Abstract
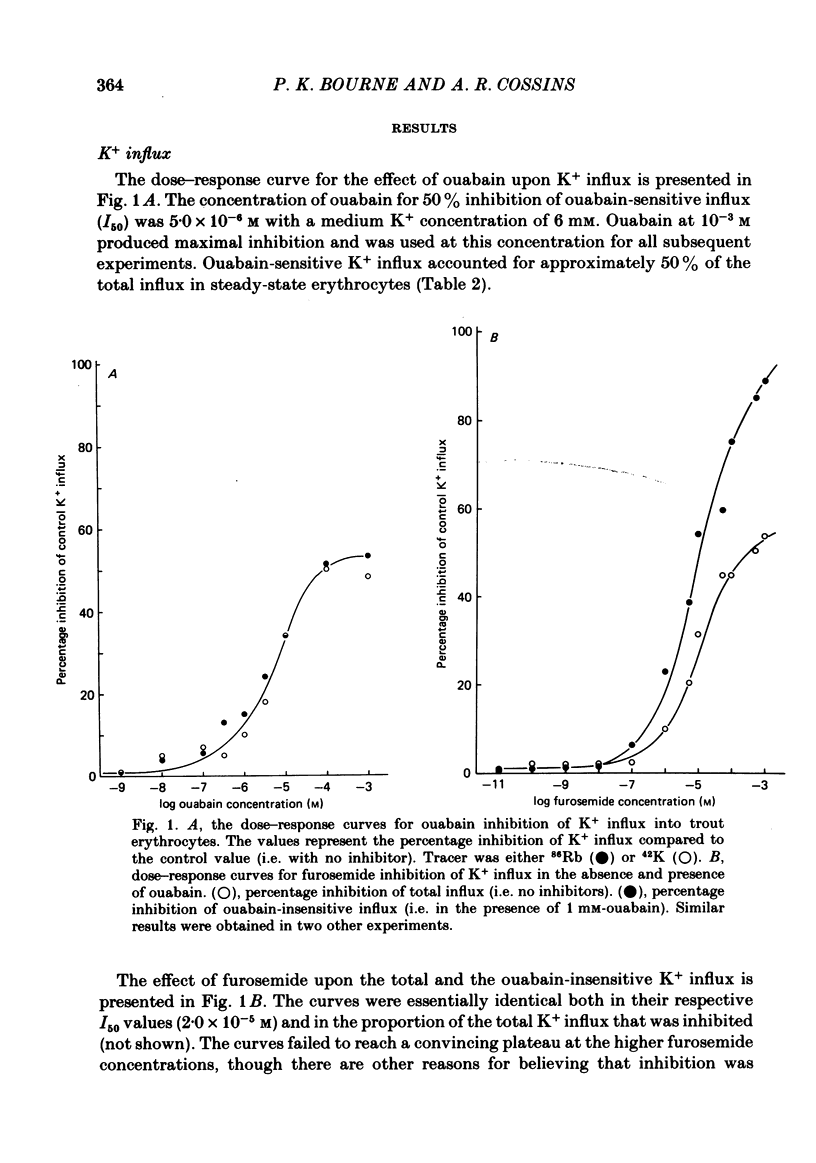
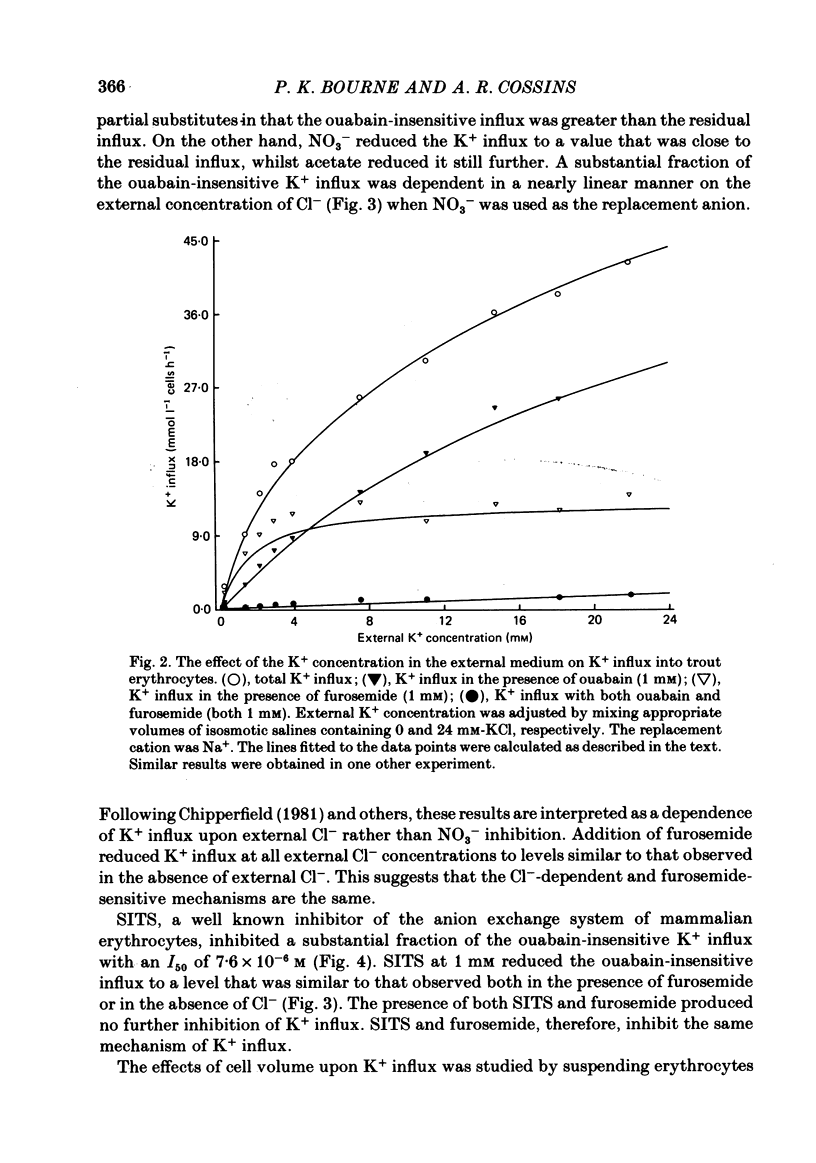
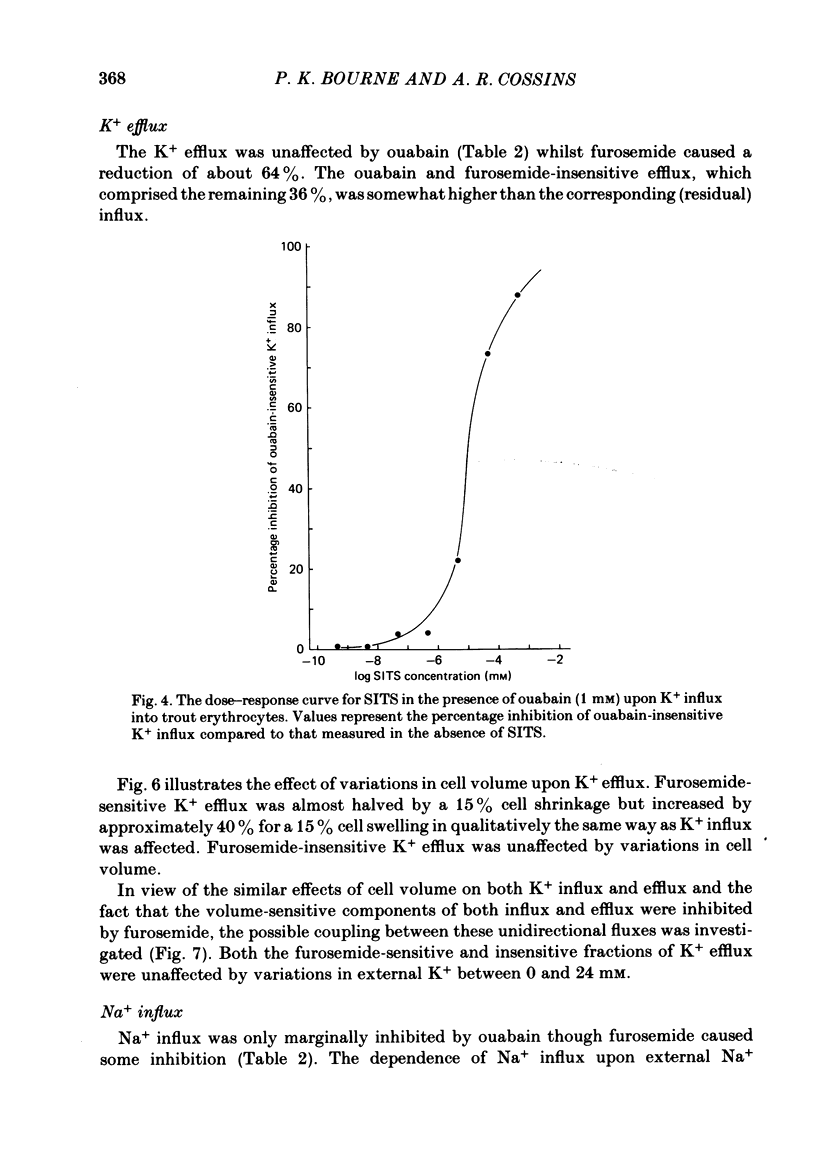
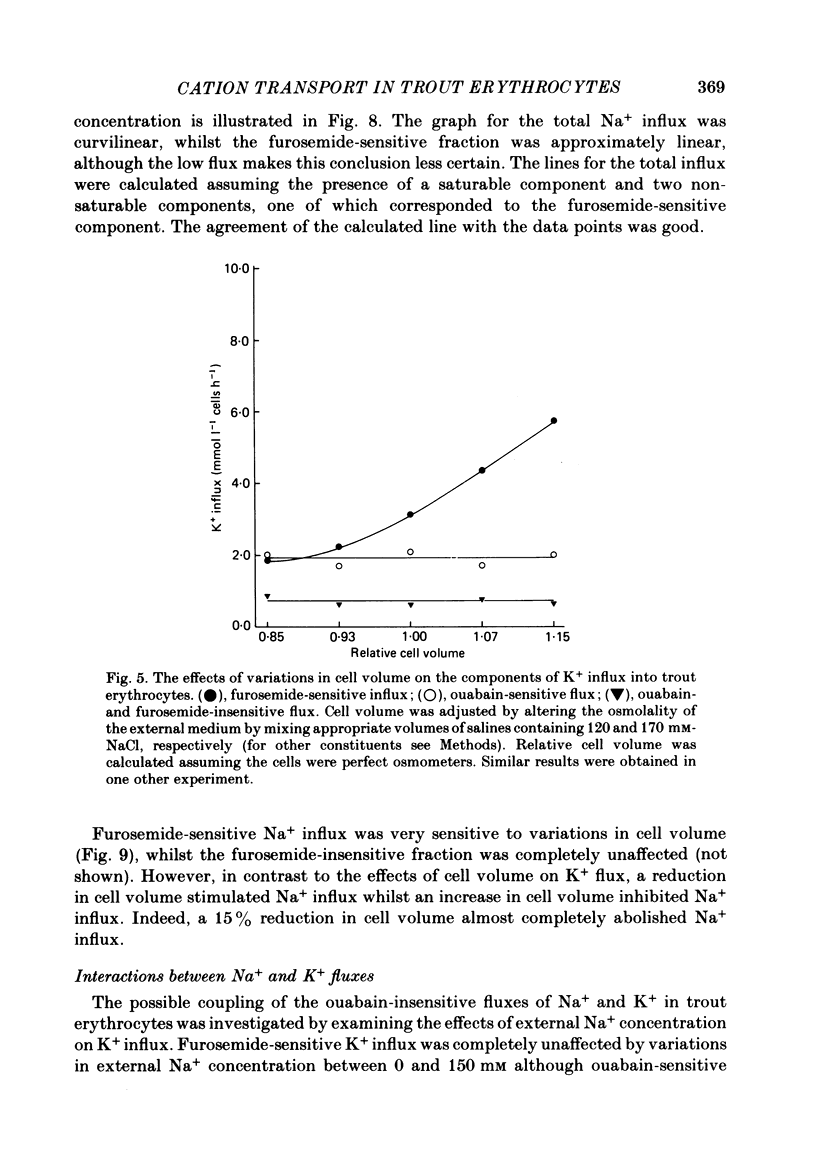
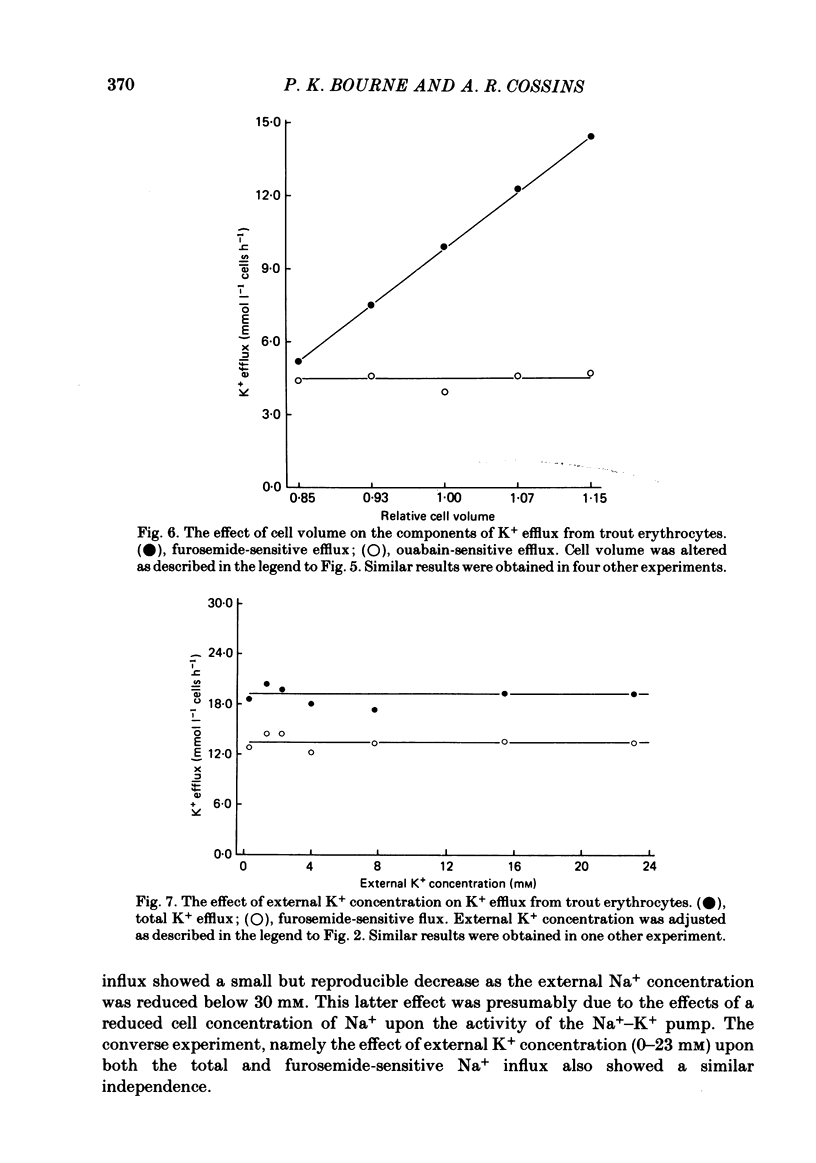
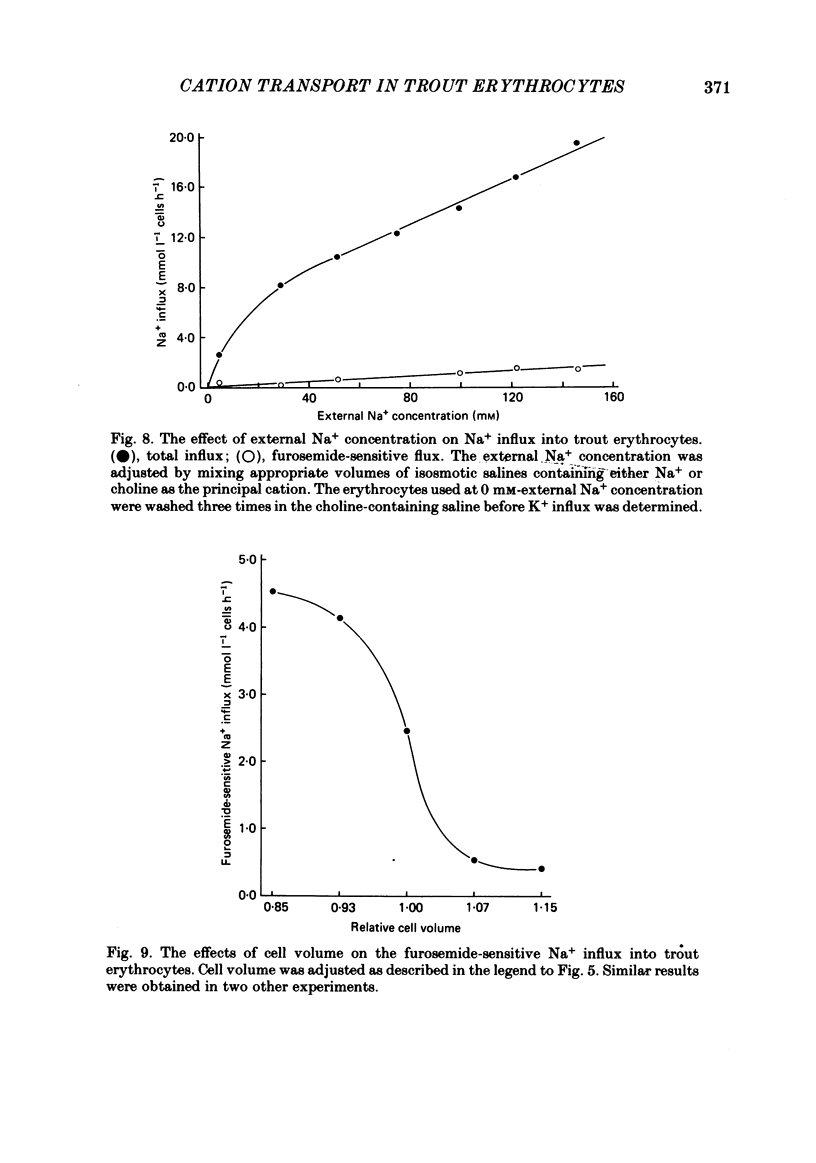
The principal pathways of Na+ and K+ transport in trout erythrocytes have been characterized. Approximately 50% of K+ influx in steady-state erythrocytes was inhibited by ouabain (1 mM) and 46% by furosemide (1 mM). Furosemide-sensitive K+ influx was a saturable function of external K+ concentration with a Km of 25 mM. This flux component was also inhibited by SITS (4-acetamido-4'-isothiocyanatostilbene-2'2-disulphonate) (concentration required for 50% inhibition, I50 = 7.6 X 10(-6)M) and by the removal of external Cl-. An increase in cell volume stimulated furosemide-sensitive K+ influx and cell shrinkage inhibited this flux. K+ efflux was mainly furosemide-sensitive (64% of total). This pathway was unaffected by variations in extracellular K+ concentration and is therefore not exchange diffusion. However, it was affected by variations in cell volume in a similar way to the furosemide-sensitive K+ influx. Na+ influx was only slightly sensitive to furosemide (13% of total) but this component