan et al. 2008; The Canola Council of Canada 2024). Within the Brassicaceae system (Zuñiga‐Mayo et al. 2018), the model plant Arabidopsis thaliana has been used primarily to translate the regulatory network governing silique development to canola and other cash crops (Liljegren et al. 2000; Rajani and Sundaresan 2001; Ogawa et al. 2009; Groszmann et al. 2011; Lenser and Theißen 2013; Braatz et al. 2018; Zuñiga‐Mayo et al. 2018; Di Vittori et al. 2019). One aspect of this dehiscence trait that has not been explored sufficiently is the lignification of specific valve layers that are critical for dehiscence. The fundamental research in A. thaliana can therefore be used as a foundation for the elucidation of genes associated with lignification in addition to other processes related to fruit dehiscence. This is of utmost importance due to our ever‐changing climate and exponential population growth, making it crucial to increase food security and sustainability.
2. Silique and Silicle Morphology in Brassicaceae
Much of our understanding of silique dehiscence is based on previous work done in A. thaliana , because it is a closely related species to many important oil and cash crops in the Brassicaceae family. Therefore, it is an ideal candidate for understanding this complex fruit development–associated process. Researchers have classified the development of the flowering and fruiting body into 20 different stages, with flower development beginning at Stages 1–12 and silique development from Stages 13–20 (Smyth et al. 1990). The developmental stages are based on landmark morphological events that define each stage (Smyth et al. 1990). An in‐depth look into fruit development within Arabidopsis and of the aforementioned stages has been conducted by Roeder and Yanofsky (2006) and Herrera‐Ubaldo and Folter (2022). Other work focuses primarily on silique development as days post anthesis (DPA), which refers to flower bud opening, and also days after pollination (DAP), after the flower has been pollinated (Vivian‐Smith and Koltunow 1999; Mizzotti et al. 2018; Nichol and Samuel 2024). Fundamentally, these two classification systems offer similar results because DPA and DAP occur concomitantly in A. thaliana (Herrera‐Ubaldo and Folter 2022). The silique matures from a gynoecium, containing the stigma, style, ovary, and gynophore (Ferrándiz et al. 1999). In brief, the gynoecium of A. thaliana is syncarpous with an axile ovary placentation, which contains two locules (Herrera‐Ubaldo and Folter 2022). For a more in‐depth description of gynoecium development, refer to the review by Herrera‐Ubaldo and Folter (2022), which offers a complete overview of the process.
In Arabidopsis, Cardamine hirsuta, and the Brassicas, the ovary space of the silique contains two chambers separated by a false partition, the septum, which attaches between two replum regions on either side (Spence et al. 1996; Dinneny et al. 2005) (Figure 1). The walls of the ovary space are made up of two crescent‐shaped valves that merge at the replum's central seam via the valve margins that run in parallel up the length of the silique from the gynophore to the base of the style (Spence et al. 1996; Dinneny et al. 2005) (Figure 1). Seeds develop within the two chambers of the ovary space, protected by the valve, and fed through the funiculus that develops from the septum (Spence et al. 1996; Dinneny et al. 2005) (Figure 1). The replum provides the main vasculature for the developing silique (Alvarez and Smyth 2002). The valve and replum merge at the valve margin, which consists of two distinct cell layers, the separation layer (SL) and the lignified layer (LL) (Ferrándiz et al. 1999; Liljegren et al. 2004; Roeder and Yanofsky 2006) (Figure 1). The SL is formed by two layers of cells that run along the length of the replum and is the site where specific cell wall degrading enzymes act (Rajani and Sundaresan 2001; Liljegren et al. 2004; Roeder and Yanofsky 2006) (Figure 1). This is often referred to as the dehiscence zone (DZ), where separation occurs allowing the silique to open (Spence et al. 1996) (Figure 1). The LL undergoes lignification on the outer side of the SL (Liljegren et al. 2004; Roeder and Yanofsky 2006) (Figure 1).
FIGURE 1.
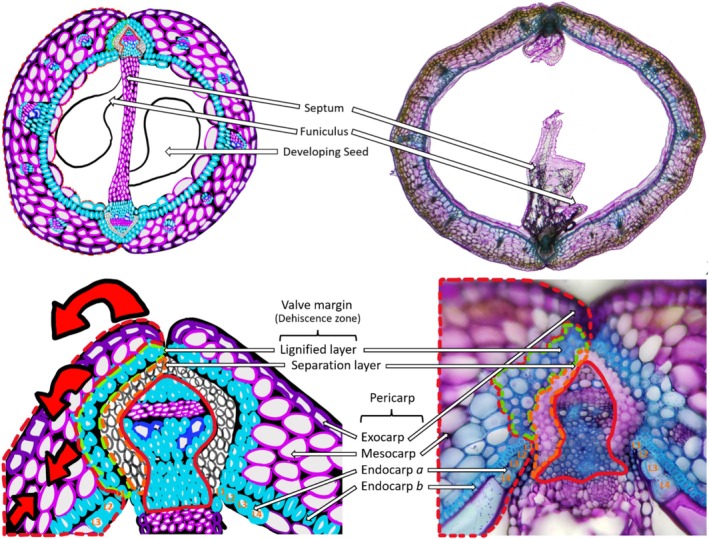
An illustrative figure of a silique cross‐section and B. napus cross‐section. The two images are representative of a silique cross‐section. The illustrative images are false‐colored to mimic toluidine blue O staining, with the pink/purple color representing the primary cell wall and the blue‐colored cells indicating secondary cell wall lignification. The real B. napus cross‐sections are stained with toluidine blue O. The red dotted line indicates the valve. The green dotted line indicates the lignified layer. The orange dotted line indicates the separation layer. The red line indicates the replum.
The valve pericarp consists of three different cell layers that vary in size, consisting of the exocarp, mesocarp, and endocarp (Rajani and Sundaresan 2001; Liljegren et al. 2004; Roeder and Yanofsky 2006) (Figure 1). The exocarp is the outermost cell layer that consists of a file of rectangular‐shaped cells, which contain a thick layer of epicuticular wax and a waxy cuticular layer protecting the silique and seeds from biotic and abiotic factors, such as pathogens, pests, and intense weather conditions (Rajani and Sundaresan 2001; Liljegren et al. 2004; Roeder and Yanofsky 2006) (Figure 1). The exocarp also contains stomata required for transpiration and respiration (Rajani and Sundaresan 2001; Liljegren et al. 2004; Roeder and Yanofsky 2006). The mesocarp is the middle cell layer that comprises most of the valve tissue, usually consisting of three layers of thin‐walled parenchyma cells (Rajani and Sundaresan 2001; Liljegren et al. 2004; Roeder and Yanofsky 2006) (Figure 1). It is the primary photosynthetic tissue layer within the valve and serves as an important cell layer during silique drying and dehiscence, providing the tensile forces necessary on the SL for silique dehiscence to occur later in maturation (Rajani and Sundaresan 2001; Liljegren et al. 2004; Roeder and Yanofsky 2006) (Figure 1).
It has been shown in the Triangle of U species that a portion of the mesocarp cells can undergo further lignification as the silique begins to mature (Nichol and Samuel 2024). This lignification process can be seen as early as 20 DAP to its maximum at 35 DAP (Nichol and Samuel 2024) in Brassica species. Within A. thaliana and the Brassicas, the endocarp cell layer consists of two different cell types that arise from a periclinal division giving rise to the endocarp a and b cell layers, with endocarp b making up small uniform lignified sclerenchyma cells and endocarp a located closest to the ovary space making up large rotund thin‐walled parenchymal cells (Spence et al. 1996; Rajani and Sundaresan 2001; Nichol and Samuel 2024) (Figure 1). The endocarp a cell layer is known to degrade within A. thaliana and other closely related species within the Triangle of U and the broader Brassicaceae family (Spence et al. 1996; Nichol and Samuel 2024). During this process of degradation, it is consistently observed that cells of the endocarp a layer closest to the replum can get lignified prior to the degradation process (Nichol and Samuel 2024) (Figure 1). The number of endocarp a cells that are lignified can be specific to the species, where the closest lignified endocarp a cell adjacent to the replum is defined as L1, with each subsequent lignified cell increasing the value numerically (Nichol and Samuel 2024). This means that the second and third endocarp a cells that are lignified would be defined as L2 and L3, respectively, until the maximum number of lignified cells is reached (Nichol and Samuel 2024). This number of endocarp a lignified cells varies from one to five depending on the stage of the maturation process and the species. In Arabidopsis, this lignification appears at 8 DAP, and by 10 DAP reaches the maximum of three cells (L1, L2, L3) lignified (Nichol and Samuel 2024) (Figure 1). Both the lignified endocarp a cells and the lignified mesocarp cells are thick‐walled parenchyma cells with identifiable plasmodesmatal pits (Nichol and Samuel 2024). Nichol and Samuel (2024) also proposed that the degradation of the endocarp a cell layer seems to follow the temporal deposition of lignification both within the endocarp b and a L1/2/3 cells within A. thaliana and the other Brassica species.
The lignification patterning within the endocarp b cell layer differs among other Brassicaceae members, such as C. hirsuta , where lignin is deposited within a V‐shaped or teardrop‐shaped pattern when looked at from a cross‐sectional point of view (Vaughn et al. 2011). Additionally, between the endocarp a and b cell layer regions, there is a large accumulation of mucilaginous pectin that is missing from A. thaliana (Vaughn et al. 2011). It is believed that this mucilaginous region could be thought of as an extension of the middle lamella, giving the endocarp b cell layer the ability to resist mechanical tensile forces (Vaughn et al. 2011). Additionally, it has been shown that in other plant species, Litchi chinensis and Dimocarpus longan , the endocarp a cell layer is made up of a waxy cuticular layer that is much thinner than the exocarp cell layer (Riederer et al. 2015). The endocarp a cell layer harbors very long‐chained aliphatic and cyclic compounds, which are believed to be the lipids associated with the makeup of this waxy layer (Riederer et al. 2015).