was very sensitive to changes in cell volume; decreased cell volume increased Na+ influx whilst increased cell volume inhibited Na+ influx. Furosemide-sensitive K+ influx was unaffected by variations in external Na+ concentration. Similarly, furosemide-sensitive Na+ influx was unaffected by variations in external K+ concentration. This indicates that the passive influxes of Na+ and K+ were not coupled, in contrast to the situation in avian erythrocytes. The opposite effects of cell volume upon passive Na+ and K+ fluxes are in good agreement with the net movements of these cations during volume regulation in erythrocytes of the flounder (Cala, 1977) and the toadfish (Lauf, 1982).
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiton J. F., Chipperfield A. R., Lamb J. F., Ogden P., Simmons N. L. Occurrence of passive furosemide-sensitive transmembrane potassium transport in cultured cells. Biochim Biophys Acta. 1981 Sep 7;646(3):389–398. doi: 10.1016/0005-2736(81)90307-2. [DOI] [PubMed] [Google Scholar]
- Bakker-Grunwald T., Ogden P., Lamb J. F. Effects of ouabain and osmolarity on bumetanide-sensitive potassium transport in simian virus-transformed 3T3 cells. Biochim Biophys Acta. 1982 May 7;687(2):333–336. doi: 10.1016/0005-2736(82)90564-8. [DOI] [PubMed] [Google Scholar]