The difference between a silique and a silicle is the width versus the length of the fruiting body, with the silique being > 3× the length than its width and a silicle being < 3× the length than its width (Simpson 2010). Although this botanical definition for a silicle changes depending on the source that is used. The Merriam‐Webster's Dictionary definition is as follows: a silicle is a silique of nearly equal length and width (Merriam‐Webster 2025). While the Missouri Botanical Garden refers to a silicle as less than twice as long as wide (Missouri Botanical Garden 2025). Regardless of these definitions, generally, the silicle is described as a globular and/or rounded flat fruit, depending on the species; containing two loculate ovary spaces that are separated by a false partition in the form of a septum, they additionally contain two valves that converge at a replum central seam, similar to what is seen in A. thaliana and the other Brassicaceae (Simpson 2010; Jankowski et al. 2019). Silicle fruit morphology differs from that of the silique, where developmental transitions early on in the gynoecium give rise to the oblate spheroid shape seen in species such as Camelina sativa (camelina), Thlaspi arrense (pennycress), and Capsella rubella (capsella) (Eldridge et al. 2016). This development of the gynoecium continues more predominantly in Capsella, where unique shoulder‐like features begin to appear that create a heart‐shaped silicle (Eldridge et al. 2016). Little is known about the inner silicle morphology of species such as Camelina, pennycress, and Capsella. Recent research conducted by Chopra et al. (2020) has shown a cross‐sectional view of a pennycress silicle where the replum and valve morphology are quite similar to that of A. thaliana siliques with a noticeable exocarp, mesocarp, and endocarp a and b cell layer as well as lignification within the endocarp b cell layer and LL. The replum and valve regions are also visibly shown to come together in a DZ‐like pattern, yet whether these regions actually function similar to A. thaliana remains unknown, and further research is needed (Chopra et al. 2020).
3. Molecular Pathway Involved in Silique Dehiscence
3.1. Cell Differentiation Genes and Silique Development, a Nexus of Repressors That Define Layer Identity
A host of genes precisely regulate silique patterning, which has been extensively studied and been the major focus of understanding the function of fruit formation in Arabidopsis (Rajani and Sundaresan 2001; Liljegren et al. 2000; Ferrándiz, Gu, et al. 2000; Dinneny et al. 2005; Arnaud et al. 2010; Groszmann et al. 2011; Jaradat et al. 2014). FRUITFULL (FUL) is a member of the MCM1 AGAMOUS DEFICIENS SRF box (MADS‐box) gene family with expression throughout the plant but playing a specific role within the valve identity (Alvarez and Smyth 1997) (Figure 2). Early research into FUL determined that it promotes normal valve differentiation and development along with controlling the extension of the silique; this is due to the ful‐1 knockout phenotype in Arabidopsis displaying shortened siliques (Gu et al. 1998; Ferrándiz, Gu, et al. 2000). The resulting ful‐1 mutants did not create noticeable phenotypes within the replum region, displaying valve specificity because it grew to normal wild‐type size, creating a zig‐zag‐like patterned seam across the silique surface due to the shortened silique size (Gu et al. 1998). Several phenotypic changes occur within the ful‐1 mutant, including ectopic lignification of the valve mesophyll cells and an increased number of endocarp a cells within the cell layer while simultaneously having reduced size and shape of the cell itself (Gu et al. 1998). The activation temporally and spatially of FUL within other Brassicaceae members such as Capsella is important in the unique heart‐shaped silicle (Eldridge et al. 2016). Crful‐1 mutants displayed no shoulder formation and remained rounded after fertilization, indicating CrFUL fruit shape is modulated during the late phase when the silicle transforms from an oblate spheroid to a heart‐shaped fruit (Eldridge et al. 2016). The MADS‐box gene family members downstream of FUL, SHATTERPROOF1 and 2 (SHP1/2) are involved in the differentiation of the DZ, promoting lignification of the LL, and promoting the formation of the valve margin (Bowman et al. 1991; Kempin et al. 1995; Purugganan 1997; Riechmann and Meyerowitz 1997; Ferrándiz, Gu, et al. 2000; Dinneny et al. 2005; Ferrándiz and Fourquin 2014) (Figure 2). It has been shown that there is no phenotypic difference between the shp1 and shp2 single mutants compared with wild‐type plants, while the double mutant of shp1/2 creates indehiscent siliques (Liljegren et al. 2000). This is often attributed to the high functional redundancy of the genes, with a shared homology of 87% at the amino acid level (Ma et al. 1991; Savidge et al. 1995; Flanagan et al. 1996). In shp1/2 double mutants, there is reduced lignification of the LL and an absence of the DZ (Liljegren et al. 2000). Studies have shown that FUL negatively regulates SHP1 and SHP2, with a 35s::FUL line showing similar phenotypic effects to that of the double mutant line of shp1/2 with reduced lignification in the LL and a disruption in the DZ formation (Liljegren et al. 2000) (Figure 2). The overexpression line of 35s::FUL and the double mutant of shp1/2 both produced siliques that were indehiscent, suggesting that FUL and SHP interact antagonistically during valve margin development (Ferrándiz, Liljegren, and Yanofsky 2000) (Figure 2). Subsequent research determined that expression of SHP1 and SHP2 is repressed by FUL in the valves and by APETALA1 (AP1) in the outer whorls of the flower (Ferrándiz, Liljegren, and Yanofsky 2000; Kaufmann et al. 2010).
FIGURE 2.
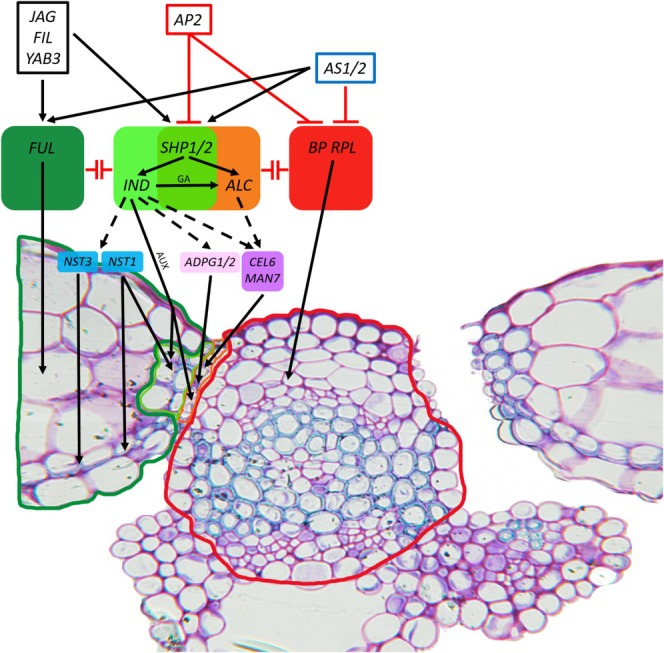
A model of Arabidopsis silique development and differentiation. The model depicts master regulatory transcription factors of silique development and differentiation, along with downstream interactions between opposing cell identity regulators creating expression gradients between the valve, valve margin, and replum. Known downstream cell wall modification enzymes and transcription factors are depicted. Solid lines indicate known pathways. Dotted lines indicate hypothetical interactions. The solid dark green line indicates the valve. The solid light green line indicates the lignified layer. The solid orange line indicates the separation layer. The solid red line indicates the replum. The silique is stained with toluidine blue O, with the pink/purple color representing the primary cell wall and the blue‐colored cells indicating secondary cell wall lignification.
The relationship between valve differentiation and valve margin formation is dependent on the formation of the replum. REPLUMLESS (RPL) encodes a transcription factor that belongs to the BEL1‐like family (Western and Haughn 1999). The activity of RPL regulates the SHP gene to control the development of the replum, similar to the antagonistic relationship between FUL and SHP mentioned above (Roeder et al. 2003; Ferrándiz, Liljegren, and Yanofsky 2000) (Figure 2). The rpl knockout had a complete loss of the replum structure, with the cells in the place of the replum resembling that of the valve margin (Roeder et al. 2003). SHP2::GUS lines demonstrated that the valve margin genes were likely ectopically expressed within the replum layer, converting them to valve margin cells in the rpl knockout (Savidge et al. 1995; Roeder et al. 2003). The triple mutant knockout with rpl and shp1/2 results in the appearance of a wild‐type replum (Roeder et al. 2003). This meant that SHP1/2 were responsible for the loss of the replum identity within the rpl mutant, demonstrating that the role of RPL is necessary to prevent the expression of SHP1/2 within the replum region (Roeder et al. 2003) (Figure 2). A class 1 KNOX (KNOTTED‐LIKE HOMEOBOX) gene BREVIPEDICELLUS (BP), also known as KNAT1, is expressed within the replum, with overexpression lines having greatly enlarged replum regions, and when there is a bp/rpl double knockout, the resulting replum‐deficient phenotype is enhanced (Lincoln et al. 1994; Chuck et al. 1996; Douglas et al. 2002; Venglat et al. 2002; Alonso‐Cantabrana et al. 2007). The MYB transcription factor ASYMMETRIC LEAVES1 (AS1), along with the LATERAL ORGAN BOUNDARY (LOB) domain protein ASYMMETRIC LEAVES2 (AS2), negatively regulates BP, with as1 and as2 mutations leading to an enlarged replum region and reduced valve size (Byrne et al. 2000; Sun et al. 2002; Iwakawa et al. 2002; Shuai et al. 2002; Alonso‐Cantabrana et al. 2007; Guo et al. 2008) (Figure 2).
A downstream regulator of valve margin identity is ALCATRAZ (ALC), with ALC encoding a myc/basic‐helix–loop–helix (bHLH)‐related transcription factor expressed within the valve margin of the silique during silique dehiscence, associated with the development of the SL (Liljegren et al. 2000; Rajani and Sundaresan 2001; Dinneny et al. 2005; Tang et al. 2007) (Figure 2). Within alc mutants, the identity of the SL seems to be split between non‐lignified replum‐like cells from the midpoint of the valve to the epidermis and lignified cells from the midpoint of the valve towards the ovary space (Gu et al. 1998). The lack of an SL identity leads to the alc mutant having indehiscent siliques (Rajani and Sundaresan 2001). The SPATULA (SPT) gene is a conserved bHLH protein that is partially redundant to ALC (Groszmann et al. 2008; Groszmann et al. 2010; Groszmann et al. 2011). Because both alc and spt single knockout mutants have abnormal valve margin identity, the contributions that ALC and SPT genes have within the valve margin can be parsed through a ful mutant background (Liljegren et al. 2004). The ful/alc double mutant has a reduced ectopic valve margin despite having a functional SPT gene (Liljegren et al. 2004). Research done by Groszmann et al. (2011) suggests that ALC and SPT redundancy does not extend to later DZ differentiation and that ALC and SPT may have a dosage dependency within early valve margin development.
Similar to ALC, the highly investigated and translationally exploited INDEHISCENT (IND) gene encodes an atypical bHLH, with an alanine residue within the DNA binding region where normally a glutamic acid resides (Fisher and Goding 1992; Buck and Atchley 2003; Toledo‐Ortiz et al. 2003; Liljegren et al. 2004). IND is responsible for the differentiation of the valve, replum, and valve margin; layers critical for seed dispersal (Liljegren et al. 2004; Wu et al. 2006; van Gelderen et al. 2016) (Figure 2). The ind mutant revealed serious defects within the valve margin with no distinctive SL and no lignification within the LL, leading to an indehiscent silique phenotype (Liljegren et al. 2000; Liljegren et al. 2004). This research suggests that IND is important in facilitating cells to adopt a valve margin identity (Liljegren et al. 2000; Liljegren et al. 2004; Rajani and Sundaresan 2001) (Figure 2). Researchers have also discovered that the interactions between IND and SPT promote valve margin development by regulating auxin formation (Girin et al. 2011) (Figure 2). Valve margin identity genes, such as IND, also play a role in negatively regulating replum identity (Girin et al. 2010) (Figure 2). Additionally, IND promotes GA accumulation directly by affecting the gibberellin biosynthesis enzyme GA3OX1, leading to GA promoting the degradation of the DELLA protein bound to ALC, allowing ALC to act on its downstream processes, leading to SL formation (Arnaud et al. 2010; Ballester and Ferrándiz 2017) (Figure 2). A master regulator upstream of BP, RPL, IND, and SHP1/2 known as APETALA2 (AP2) controls the replum growth by inhibiting BP and RPL expression because in ap2 knockouts the size of the replum increased compared with wild‐type plants (Ripoll et al. 2011) (Figure 2). Additionally, the research by Ripoll et al. (2011) determined that AP2 negatively regulates valve margin formation through IND and SHP1/2 (Figure 2). Reporter expression of SHP and IND is increased within the ap2 knockout mutant compared with wild‐type plants, with the ap2 mutant also leading to increases in both the size and number of lignified cells within the valve margin (Ripoll et al. 2011). The loss of replum and valve margin formation in the 35s::FUL overexpression line was overcome by placing the overexpression line within the ap2 background because AP2 was no longer able to suppress BP/RPL and IND/SHP required for replum valve margin formation, respectively (Ripoll et al. 2011) (Figure 2).
An overview of the work done above can be represented briefly here. The main tissues within the silique are the valve, valve margin, and replum (Figure 1). The expression of FUL is important for valve development and differentiation, additionally playing a role in the negative regulation of SHP1 and SHP2, preventing valve cells from adopting valve margin cell identity (Gu et al. 1998; Ferrándiz, Liljegren, and Yanofsky 2000) (Figure 2). For proper replum development to occur, RPL and BP expression must be present to prevent the formation of valve margin cells within the replum region from SHP1 and SHP2 (Douglas et al. 2002; Venglat et al. 2002; Chuck et al. 1996; Lincoln et al. 1994; Alonso‐Cantabrana et al. 2007; Ripoll et al. 2011) (Figure 2). SHP1 and SHP2 regulate downstream genes IND, regulating the identity of the valve margin and subsequent differentiation into the LL and SL, and ALC, regulating the identity of the SL (Bowman et al. 1991; Kempin et al. 1995; Purugganan 1997; Riechmann and Meyerowitz 1997; Ferrándiz, Gu, et al. 2000; Dinneny et al. 2005; Tang et al. 2007; Ferrándiz and Fourquin 2014) (Figure 2). FUL negatively regulates SHP1 and SHP2 expression so that valve identity is not lost (Liljegren et al. 2000) (Figure 2). FUL and SHP expression is also promoted by JAGGED (JAG), FILAMENTOUS FLOWER (FIL), YABBY3 (YAB3), AS1, and AS2 (Sawa et al. 1999; Eshed et al. 2004; Dinneny et al. 2004; Ohno et al. 2004; Alonso‐Cantabrana et al. 2007) (Figure 2). While the expression of BP is negatively regulated by AS1 and AS2 (Alonso‐Cantabrana et al. 2007) (Figure 2). AP2 negatively regulates SHP and IND to regulate valve margin formation (Ripoll et al. 2011) (Figure 2). Additionally, AP2 negatively regulates BP and RPL to reduce the size of the replum (Ripoll et al. 2011) (Figure 2). A model proposed by Ripoll et al. (2011) suggests that the development of the valve, valve margin, and replum in plants is driven by the interaction of opposing factors, each promoting either valve or replum formation. These factors create gradients of activity; high valve factor activity defines valve identity, high replum factor activity defines replum identity, and valve margins form where both factors are weakly expressed. When valve factors are mis‐expressed, they repress replum and valve margin identities. Conversely, misexpression of replum factors represses valve identity.
3.2. Genes Associated With the Process of Silique Dehiscence
Silique dehiscence is primarily coordinated through the proper cell development and specification events from the DZ, but additional effector genes downstream add to the secondary coordinated level of silique dehiscence. Although several transcription factors define the intricate organization of the valve layer, the major process of silique dehiscence is primarily coordinated through cell wall rupture of cells in SL via cell wall degrading enzymes, which play a major role in this process. The coordinated cellular degradation of the SL is performed by degrading the cellulose, hemicellulose, pectin, and xyloglucan of the cells residing within this region. The first instance of a cell wall degrading enzyme was polygalacturonase (PG) activity documented within the DZ of Brassica napus by Jenkins et al. 1996 and Petersen et al. 1996. The expression patterning of PGs within the SL just prior to silique dehiscence was first demonstrated by Sander et al. 2001. In Arabidopsis, ARABIDOPSIS DEHISCENCE ZONE POLYGALACTURONASE1 (ADPG1) was initially suggested to be involved in silique dehiscence based on its expression pattern within the DZ (González‐Carranza et al. 2007). Additional evidence was provided by the lack of expression of ADPG1 present within ind mutants (Ogawa et al. 2009). In 2009, Ogawa et al. (2009) demonstrated that two PGs, ADPG1 and ADPG2, expressed within the DZ of mature siliques, were involved in silique dehiscence. The double knockout of adpg1/2 resulted in an indehiscent silique due to the lack of pectinase activity within the DZ (Ogawa et al. 2009; He et al. 2018). Ogawa et al. (2009) also determined that the role of ADPG1 and ADPG2 differed in silique dehiscence due to expression levels, with ADPG1 having a major impact compared with ADPG2. Additional work completed by He et al. (2018) confirmed this with an adpg1 knockout demonstrating incomplete dehiscent siliques and an adpg2 knockout that exhibited complete dehiscence with collapsed cells within the SL. Moreover, post‐translational modifications of PGs were demonstrated by Degan et al. (2001), where a cleavable N‐terminal domain is removed, leading to subsequent mature protein to be transported extracellularly. Pectin methylesterases may be associated with middle lamella degradation during dehiscence, although no direct evidence of this has been demonstrated (Jaradat et al. 2014).
He et al. (2018) expanded our knowledge of the various cell wall degrading enzymes functioning within the DZ of the silique with the addition of the cellulase gene CELLULASE6 (CEL6) and the hemicellulase gene MANNANASE7 (MAN7). The expression of CEL6 and MAN7 was partially dependent on the IND and ALC transcription factors (He et al. 2018). Cell wall degradation in the SL in nearly mature siliques was promoted by CEL6 and MAN7 because cel6 and man7 single knockouts along with the cel6/man7 double knockout displayed an intact SL, but the siliques could still dehisce but at low rates (He et al. 2018). The degree to which the SL was degraded and the level to which the silique can dehisce was also manipulated through a set of single, double, and triple mutants of the various cell wall degrading enzymes (He et al. 2018). The effects of CEL6, MAN7, and ADPG1 are additive because the man7/adpg1 double knockout showed more intact cells in the SL than adpg1 alone, while the cel6/adpg1 double knockout had mostly intact cells at the SL (He et al. 2018). The triple mutant cel6/man7/adpg1 closely resembled the cel6/adpg1 double mutant (He et al. 2018). Comparatively, ADPG1 is of critical importance in middle lamella degradation, while CEL6 and MAN7 are specific to cell lysis, with CEL6 having a greater role in this process than MAN7 (He et al. 2018). RNAi lines of MAN7 in B. napus were also shown to promote silique indehiscence (Li et al. 2021). Xyloglucan endotransglycosylase was determined to be another cell wall degrading enzyme shown within the DZ of B. napus siliques (Fry et al. 1992; Roberts et al. 2000). The antagonistic relationship between known plant hormones, ethylene and auxin, aids in promoting and inhibiting the DZ through cell separation timing, respectively (González‐Carranza et al. 1998; Child et al. 1998). A decrease in the auxin concentration increases cellulase activity within the DZ, while the application of exogenous auxin analogs inhibits cellulase activity and pectinase secretion, ultimately delaying DZ cell separation (Degan et al. 2001).
Another essential process necessary to allow for proper seed dispersal occurs through NAC SECONDARY WALL THICKENING PROMOTING FACTOR 1 and 3 (NST1/3) genes, which have been shown to play important roles in the SCW thickening within specific tissues (Mitsuda et al. 2005; Mitsuda et al. 2007; Mitsuda and Ohme‐Takagi 2008). NST1 promotes SCW thickening and formation within the LL of the valve margin and the endocarp b cell layer within A. thaliana (Mitsuda and Ohme‐Takagi 2008). Research done by Zhong et al. (2006) described a role for the SECONDARY WALL–ASSOCIATED NAC DOMAIN PROTEIN‐1 (SND1) in endocarp b lignification. It should be noted that NST3 and SND1 are the same gene but were designated differently by the two groups (Mitsuda and Ohme‐Takagi 2008). SND1/NST3 promotes SCW thickening and formation within the endocarp b cell layer (Mitsuda and Ohme‐Takagi 2008; Zhong et al. 2006). The NST1 and SND1/NST3 genes function as the first layer of the regulatory network for SCW deposition, classified by Zhang et al. (2018). The double knockout of nst1/3 leads to a silique with a complete lack of lignification within the LL and endocarp b cell layer, leading to silique indehiscence (Mitsuda and Ohme‐Takagi 2008). Studies conducted by Zhong et al. (2007) and McCarthy et al. (2009) revealed that MYB46 and MYB83 are direct targets of SND1/NST3, which play a key role in activating SCW biosynthesis. This was demonstrated through the knockout lines of myb46 and myb83, where a marked reduction in SCW size was observed (Zhong et al. 2007; McCarthy et al. 2009).