- Bakker-Grunwald T., Sinensky M. 86Rb+ fluxes in Chinese hamster ovary cells as a function of membrane cholesterol content. Biochim Biophys Acta. 1979 Dec 12;558(3):296–306. doi: 10.1016/0005-2736(79)90264-5. [DOI] [PubMed] [Google Scholar]
- Bourne P. K., Cossins A. R. On the instability of K+ influx in erythrocytes of the rainbow trout, Salmo gairdneri, and the role of catecholamine hormones in maintaining in vivo influx activity. J Exp Biol. 1982 Dec;101:93–104. doi: 10.1242/jeb.101.1.93. [DOI] [PubMed] [Google Scholar]
- Cala P. M. Volume regulation by Amphiuma red blood cells. The membrane potential and its implications regarding the nature of the ion-flux pathways. J Gen Physiol. 1980 Dec;76(6):683–708. doi: 10.1085/jgp.76.6.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cala P. M. Volume regulation by flounder red blood cells in anisotonic media. J Gen Physiol. 1977 May;69(5):537–552. doi: 10.1085/jgp.69.5.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet E., Verdonck F. Reduction of potassium permeability by chloride substitution in cardiac cells. J Physiol. 1977 Feb;265(1):193–206. doi: 10.1113/jphysiol.1977.sp011712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipperfield A. R. Chloride dependence of frusemide- and phloretin-sensitive passive sodium and potassium fluxes in human red cells. J Physiol. 