In C. hirsuta , unique asymmetrical polar deposition of lignin within the endocarp b cell layer is controlled through LACCASES (LACs) 4, 11, and 17 (Pérez‐Antón et al. 2022). LAC4/11/17 localize in the zones with asymmetric lignification within endocarp b cells (Pérez‐Antón et al. 2022). Interestingly, when LAC4 and LAC17 were expressed under their native promoters in A. thaliana , non‐polar localization of lignification was seen (Pérez‐Antón et al. 2022). A known transcription factor, SQUAMOSA PROMOTER‐BINDING PROTEIN‐LIKE 7 (SPL7), that regulates copper (Cu) homeostasis, when mutated, was shown to limit the range of the explosive seed dispersal force (Pérez‐Antón et al. 2022). This was due to spl7 exhibiting a silique bulking phenotype that affects the amount of stored potential elastic energy for explosive seed dispersal (Pérez‐Antón et al. 2022). This Cu‐dependency through LAC4 and LAC17 determines the endocarp b lignification within spl7 mutants. Cu homeostasis through SPL7 is required to activate Cu‐requiring laccases to regulate lignification within the endocarp b cell layer, leading to the explosive seed dispersal, demonstrating mineral nutrition with polar lignification in endocarp b (Pérez‐Antón et al. 2022).
4. Understanding the Process of Silique Dehiscence
The process of silique dehiscence is essential for the proper dispersal of seeds at the end of a dehiscent plant's lifecycle (Howe and Smallwood 1982; Howe and Miriti 2004; Moles and Westoby 2006). Silique dehiscence occurs through the spatial and temporal relationships of many silique morphology genes and downstream cell wall degrading enzymes as mentioned above (Rajani and Sundaresan 2001; Liljegren et al. 2000; Ferrándiz, Gu, et al. 2000; Dinneny et al. 2005; Arnaud et al. 2010; Groszmann et al. 2011; Jaradat et al. 2014) (Figure 2). Once the identity of the replum region and valve region has been established, the silique of Brassica species and A. thaliana will undergo what we classify here as two priming events that occur almost simultaneously to allow for dehiscence to occur (Squires et al. 2003) (Figure 3a). The first priming event is the development of the DZ with lignification occurring within the LL and the cell wall degrading enzymes acting on the SL (Ferrándiz et al. 1999; Liljegren et al. 2004; Roeder and Yanofsky 2006) (Figure 3a). While lignification of the LL allows for additional stability of the silique, it also allows for tensile forces from upper drying layers to converge on the LL (Ferrándiz et al. 1999; Liljegren et al. 2004; Roeder and Yanofsky 2006) (Figure 3a). Cellular degradation of SL weakens this particular section of the silique, allowing for a location where the splitting of the valve away from the replum can occur (Ferrándiz et al. 1999; Liljegren et al. 2004; Roeder and Yanofsky 2006) (Figure 3a,b). These two processes in conjunction act as the primary priming step of silique dehiscence. The second priming step occurs through the lignification of the endocarp b and endocarp a L1–3 cells and the mesocarp layer's additional lignification (Spence et al. 1996; Rajani and Sundaresan 2001; Nichol and Samuel 2024) (Figure 3a). Once these two priming steps are complete, the silique is ready to begin the process of dehiscence (Figure 3b). Near the end of the silique lifecycle, depicted at Stage 18 using the classical staging classification or after 12 DAP/DPA using the alternate staging classification, the silique begins the process of drying (Spence et al. 1996; Rajani and Sundaresan 2001; Nichol and Samuel 2024). Through the process of drying, the collapsing, non‐lignified cells of the mesocarp layer begin to create tensile forces that apply to the radial walls of the cell because the tangential walls start to collapse (Vaughn et al. 2011). The tensile forces created from the mesocarp layer are represented by the smaller two arrows facing each other in Figure 3a–d. The tensile force is amplified because of the LL, the endocarp a L1–3 cells, and the rigidity of the endocarp b cell layer (Vaughn et al. 2011; Nichol and Samuel 2024). This is visualized in Figure 3a. where the large red arrow on top of the replum is the active mechanical force being applied to the valve. The mechanical tensile forces pull the top of the valve towards the middle of the valve region in a spring‐like fashion (Figure 3a). Once the applied mechanical force is large enough, the weakest point of the silique, which is the SL, will begin to separate because there is no longer a strengthened cell wall or middle lamella providing support (Ferrándiz et al. 1999; Liljegren et al. 2004; Roeder and Yanofsky 2006) (Figure 3b). Once the SL separates, the silique will begin to dehisce, and subsequent seed dispersal can occur (Ferrándiz et al. 1999; Liljegren et al. 2004; Roeder and Yanofsky 2006) (Figure 3b).
FIGURE 3.
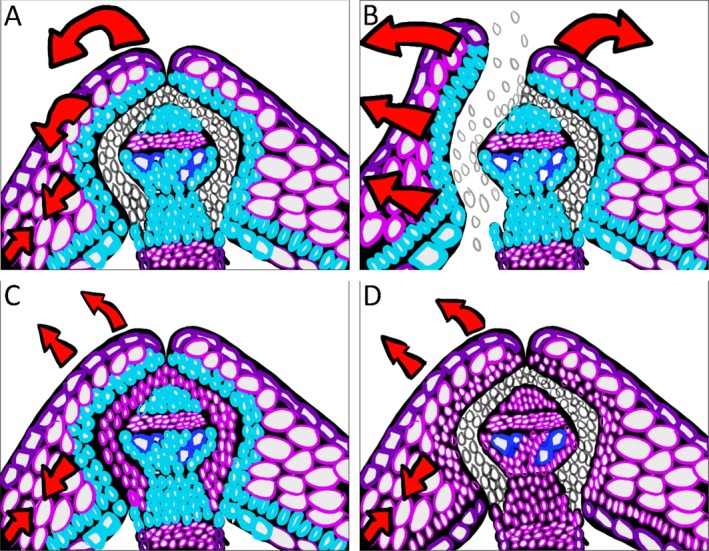
An illustrative figure of a wild‐type Brassica napus silique DZ and mutant variations. The siliques are false‐colored to mimic toluidine blue O staining, with the pink/purple color representing the primary cell wall and the blue‐colored cells indicating secondary cell wall lignification (A–D). The large red arrows represent forces applied onto the silique structure (A–D). A representative wild‐type Brassica napus silique that has not undergone dehiscence (A). A representative Brassica napus wild‐type silique that has undergone dehiscence within the DZ, illustrates the separation of the valve from the replum (B). A mutant Brassica napus silique that no longer has proper cell wall and middle lamella degradation due to hypothetical knockouts in cell wall degrading enzymes (C). A mutant Brassica napus silique that no longer has secondary cell wall deposition within the endocarp b cells and lignified layer due to hypothetical knockouts in lignification deposition/patterning (D).
There are commonalities and differences in silique dehiscence observed in other Brassicaceae members, wherein C. hirsuta , priming events occur similar to Brassicas and A. thaliana , as mentioned above, with lignification of the endocarp b cell layer and LL along with degradation of the secondary cell wall within the SL as demonstrated by Cullen and Hay (2024). C. hirsuta differs in having asymmetric polar deposition of lignification in the form of a V‐shaped or teardrop‐shaped endocarp b and a mucilaginous layer deposited within the endocarp a and b cell layer (Vaughn et al. 2011; Cullen and Hay 2024). C. hirsuta undergoes similar outer pericarp drying with the non‐lignified cells of the mesocarp layer creating similar tensile forces across the radial walls (Vaughn et al. 2011). Due to the V‐shaped lignification of the endocarp b cell layer, collapse of the upper endocarp b cell wall leads to an ultrafast coiling of the silique valve, releasing the seeds in an explosive manner (Cullen and Hay 2024). In camelina and pennycress, although little is known about the mechanisms behind silicle dehiscence, we can infer from the similarities to A. thaliana in cell layer morphology and patterning, that the overall mechanism functions in a similar manner. However, ultimately, more research in this area is needed to verify the developmental patterning and inner silicle morphology to further determine the actual mechanisms at play.
The absence of either of the priming effects disrupts the action of silique dehiscence. This is either through disruptions of the cell wall degrading enzymes or the lignification pathway and downstream genes associated with silique lignification (Ogawa et al. 2009; Mitsuda and Ohme‐Takagi 2008; He et al. 2018) (Figure 3c,d). If the cell wall degrading enzymes mentioned previously in Section 3.2 are disrupted as illustrated in Figure 3c, the SL no longer contains weakened cells because the cell wall and middle lamella are left intact (Ogawa et al. 2009; He et al. 2018). This creates a scenario where the mechanical tensile forces from mesocarp drying are insufficient to break open the SL and split the valve from the replum, leading to a partial or fully indehiscent silique (Ogawa et al. 2009; He et al. 2018) (Figure 3c). If the lignification pathway or downstream genes associated with silique lignification deposition are disrupted, as illustrated in Figure 3d, the mechanical tensile forces are insufficient to break open the SL, even though the SL has undergone proper cell wall degradation (Mitsuda and Ohme‐Takagi 2008). The lignification of the LL, endocarp a L1–3 cells, and endocarp b cells are necessary because the provided rigidity of the lignified cells is essential for mechanical tensile forces to be applied to the valve (Mitsuda and Ohme‐Takagi 2008; Nichol and Samuel 2024) (Figure 3d). This is because the applied force on the radial wall is dispersed throughout the valve and not focused on the LLs (Mitsuda and Ohme‐Takagi 2008) (Figure 3d). This creates a scenario where the valve cannot separate from the replum, leading to a partial or fully indehiscent silique (Mitsuda and Ohme‐Takagi 2008) (Figure 3d). Any approach to creating shatter tolerance should consider establishing the necessary valve identities so that the siliques are shatter tolerant and not shatter resistant. In simple terms, creating a silique that is shatter resistant from loss of DZ identity will be difficult to break open by the harvesting machinery due to the lack of the SL, leading to separation in planes other than the valve margins to release the seeds. This approach, although can result in shatter‐resistant siliques, may not result in the yield advantage as the valve is unable to open along the natural plane to release all the seeds. The better approach would be to create strong, indehiscent siliques with proper valve identities that can be broken open with added pressures from the harvesting machinery.
5. Effects of Shattering on Oilseed Crops
Weather and climate‐related abiotic factors such as periods of drought and hail can exacerbate the natural dehiscence process, resulting in lost yield (Bara et al. 2013; Steponavičius et al. 2019). A previous study by Kutcher et al. (2010) has reported that hot and dry climates can reduce the number of fertile siliques on the main branch, seed weight, and number of seeds per silique. Additionally, these conditions cause tensile force to build up rapidly, resulting in silique shatter. Similar oilseed crop harvest loss percentages are seen throughout Canada, Europe, and China, with average losses due to shatter hovering around 6%–10% of the total yield, with this reaching as high as 20% (Kadkol et al. 1984; Price et al. 1996; Pekrun et al. 1998; Gulden et al. 2003; Weber et al. 2009; Pari et al. 2012; Peltonen‐Sainio et al. 2014; Cavalieri et al. 2016; Kuai et al. 2016). In 2021, the Canadian prairies experienced the greatest reduction in yield due to intense drought conditions (StatisticsCanada 2024). Canola yields decreased by 45%, 31%, and 28% in Saskatchewan, Alberta, and Manitoba, respectively (StatisticsCanada 2024). Specifically, in 1996, up to 50% of canola yield was lost due to what is agriculturally known as “pod shatter,” from a combination of non‐ideal weather conditions, natural dehiscence, and mechanical harvesting mechanisms (Price et al. 1996; Maity et al. 2021). By 2003, 6% of canola yield was lost, which amounted to roughly 3000m2 of seeds, all of which would then become volunteer plants and would have to be treated as pests (Gulden et al. 2003; Maity et al. 2021). The reduction in yield loss has been attributed to the development of shatter‐tolerant varieties through a combination of traditional breeding, hybridization, and other genetic modification (GM) techniques, although even a 6% loss of yield approximately results in a $1.8B CAD loss in revenue in Canada (The Canola Council of Canada 2024; Alberta Canola 2021; CBAN 2024).
Camelina has been receiving much attention in the past decade as it has shown potential for becoming a valuable oilseed crop that is not susceptible to common Brassica pests with a desirable seed oil composition, making it applicable for both feed and non‐feed uses (i.e., livestock feed and biodiesel) (Guy et al. 2014; Zanetti et al. 2021). Despite these economically valuable traits, it remains a species susceptible to drought stress, with yield losses increasing to 29% when combined directly due to silicle dehiscence from mechanical disturbance produced by the combine (Sintim et al. 2016). Similarly, pennycress yield loss significantly increases to 70% during harvest due to low moisture content within the silicle at maturity (Cubins et al. 2019). This translates to a loss of 300 times the seeding rate of pennycress, and because each pennycress crop can lose up to 15,000 seeds from shatter, it results in large‐scale growth of volunteer plants for farmers to then manage (Cubins et al. 2019).
6. Mitigating Silique Dehiscence
6.1. Classical Methods for Reducing Silique Dehiscence
Traditionally, the starting point at which crop growers will attempt to minimize seed loss through silique dehiscence during harvest is by optimizing the time of harvest and the speed of the combine (Price et al. 1996; Jeschke 2017; The Canola Council of Canada 2024). Checking the seed color and moisture content of siliques is also necessary to learn of silique maturity and prevent delayed harvest. Post‐maturity harvesting makes oilseed crops significantly susceptible to shattering from any mechanical stress caused by wind, rain, and combine use (Sintim et al. 2016). Specifically, it is recommended to harvest pennycress when it reaches physiological maturity about 2 weeks prior to harvest when silicle moisture is low enough for an efficient harvest, and this could mitigate large yield losses to shatter mentioned earlier (Cubins et al. 2019). In Brassica cultivation, fruit sealants consisting of various latex and resin ingredients have been used to prevent siliques from shattering by limiting water movement (Serafin‐Andrzejewska et al. 2021). This allows seeds to further develop and mature in an even manner (Serafin‐Andrzejewska et al. 2021). Additionally, swathes are placed beneath the plants for 7–14 days prior to combining, instead of direct cutting or combining (Price et al. 1996; Summers et al. 2003; Ogutcen et al. 2018). This has demonstrated increased results in minimizing seed loss (Price et al. 1996; Summers et al. 2003; Ogutcen et al. 2018). This method also prevents issues of uneven seed maturity and harvesting of low‐quality seeds (Ogutcen et al. 2018). Desiccant sprays are also routinely applied after swathing to increase the rate of maturation (Price et al. 1996; Summers et al. 2003).
6.2. Reducing Silique Dehiscence: Lignification Approaches
One promising approach to mitigate the seed dispersal mechanisms problem is by increasing lignification, which enhances the structural integrity of silique walls, making them less prone to shattering. Chu et al. (2022) determined that a B. napus single recessive gene controlled a lignified bridge between the two LLs of the silique, leading to indehiscence and high shatter tolerance. Conversely, when lignification is completely absent within the silique walls, such as in the nst1/3 double knockout mutant, the silique no longer has mechanical tensile forces being applied, leading to indehiscent siliques (Mitsuda and Ohme‐Takagi 2008). This may also be the case for the lignin biosynthetic transcription factors of MYB46 and MYB83, where a potential double knockout mutant could lead to silique indehiscence due to a complete loss of lignification within the silique walls. Focusing efforts on the genetic manipulation of the lignification pathway and lignin biosynthesis genes can allow for enhancing the mechanical strength of fruiting bodies. Hypothetically, increasing lignification in mesocarp could lead to a reduction in the tensile forces from the mesocarp drying, leading to reduced shatter. Conversely, eliminating lignification in the endocarp b can also lead to indehiscent siliques by distributing these mechanical tensile forces or preventing the tension from the forces from impinging on the LL to break the silique. This structural reinforcement, whether through increased lignification or complete elimination, can help ensure siliques remain intact under various environmental conditions. Ultimately, this approach minimizes seed loss and improves overall crop yield and stability.
6.3. Reducing Silique Dehiscence: Non‐Lignification Approaches
Similar to lignification, bioengineering plants using non‐lignifying approaches is another potential strategy to mitigate seed dispersal mechanisms. Research within this area has focused primarily on the manipulation of the main genes associated with morphological development and cell identity. Knockouts within the IND, SHP1/2, and ALC have been shown to lead to indehiscent siliques (Ferrándiz, Liljegren, and Yanofsky 2000; Liljegren et al. 2000; Rajani and Sundaresan 2001; Liljegren et al. 2004). While overexpression of FUL also leads to an indehiscent phenotype (Ferrándiz, Liljegren, and Yanofsky 2000). This work has led to the development of agricultural technologies that resulted in both accepted patents and patents pending approval. In 2008, an original patent (US20110030106A1) was filed by Bayer CropScience NV. This patent was based on a full phenotypic knockout IND. This technology was later patented under US9475849B2, with the current assignee being BASF Agricultural Solutions Seed US LLC, with this additional patent extending the full phenotypic knockout of IND to two genes within B. napus plants. In regards to pennycress, two recent patent filings within 2023, (US20240065193A1) and (US20240147929A1), have been filed by the University of Minnesota and Covercress Inc., respectively, with both patents having a pending status for the implementation of IND knockouts. Patent (US20230057587A1) has been filed with a pending status for gene knockouts within SHP by Cibus Europe BV, Cibus US LLC. This claim has been extended to many cash crop varieties, such as B. napus , Brassica rapa , Brassica oleracea , Brassica juncea , Brassica species, Raphanus sativus , Pisum sativum , Phaseolus vulgaris , Lens culinaris , Glycine max , and Fabaceae species. Limagrain Europe SA has filed pending patents in both Canada and the United States (US20210169029A1 and CA2994405A1; WO2017025420A1) trying to create a shatter‐tolerant line (POSH+) through manipulation of a single nucleotide polymorphism within the FUL gene of B. napus , B. juncea, and B. rapa through the addition of a Raphanus genomic fragment. These examples are a small subset of patent filings that demonstrate agricultural applications of shatter‐tolerant crop varieties based on the genes associated with development and cell identity.
There is potential for future efforts focusing on creating agricultural resilient crops conferring shatter tolerance through the disruption of cell wall degrading enzymes within the DZ. Research done by Li et al. (2021) has provided insights using RNAi technology targeting MAN7 within B. napus creating lines that are indehiscent. Future genetic approaches could use CEL6 and ADPG1/2 as potential targets for silique shatter–tolerant crops because it is known within Arabidopsis that knockouts within these genes lead to indehiscent siliques (Ogawa et al. 2009; He et al. 2018).
7. (Conclusion) Future Applications for Food Security and Sustainability
Silique dehiscence in Arabidopsis involves a tightly coordinated set of events regulated by master controllers of morphological development and cell identity, followed by secondary cell wall‐activating transcription factors and cell wall‐degrading enzymes. Each level of regulation is crucial for natural seed dispersal. However, from an agricultural and economic perspective, silique dehiscence is unfavorable as it leads to significant pre‐harvest seed loss, reducing crop yield and profitability in crops like canola, pennycress, and Camelina.
Research into seed dispersal mechanisms has enabled the development of more efficient and resilient crop varieties, addressing critical challenges in oilseed crop loss. Researchers have successfully created genetically modified plants within Brassicaceae species that exhibit indehiscent siliques by reducing allele function within genes such as IND, ALC, FUL, and SHP, with patents pending approval. Future genetic approaches may focus on increasing silique strength by enhancing secondary cell walls through increased lignification or reducing cell wall‐degrading enzymes to achieve shatter tolerance in oilseed crops.
Although the current knowledge presented is limited to Brassicaceae, it can be extended to other crop varieties depending on their own similarities of silique/silicle morphology to the Brassicaceae family. Overall, the understanding of silique dehiscence has laid the groundwork for broader applications across diverse oilseed crops and beyond.
Author Contributions
J.N. and M.S. conceived the idea. J.N. wrote a majority of the work. S.D. wrote Sections 5 and 6.1. J.N., S.D., and M.S. all contributed to editing.
Conflicts of Interest
The authors declare no conflicts of interest.
Acknowledgments
We would like to thank Courtney Pham for her invaluable legal knowledge in patent filings. We acknowledge NSERC discovery grant (RT735240) for funding for this work.
Funding: This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC) (RT735240).
Data Availability Statement
No restrictions on the data.
References
- Alberta Canola . 2021. “Canola Statistics.” https://albertacanola.com/about/canola‐statistics/.