1981 Mar;312:435–444. doi: 10.1113/jphysiol.1981.sp013636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham P. B., Ellory J. C. Passive potassium transport in low potassium sheep red cells: dependence upon cell volume and chloride. J Physiol. 1981 Sep;318:511–530. doi: 10.1113/jphysiol.1981.sp013881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham P. B., Stewart G. W., Ellory J. C. Chloride-activated passive potassium transport in human erythrocytes. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1711–1715. doi: 10.1073/pnas.77.3.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fugelli K. Regulation of cell volume in flounder (Pleuronectes flesus) erythrocytes accompanying a decrease in plasma osmolarity. Comp Biochem Physiol. 1967 Jul;22(1):253–260. doi: 10.1016/0010-406x(67)90185-5. [DOI] [PubMed] [Google Scholar]
- Funder J., Wieth J. O. Effects of some monovalent anions on fluxes of Na and K, and on glucose metabolism of ouabain treated human red cells. Acta Physiol Scand. 1967 Oct-Nov;71(2):168–185. doi: 10.1111/j.1748-1716.1967.tb03723.x. [DOI] [PubMed] [Google Scholar]
- Geck P., Pietrzyk C., Burckhardt B. C., Pfeiffer B., Heinz E. Electrically silent cotransport on Na+, K+ and Cl- in Ehrlich cells. Biochim Biophys Acta. 1980 Aug 4;600(2):432–447. doi: 10.1016/0005-2736(80)90446-0. [DOI] [PubMed] [Google Scholar]
- Greaney G. S., Powers D. A. Allosteric modifiers of fish hemoglobins: in vitro and in vivo studies of the effect of ambient oxygen and pH on erythrocyte ATP concentrations. J Exp Zool. 1978 Mar;203(3):339–350. doi: 10.1002/jez.1402030302. [DOI] [PubMed] [Google Scholar]
- Haas M., Schmidt W. F., 3rd, McManus T. J. Catecholamine-stimulated ion transport in duck red cells. Gradient effects in electrically neutral [Na + K + 2Cl] Co-transport. J Gen Physiol. 1982 Jul;80(1):125–147. doi: 10.1085/jgp.80.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlish S. J., Ellory J. C., Lew V. L. Evidence against Na+-pump mediation of Ca2+-activated K+ transport and diuretic-sensitive (Na+/K+)-cotransport. Biochim Biophys Acta. 1981 Aug 20;646(2):353–355. doi: 10.1016/0005-2736(81)90343-6. [DOI] [PubMed] [Google Scholar]
- Kregenow F. M. Osmoregulatory salt transporting mechanisms: control of cell volume in anisotonic media. Annu Rev Physiol. 1981;43:493–505. doi: 10.1146/annurev.ph.43.030181.002425. [DOI] [PubMed] [Google Scholar]