- Alonso‐Cantabrana, H. , Ripoll J. J., Ochando I., Vera A., Ferrándiz C., and Martínez‐Laborda A.. 2007. “Common Regulatory Networks in Leaf and Fruit Patterning Revealed by Mutations in the Arabidopsis ASYMMETRIC LEAVES1 Gene.” Development 134: 2663–2671. [DOI] [PubMed] [Google Scholar]
- Alvarez, J. , and Smyth D. R.. 1997. “Carpel Development Genes in ‘Arabidopsis’.” Flowering Newsletter 23: 12–17. [Google Scholar]
- Alvarez, J. , and Smyth D. R.. 2002. “ CRABS CLAW and SPATULA Genes Regulate Growth and Pattern Formation During Gynoecium Development in Arabidopsis thaliana .” International Journal of Plant Sciences 163: 17–41. [Google Scholar]
- Arnaud, N. , Girin T., Sorefan K., et al. 2010. “Gibberellins Control Fruit Patterning in Arabidopsis thaliana .” Genes & Development 24: 2127–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballester, P. , and Ferrándiz C.. 2017. “Shattering Fruits: Variations on a Dehiscent Theme.” Current Opinion in Plant Biology 35: 68–75. [DOI] [PubMed] [Google Scholar]
- Bara, N. , Khare D., and Shrivastava A. N.. 2013. “Studies on the Factors Affecting Pod Shattering in Soybean.” Indian Journal Of Genetics And Plant Breeding 73, no. 3: 270–277. [Google Scholar]
- Bowman, J. L. , Smyth D. R., and Meyerowitz E. M.. 1991. “Genetic Interactions Among Floral Homeotic Genes of Arabidopsis .” Development 112: 1–20. [DOI] [PubMed] [Google Scholar]
- Braatz, J. , Harloff H.‐J., Emrani N., et al. 2018. “The Effect of INDEHISCENT Point Mutations on Silique Shatter Resistance in Oilseed Rape (Brassica napus).” Theoretical and Applied Genetics 131: 959–971. [DOI] [PubMed] [Google Scholar]
- Buck, M. J. , and Atchley W. R.. 2003. “Phylogenetic Analysis of Plant Basic Helix‐Loop‐Helix Proteins.” Journal of Molecular Evolution 56: 742–750. [DOI] [PubMed] [Google Scholar]
- Byrne, M. E. , Barley R., Curtis M., et al. 2000. “ Asymmetric leaves1 Mediates Leaf Patterning and Stem Cell Function in Arabidopsis .” Nature 408: 967–971. [DOI] [PubMed] [Google Scholar]
- Canadian Biotechnology Action Network (CBAN) . 2024. “Canola on the Market.” https://cban.ca/gmos/products/on‐the‐market/canola/.
- Cavalieri, A. , Harker K. N., Hall L. M., et al. 2016. “Evaluation of the Causes of On‐Farm Harvest Losses in Canola in the Northern Great Plains.” Crop Science 56: 2005–2015. [Google Scholar]
- Child, R. D. , Chauvaux N., John K., Van Onckelen H. A., and Ulvskov P.. 1998. “Ethylene Biosynthesis in Oilseed Rape Pods in Relation to Pod Shatter.” Journal of Experimental Botany 49: 829–838. [Google Scholar]
- Chopra, R. , Johnson E. B., Emenecker R., et al. 2020. “Identification and Stacking of Crucial Traits Required for the Domestication of Pennycress.” Nature Food 1: 84–91. [Google Scholar]
- Chu, W. , Liu J., Cheng H., et al. 2022. “A Lignified‐Layer Bridge Controlled by a Single Recessive Gene Is Associated With High Pod‐Shatter Resistance in Brassica napus L.” Crop Journal 10: 638–646. [Google Scholar]
- Chuck, G. , Lincoln C., and Hake S.. 1996. “KNAT1 Induces Lobed Leaves With Ectopic Meristems When Overexpressed in Arabidopsis.” Plant Cell 8: 1277–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubins, J. A. , Wells M. S., Frels K., et al. 2019. “Management of Pennycress as a Winter Annual Cash Cover Crop. A review.” Agronomy for Sustainable Development 39: 1–11.30881486 [Google Scholar]
- Cullen, E. , and Hay A.. 2024. “Creating an Explosion: Form and Function in Explosive Fruit.” Current Opinion in Plant Biology 79: 102543. [DOI] [PubMed] [Google Scholar]
- Degan, F. D. , Child R., Svendsen I., and Ulvskov P.. 2001. “The Cleavable N‐Terminal Domain of Plant Endopolygalacturonases From Clade B May Be Involved in a Regulated Secretion Mechanism.” Journal of Biological Chemistry 276: 35297–35304. [DOI] [PubMed] [Google Scholar]
- Di Vittori, V. , Gioia T., Rodriguez M., et al. 2019. “Convergent Evolution of the Seed Shattering Trait.” Genes 10, no. 1: 68. 10.3390/genes10010068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinneny, J. R. , Weigel D., and Yanofsky M. F.. 2005. “A Genetic Framework for Fruit Patterning in Arabidopsis thaliana .” Development 132: 4687–4696. [DOI] [PubMed] [Google Scholar]
- Dinneny, J. R. , Yadegari R., Fischer R. L., Yanofsky M. F., and Weigel D.. 2004. “The Role of JAGGED in Shaping Lateral Organs.” Development 131: 1101–1110. [DOI] [PubMed] [Google Scholar]
- Douglas, S. J. , Chuck G., Dengler R. E., Pelecanda L., and Riggs C. D.. 2002. “ KNAT1 and ERECTA Regulate Inflorescence Architecture in Arabidopsis.” Plant Cell 14: 547–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldridge, T. , Łangowski Ł., Stacey N., et al. 2016. “Fruit Shape Diversity in the Brassicaceae Is Generated by Varying Patterns of Anisotropy.” Development 143: 3394–3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshed, Y. , Izhaki A., Baum S. F., Floyd S. K., and Bowman J. L.. 2004. “Asymmetric Leaf Development and Blade Expansion in Arabidopsis Are Mediated by KANADI and YABBY Activities.” Development 131: 2997–3006. [DOI] [PubMed] [Google Scholar]
- Ferrándiz, C. , and Fourquin C.. 2014. “Role of the FUL‐SHP Network in the Evolution of Fruit Morphology and Function.” Journal of Experimental Botany 65: 4505–4513. [DOI] [PubMed] [Google Scholar]
- Ferrándiz, C. , Gu Q., Martienssen R., and Yanofsky M. F.. 2000. “Redundant Regulation of Meristem Identity and Plant Architecture by FRUITFULL, APETALA1 and CAULIFLOWER .” Development 127: 725–734. [DOI] [PubMed] [Google Scholar]
- Ferrándiz, C. , Liljegren S. J., and Yanofsky M. F.. 2000. “Negative Regulation of the SHATTERPROOF Genes by FRUITFULL During Arabidopsis Fruit Development.” Science 289: 436–438. [DOI] [PubMed] [Google Scholar]
- Ferrándiz, C. , Pelaz S., and Yanofsky M. F.. 1999. “Control of Carpel and Fruit Development in Arabidopsis.” Annual Review of Biochemistry 68: 321–354. [DOI] [PubMed] [Google Scholar]
- Fisher, F. , and Goding C. R.. 1992. “Single Amino Acid Substitutions Alter Helix‐Loop‐Helix Protein Specificity for Bases Flanking the Core CANNTG Motif.” EMBO Journal 11: 4103–4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan, C. A. , Hu Y., and Ma H.. 1996. “Specific Expression of the AGL1 MADS‐Box Gene Suggests Regulatory Functions in Arabidopsis Gynoecium and Ovule Development.” Plant Journal: For Cell and Molecular Biology 10: 343–353. [DOI] [PubMed] [Google Scholar]
- Fry, S. C. , Smith R. C., Renwick K. F., Martin D. J., Hodge S. K., and Matthews K. J.. 1992. “Xyloglucan Endotransglycosylase, a New Wall‐Loosening Enzyme Activity From Plants.” Biochemical Journal 282, no. Pt 3: 821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan, Y. , Malhi S., Brandt S., and McDonald C.. 2008. “Assessment of Seed Shattering Resistance and Yield Loss in Five Oilseed Crops.” Canadian Journal of Plant Science 88: 267–270. [Google Scholar]
- Girin, T. , Paicu T., Stephenson P., et al. 2011. “INDEHISCENT and SPATULA Interact to Specify Carpel and Valve Margin Tissue and Thus Promote Seed Dispersal in Arabidopsis .” Plant Cell 23: 3641–3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girin, T. , Stephenson P., Goldsack C. M. P., et al. 2010. “Brassicaceae INDEHISCENT Genes Specify Valve Margin Cell Fate and Repress Replum Formation.” Plant Journal: For Cell and Molecular Biology 63: 329–338. [DOI] [PubMed] [Google Scholar]
- González‐Carranza, Z. H. , Elliott K. A., and Roberts J. A.. 2007. “Expression of Polygalacturonases and Evidence to Support Their Role During Cell Separation Processes in Arabidopsis thaliana .” Journal of Experimental Botany 58: 3719–3730. [DOI] [PubMed] [Google Scholar]
- González‐Carranza, Z. H. , Lozoya‐Gloria E., and Roberts J. A.. 1998. “Recent Developments in Abscission: Shedding Light on the Shedding Process.” Trends in Plant Science 3: 10–14. [Google Scholar]
- Groszmann, M. , Bylstra Y., Lampugnani E. R., and Smyth D. R.. 2010. “Regulation of Tissue‐Specific Expression of SPATULA, a bHLH Gene Involved in Carpel Development, Seedling Germination, and Lateral Organ Growth in Arabidopsis .” Journal of Experimental Botany 61: 1495–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groszmann, M. , Paicu T., Alvarez J. P., Swain S. M., and Smyth D. R.. 2011. “ SPATULA and ALCATRAZ, Are Partially Redundant, Functionally Diverging bHLH Genes Required for Arabidopsis Gynoecium and Fruit Development.” Plant Journal: For Cell and Molecular Biology 68: 816–829. [DOI] [PubMed] [Google Scholar]
- Groszmann, M. , Paicu T., and Smyth D. R.. 2008. “Functional Domains of SPATULA, a bHLH Transcription Factor Involved in Carpel and Fruit Development in Arabidopsis.” Plant Journal: For Cell and Molecular Biology 55: 40–52. [DOI] [PubMed] [Google Scholar]
- Gu, Q. , Ferrándiz C., Yanofsky M. F., and Martienssen R.. 1998. “The FRUITFULL MADS‐Box Gene Mediates Cell Differentiation During Arabidopsis Fruit Development.” Development 125: 1509–1517. [DOI] [PubMed] [Google Scholar]
- Gulden, R. H. , Shirtliffe S. J., and Gordon Thomas A.. 2003. “Harvest Losses of Canola (Brassica napus) Cause Large Seedbank Inputs.” Weed Science 51: 83–86. [Google Scholar]
- Guo, M. , Thomas J., Collins G., and Timmermans M. C. P.. 2008. “Direct Repression of KNOX Loci by the ASYMMETRIC LEAVES1 Complex of Arabidopsis .” Plant Cell 20: 48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy, S. O. , Wysocki D. J., Schillinger W. F., et al. 2014. “Camelina: Adaptation and Performance of Genotypes.” Field Crops Research 155: 224–232. [Google Scholar]
- He, H. , Bai M., Tong P., Hu Y., Yang M., and Wu H.. 2018. “CELLULASE6 and MANNANASE7 Affect Cell Differentiation and Silique Dehiscence.” Plant Physiology 176: 2186–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera‐Ubaldo, H. , and de Folter S.. 2022. “Gynoecium and Fruit Development in Arabidopsis .” Development 149, no. 5: dev200120. 10.1242/dev.200120. [DOI] [PubMed] [Google Scholar]
- Howe, H. F. , and Miriti M. N.. 2004. “When Seed Dispersal Matters.” Bioscience 54: 651–660. [Google Scholar]
- Howe, H. F. , and Smallwood J.. 1982. “Ecology of Seed Dispersal.” Annual Review of Ecology, Evolution, and Systematics 13: 201–228. [Google Scholar]
- Iwakawa, H. , Ueno Y., Semiarti E., et al. 2002. “The ASYMMETRIC LEAVES2 Gene of Arabidopsis thaliana, Required for Formation of a Symmetric Flat Leaf lamina, Encodes a Member of a Novel Family of Proteins Characterized by Cysteine Repeats and a Leucine Zipper.” Plant & Cell Physiology 43: 467–478. [DOI] [PubMed] [Google Scholar]
- Jankowski, K. J. , Sokólski M., and Kordan B.. 2019. “Camelina: Yield and Quality Response to Nitrogen and Sulfur Fertilization in Poland.” Industrial Crops and Products 141: 111776. [Google Scholar]
- Jaradat, M. R. , Ruegger M., Bowling A., Butler H., and Cutler A. J.. 2014. “A Comprehensive Transcriptome Analysis of Silique Development and Dehiscence in Arabidopsis and Brassica Integrating Genotypic, Interspecies and Developmental Comparisons.” GM Crops & Food 5: 302–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins, E. S. , Paul W., Coupe S. A., Bell S. J., Davies E. C., and Roberts J. A.. 1996. “Characterization of an mRNA Encoding a Polygalacturonase Expressed During Pod Development in Oilseed Rape (Brassica napus L.).” Journal of Experimental Botany 47: 111–115. [Google Scholar]
- Jeschke, M . 2017. “Reducing Yield Loss from Pod Shattering in Soybean.” https://www.pioneer.com/ca‐en/agronomy/reducing‐yield‐loss‐from‐pod‐shattering‐in‐soybean.html.