- Riddick D. H., Kregenow F. M., Orloff J. The effect of norepinephrine and dibutyryl cyclic adenosine monophosphate on cation transport in duck erythrocytes. J Gen Physiol. 1971 Jun;57(6):752–766. doi: 10.1085/jgp.57.6.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rindler M. J., McRoberts J. A., Saier M. H., Jr (Na+,K+)-cotransport in the Madin-Darby canine kidney cell line. Kinetic characterization of the interaction between Na+ and K+. J Biol Chem. 1982 Mar 10;257(5):2254–2259. [PubMed] [Google Scholar]
- Schmidt W. F., 3rd, McManus T. J. Ouabain-insensitive salt and water movements in duck red cells. I. Kinetics of cation transport under hypertonic conditions. J Gen Physiol. 1977 Jul;70(1):59–79. doi: 10.1085/jgp.70.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt W. F., 3rd, McManus T. J. Ouabain-insensitive salt and water movements in duck red cells. III. The role of chloride in the volume response. J Gen Physiol. 1977 Jul;70(1):99–121. doi: 10.1085/jgp.70.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley J. S., Cooper R. A. A furosemide-sensitive cotransport of sodium plus potassium in the human red cell. J Clin Invest. 1974 Mar;53(3):745–755. doi: 10.1172/JCI107613. [DOI] [PMC free article] [PubMed] [Google Scholar]