- Kadkol, G. P. , Macmillan R. H., Burrow R. P., and Halloran G. M.. 1984. “Evaluation of Brassica Genotypes for Resistance to Shatter. I. Development of a Laboratory Test.” Euphytica 33: 63–73. [Google Scholar]
- Kaufmann, K. , Wellmer F., Muiño J. M., et al. 2010. “Orchestration of Floral Initiation by APETALA1.” Science 328: 85–89. [DOI] [PubMed] [Google Scholar]
- Kempin, S. A. , Savidge B., and Yanofsky M. F.. 1995. “Molecular Basis of the Cauliflower Phenotype in Arabidopsis .” Science 267: 522–525. [DOI] [PubMed] [Google Scholar]
- Kuai, J. , Sun Y., Liu T., et al. 2016. “Physiological Mechanisms Behind Differences in Pod Shattering Resistance in Rapeseed (Brassica napus L.) Varieties.” PLoS ONE 11: e0157341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutcher, H. R. , Warland J. S., and Brandt S. A.. 2010. “Temperature and Precipitation Effects on Canola Yields in Saskatchewan, Canada.” Agricultural and Forest Meteorology 150: 161–165. [Google Scholar]
- Lenser, T. , and Theißen G.. 2013. “Molecular Mechanisms Involved in Convergent Crop Domestication.” Trends in Plant Science 18: 704–714. [DOI] [PubMed] [Google Scholar]
- Li, Y.‐L. , Yu Y.‐K., Zhu K.‐M., et al. 2021. “Down‐Regulation of MANNANASE7 Gene in Brassica napus L. Enhances Silique Dehiscence‐Resistance.” Plant Cell Reports 40: 361–374. [DOI] [PubMed] [Google Scholar]
- Liljegren, S. J. , Ditta G. S., Eshed Y., Savidge B., Bowman J. L., and Yanofsky M. F.. 2000. “ SHATTERPROOF MADS‐Box Genes Control Seed Dispersal in Arabidopsis .” Nature 404: 766–770. [DOI] [PubMed] [Google Scholar]
- Liljegren, S. J. , Roeder A. H. K., Kempin S. A., et al. 2004. “Control of Fruit Patterning in Arabidopsis by INDEHISCENT.” Cell 116: 843–853. [DOI] [PubMed] [Google Scholar]
- Lincoln, C. , Long J., Yamaguchi J., Serikawa K., and Hake S.. 1994. “A Knotted1‐Like Homeobox Gene in Arabidopsis Is Expressed in the Vegetative Meristem and Dramatically Alters Leaf Morphology When Overexpressed in Transgenic Plants.” Plant Cell 6: 1859–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, H. , Yanofsky M. F., and Meyerowitz E. M.. 1991. “AGL1‐AGL6, an Arabidopsis Gene Family With Similarity to Floral Homeotic and Transcription Factor Genes.” Genes & Development 5: 484–495. [DOI] [PubMed] [Google Scholar]
- Maity, A. , Lamichaney A., Joshi D. C., et al. 2021. “Seed Shattering: A Trait of Evolutionary Importance in Plants.” Frontiers in Plant Science 12: 657773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy, R. L. , Zhong R., and Ye Z.‐H.. 2009. “MYB83 Is a Direct Target of SND1 and Acts Redundantly With MYB46 in the Regulation of Secondary Cell Wall Biosynthesis in Arabidopsis.” Plant & Cell Physiology 50: 1950–1964. [DOI] [PubMed] [Google Scholar]
- Merriam‐Webster . 2025. “Silicle. In Merriam‐Webster.com Dictionary.” https://www.merriam‐webster.com/dictionary/silicle.
- Missouri Botanical Garden . 2025. “ “Silicle”, Missouri Botanical Garden Plant Finder.” http://www.missouribotanicalgarden.org/plantfinder/plantfindersearch.aspx.
- Mitsuda, N. , Iwase A., Yamamoto H., et al. 2007. “NAC Transcription Factors, NST1 and NST3, Are Key Regulators of the Formation of Secondary Walls in Woody Tissues of Arabidopsis .” Plant Cell 19: 270–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuda, N. , and Ohme‐Takagi M.. 2008. “NAC Transcription Factors NST1 and NST3 Regulate Pod Shattering in a Partially Redundant Manner by Promoting Secondary Wall Formation After the Establishment of Tissue Identity.” Plant Journal: For Cell and Molecular Biology 56: 768–778. [DOI] [PubMed] [Google Scholar]
- Mitsuda, N. , Seki M., Shinozaki K., and Ohme‐Takagi M.. 2005. “The NAC Transcription Factors NST1 and NST2 of Arabidopsis Regulate Secondary Wall Thickenings and Are Required for Anther Dehiscence.” Plant Cell 17: 2993–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizzotti, C. , Rotasperti L., Moretto M., et al. 2018. “Time‐Course Transcriptome Analysis of Arabidopsis Siliques Discloses Genes Essential for Fruit Development and Maturation.” Plant Physiology 178: 1249–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moles, A. T. , and Westoby M.. 2006. “Seed Size and Plant Strategy Across the Whole Life Cycle.” Oikos 113: 91–105. [Google Scholar]
- Nichol, J. B. , and Samuel M. A.. 2024. “Characterizing the Role of Endocarp a and b Cells Layers During Pod (Silique) Development in Brassicaceae.” Plant Signaling & Behavior 19, no. 1: 2384243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa, M. , Kay P., Wilson S., and Swain S. M.. 2009. “ARABIDOPSIS DEHISCENCE ZONE POLYGALACTURONASE1 (ADPG1), ADPG2, and QUARTET2 Are Polygalacturonases Required for Cell Separation During Reproductive Development in Arabidopsis .” Plant Cell 21: 216–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogutcen, E. , Pandey A., Khan M. K., et al. 2018. “Pod Shattering: A Homologous Series of Variation Underlying Domestication and an Avenue for Crop Improvement.” Agronomy (Basel, Switzerland) 8: 137. [Google Scholar]
- Ohno, C. K. , Reddy G. V., Heisler M. G. B., and Meyerowitz E. M.. 2004. “The Arabidopsis JAGGED Gene Encodes a Zinc Finger Protein That Promotes Leaf Tissue Development.” Development 131: 1111–1122. [DOI] [PubMed] [Google Scholar]
- Pari, L. , Assirelli A., Suardi A., et al. 2012. “The Harvest of Oilseed Rape (Brassica napus L.): The Effective Yield Losses at On‐Farm Scale in the Italian Area.” Biomass and Bioenergy 46: 453–458. [Google Scholar]
- Pekrun, C. , Lutman P. J. W., and Baeumer K.. 1998. “Research on Volunteer Rape: A Review.” Pflanzenbauwissenschaften 2: 84–90. [Google Scholar]
- Peltonen‐Sainio, P. , Pahkala K., Mikkola H., and Jauhiainen L.. 2014. “Seed Loss and Volunteer Seedling Establishment of Rapeseed in the Northernmost European Conditions: Potential for Weed Infestation and GM Risks.” Agricultural and Food Science 23: 327–339. [Google Scholar]
- Pérez‐Antón, M. , Schneider I., Kroll P., et al. 2022. “Explosive Seed Dispersal Depends on SPL7 to Ensure Sufficient Copper for Localized Lignin Deposition via Laccases.” Proceedings of the National Academy of Sciences 119, no. 24: e2202287119. 10.1073/pnas.2202287119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen, M. , Sander L., Child R., van Onckelen H., Ulvskov P., and Borkhardt B.. 1996. “Isolation and Characterisation of a Pod Dehiscence Zone‐Specific Polygalacturonase from Brassica napus .” Plant Molecular Biology 31: 517–527. [DOI] [PubMed] [Google Scholar]
- Price, J. S. , Hobson R. N., Neale M. A., and Bruce D. M.. 1996. “Seed Losses in Commercial Harvesting of Oilseed Rape.” Journal of Agricultural Engineering Research 65: 183–191. [Google Scholar]
- Purugganan, M. D. 1997. “The MADS‐Box Floral Homeotic Gene Lineages Predate the Origin of Seed Plants: Phylogenetic and Molecular Clock Estimates.” Journal of Molecular Evolution 45: 392–396. [DOI] [PubMed] [Google Scholar]
- Rajani, S. , and Sundaresan V.. 2001. “The Arabidopsis myc/bHLH Gene ALCATRAZ Enables Cell Separation in Fruit Dehiscence.” Current Biology: CB 11: 1914–1922. [DOI] [PubMed] [Google Scholar]
- Riechmann, J. L. , and Meyerowitz E. M.. 1997. “Determination of Floral Organ Identity by Arabidopsis MADS Domain Homeotic Proteins AP1, AP3, PI, and AG Is Independent of Their DNA‐Binding Specificity.” Molecular Biology of the Cell 8: 1243–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riederer, M. , Arand K., Burghardt M., et al. 2015. “Water Loss From Litchi (Litchi chinensis) and Longan (Dimocarpus longan) Fruits Is Biphasic and Controlled by a Complex Pericarpal Transpiration Barrier.” Planta 242: 1207–1219. [DOI] [PubMed] [Google Scholar]
- Ripoll, J. J. , Roeder A. H. K., Ditta G. S., and Yanofsky M. F.. 2011. “A Novel Role for the Floral Homeotic Gene APETALA2 During Arabidopsis Fruit Development.” Development 138: 5167–5176. [DOI] [PubMed] [Google Scholar]
- Roberts, J. A. , Whitelaw C. A., Gonzalez‐Carranza Z. H., and McManus M. T.. 2000. “Cell Separation Processes in Plants—Models, Mechanisms and Manipulation.” Annals of Botany 86: 223–235. [Google Scholar]
- Roeder, A. H. K. , Ferrándiz C., and Yanofsky M. F.. 2003. “The Role of the REPLUMLESS Homeodomain Protein in Patterning the Arabidopsis Fruit.” Current Biology: CB 13: 1630–1635. [DOI] [PubMed] [Google Scholar]
- Roeder, A. H. K. , and Yanofsky M. F.. 2006. “Fruit Development in Arabidopsis .” Arabidopsis Book/American Society of Plant Biologists 4: e0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander, L. , Child R., Ulvskov P., Albrechtsen M., and Borkhardt B.. 2001. “Analysis of a Dehiscence Zone Endo‐Polygalacturonase in Oilseed Rape (Brassica napus) and Arabidopsis thaliana: Evidence for Roles in Cell Separation in Dehiscence and Abscission Zones, and in Stylar Tissues During Pollen Tube Growth.” Plant Molecular Biology 46: 469–479. [DOI] [PubMed] [Google Scholar]
- Savidge, B. , Rounsley S. D., and Yanofsky M. F.. 1995. “Temporal Relationship Between the Transcription of Two Arabidopsis MADS Box Genes and the Floral Organ Identity Genes.” Plant Cell 7: 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa, S. , Watanabe K., Goto K., et al. 1999. “ FILAMENTOUS FLOWER, a Meristem and Organ Identity Gene of Arabidopsis, Encodes a Protein With a Zinc Finger and HMG‐Related Domains.” Genes & Development 13: 1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafin‐Andrzejewska, M. , Kozak M., and Kotecki A.. 2021. “Effect of Pod Sealant Application on the Quantitative and Qualitative Traits of Field Pea (Pisum sativum L.) Seed Yield.” Collection FAO: Agriculture 11: 1–9. [Google Scholar]
- Shuai, B. , Reynaga‐Peña C. G., and Springer P. S.. 2002. “The Lateral Organ Boundaries Gene Defines a Novel, Plant‐Specific Gene Family.” Plant Physiology 129: 747–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson, M. G. 2010. “Plant Systematics, Diversity and Classification of Flowering Plants: Eudicots.” 275–448.
- Sintim, H. Y. , Zheljazkov V. D., Obour A. K., and Garcia y Garcia A.. 2016. “Managing Harvest Time to Control Pod Shattering in Oilseed Camelina.” Agronomy Journal 108: 656–661. [Google Scholar]
- Smyth, D. R. , Bowman J. L., and Meyerowitz E. M.. 1990. “Early Flower Development in Arabidopsis.” Plant Cell 2: 755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence, J. , Vercher Y., Gates P., and Harris N.. 1996. “‘Pod Shatter’ in Arabidopsis thaliana, Brassica napus and B. juncea .” Journal of Microscopy 181: 195–203. [Google Scholar]
- Squires, T. M. , Gruwel M. L. H., Zhou R., Sokhansanj S., Abrams S. R., and Cutler A. J.. 2003. “Dehydration and Dehiscence in Siliques of Brassica napus and Brassica rapa .” Canadian Journal of Botany 81: 248–254. [Google Scholar]
- StatisticsCanada . 2024. https://www150.statcan.gc.ca/n1/daily‐quotidien/221202/dq221202b‐eng.htm.
- Steponavičius, D. , Kemzūraitė A., Bauša L., and Zaleckas E.. 2019. “Evaluation of the Effectiveness of Pod Sealants in Increasing Pod Shattering Resistance in Oilseed Rape (Brassica napus L.).” Energies 12: 2256. [Google Scholar]
- Summers, J. E. , Bruce D. M., Vancanneyt G., et al. 2003. “Pod Shatter Resistance in the Resynthesized Brassica napus Line DK142.” Journal of Agricultural Science 140: 43–52. [Google Scholar]
- Sun, Y. , Zhou Q., Zhang W., Fu Y., and Huang H.. 2002. “ ASYMMETRIC LEAVES1, an Arabidopsis Gene That Is Involved in the Control of Cell Differentiation in LEAVES.” Planta 214: 694–702. [DOI] [PubMed] [Google Scholar]
- Tang, G.‐X. , Qin Y.‐B., Song W.‐J., and Zhou W.‐J.. 2007. “Mechanism to Control Fruit Development and Disperse in Arabidopsis and the Application for Rapeseed (Brassica napus) Breeding.” Chinese Journal of Cell Biology 29: 864–868. [Google Scholar]
- The Canola Council of Canada . 2024. “Canada's Top Canola Markets.” https://www.canolacouncil.org/markets‐stats/top‐markets/.
- Toledo‐Ortiz, G. , Huq E., and Quail P. H.. 2003. “The Arabidopsis Basic/Helix‐Loop‐Helix Transcription Factor Family.” Plant Cell 15: 1749–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gelderen, K. , van Rongen M., Liu A., Otten A., and Offringa R.. 2016. “An INDEHISCENT‐Controlled Auxin Response Specifies the Separation Layer in Early Arabidopsis Fruit.” Molecular Plant 6: 857–869. [DOI] [PubMed] [Google Scholar]
- Vaughn, K. C. , Bowling A. J., and Ruel K. J.. 2011. “The Mechanism for Explosive Seed Dispersal in Cardamine hirsuta (Brassicaceae).” American Journal of Botany 98: 1276–1285. [DOI] [PubMed] [Google Scholar]
- Venglat, S. P. , Dumonceaux T., Rozwadowski K., et al. 2002. “The Homeobox Gene BREVIPEDICELLUS Is a Key Regulator of Inflorescence Architecture in Arabidopsis .” Proceedings of the National Academy of Sciences of the United States of America 99: 4730–4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivian‐Smith, A. , and Koltunow A. M.. 1999. “Genetic Analysis of Growth‐Regulator‐Induced Parthenocarpy in Arabidopsis.” Plant Physiology 121: 437–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, E. A. , Frick K., Gruber S., and Claupein W.. 2009. “Entwicklung eines Schätzrahmens für Durchwuchsraps auf Praxisflächen‐Erste Ergebnisse zu Ausfallverlusten und Bodensamenvorrat.” Mitt. Ges. Pflanzenbauwiss. in press.
- Western, T. L. , and Haughn G. W.. 1999. “ BELL1 and AGAMOUS Genes Promote Ovule Identity in Arabidopsis thaliana .” Plant Journal: For Cell and Molecular Biology 18: 329–336. [DOI] [PubMed] [Google Scholar]
- Wu, H. , Mori A., Jiang X., Wang Y., and Yang M.. 2006. “The INDEHISCENT Protein Regulates Unequal Cell Divisions in Arabidopsis Fruit.” Planta 224: 971–979. [DOI] [PubMed] [Google Scholar]
- Zanetti, F. , Alberghini B., Marjanović Jeromela A., et al. 2021. “Camelina, an Ancient Oilseed Crop Actively Contributing to the Rural Renaissance in Europe. A review.” Agronomy for Sustainable Development 41: 1–18. [Google Scholar]
- Zhang, J. , Xie M., Tuskan G. A., Muchero W., and Chen J.‐G.. 2018. “Recent Advances in the Transcriptional Regulation of Secondary Cell Wall Biosynthesis in the Woody Plants.” Frontiers in Plant Science 9: 1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, R. , Demura T., and Ye Z.‐H.. 2006. “SND1, a NAC Domain Transcription Factor, Is a Key Regulator of Secondary Wall Synthesis in Fibers of Arabidopsis .” Plant Cell 18: 3158–3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, R. , Richardson E. A., and Ye Z.‐H.. 2007. “Two NAC Domain Transcription Factors, SND1 and NST1, Function Redundantly in Regulation of Secondary Wall Synthesis in Fibers of Arabidopsis .” Planta 225: 1603–1611. [DOI] [PubMed] [Google Scholar]
- Zuñiga‐Mayo, V. M. , Baños‐Bayardo C. R., Díaz‐Ramírez D., Marsch‐Martínez N., and de Folter S.. 2018. “Conserved and Novel Responses to Cytokinin Treatments During Flower and Fruit Development in Brassica napus and Arabidopsis thaliana .” Scientific Reports 8: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No restrictions on the data.


