Abstract
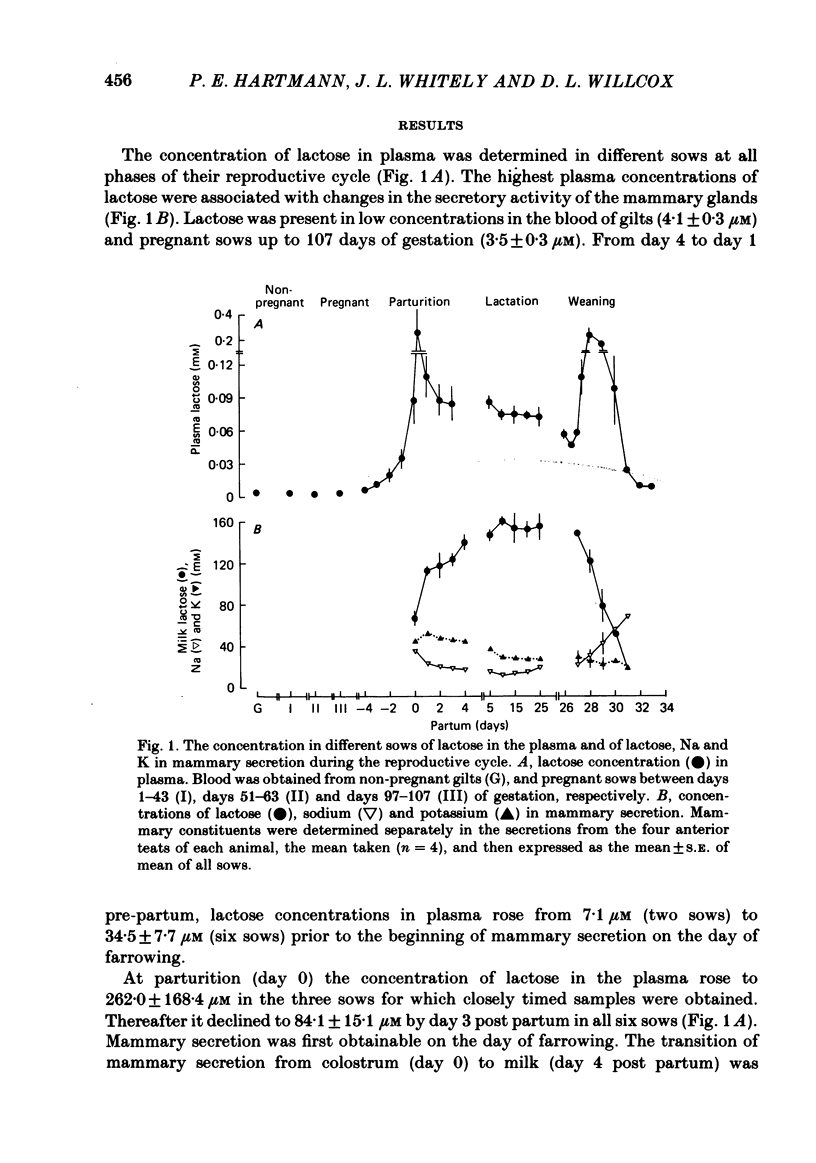
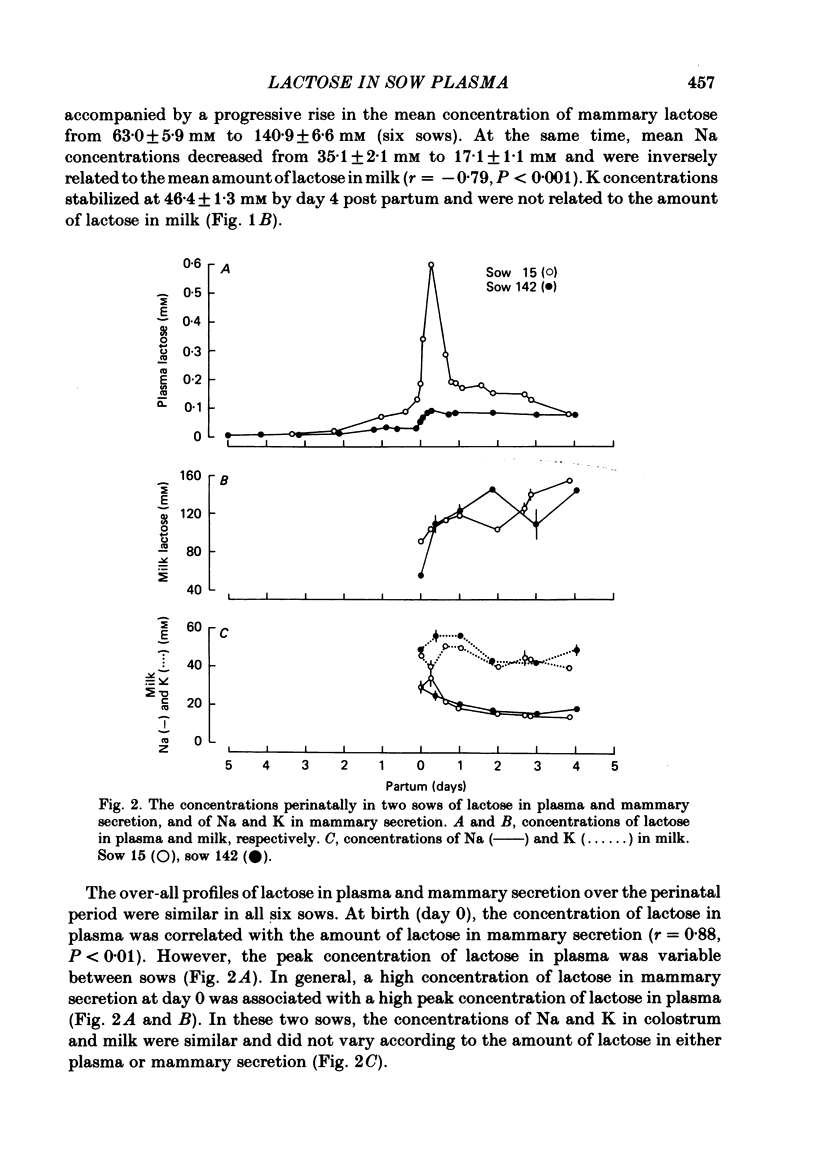
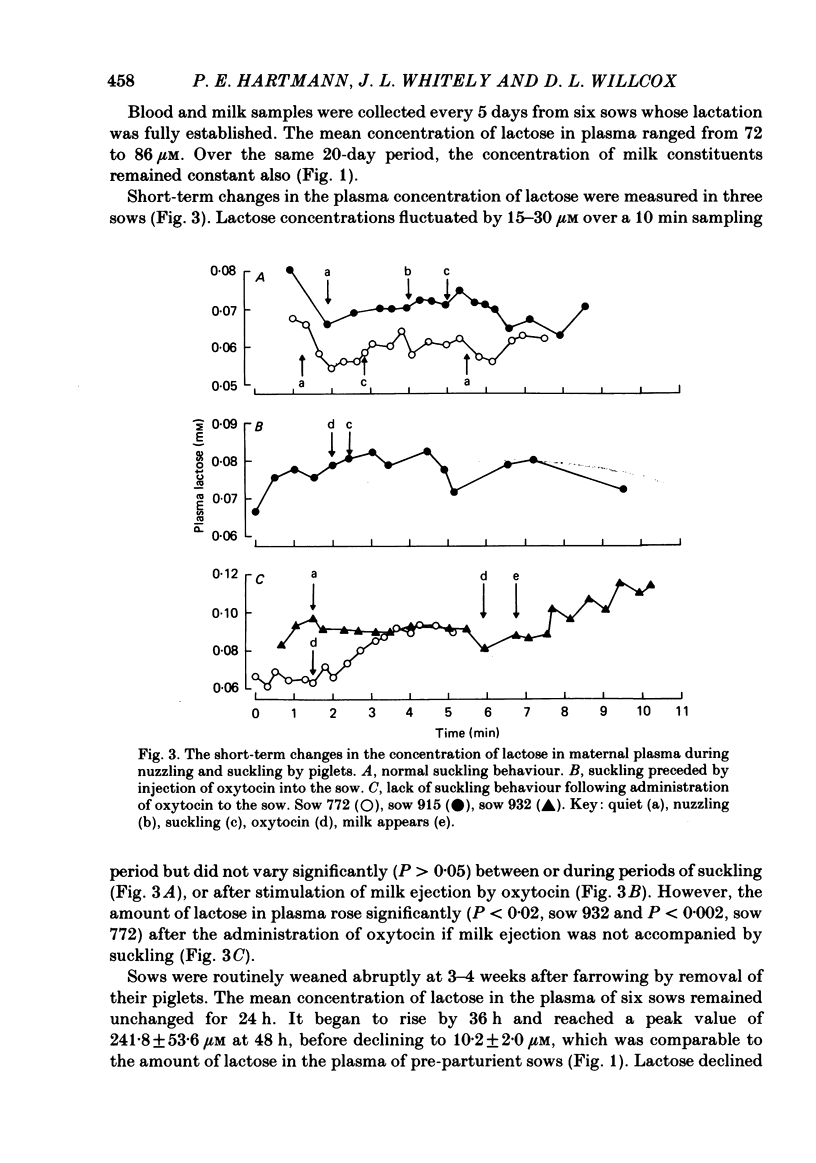
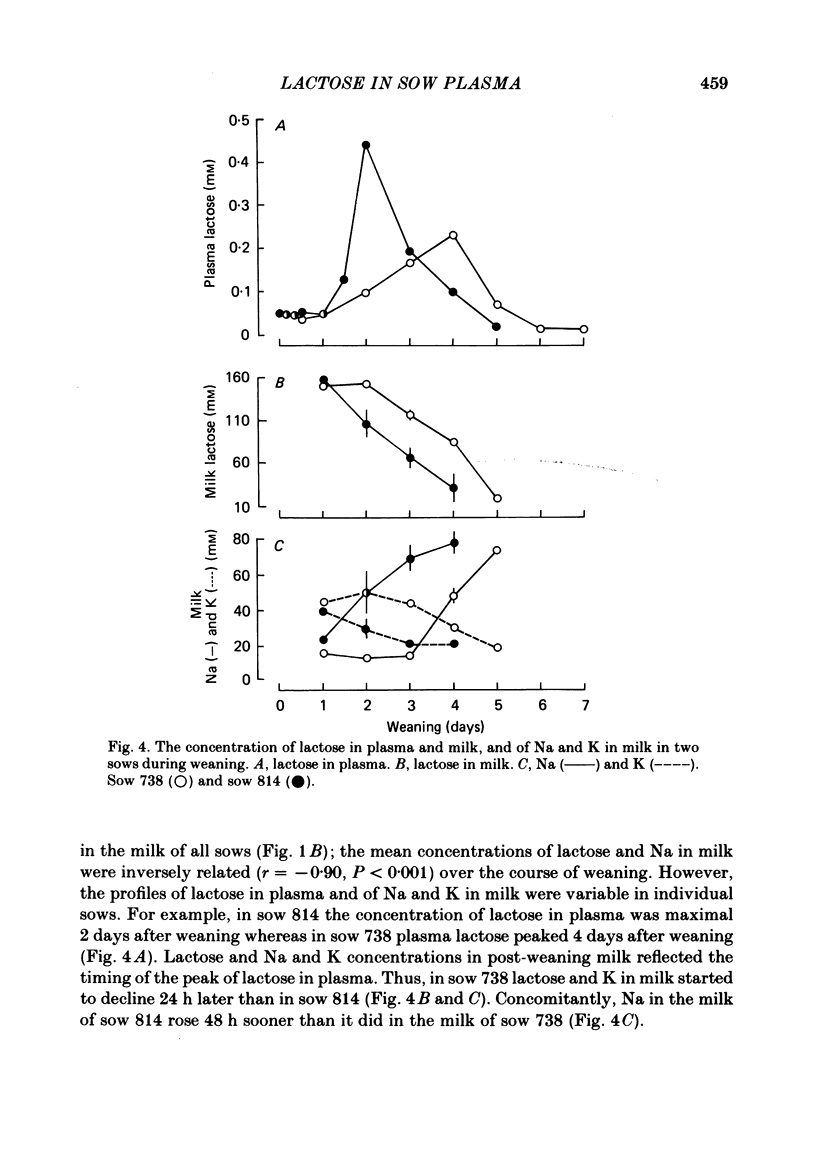
The concentration of lactose in plasma was determined in different sows at all phases of their reproductive cycle and related to the compositional changes in mammary secretion during lactogenesis, established lactation and weaning. Lactose was present in low concentrations (3-4 microM) in the blood of virgin sows and pregnant sows up to 107 days of gestation. From day 4 pre-partum to day 1 pre-partum circulating lactose rose gradually to 34.5 +/- 7.7 microM (mean +/- S.E. of mean). Maximal concentrations of 262 +/- 168.4 microM were reached 6 h after parturition. The concentration of lactose in plasma was correlated with the amount of lactose in mammary secretion (r = 0.88, P less than 0.01) at the beginning of farrowing. During established lactation the concentrations of lactose, Na and K in milk, and of lactose in plasma (72-86 microM), were constant. The concentration of lactose in plasma did not vary significantly during periods of suckling, or after stimulation of milk ejection by oxytocin. However, the amount of lactose in plasma rose significantly (P less than 0.02) after the administration of oxytocin if milk ejection was not accompanied by suckling. The mean plasma concentration of lactose began to rise 36 h after weaning to a peak value of 241.8 +/- 53.6 microM at 48 h; thereafter it declined to 10.2 +/- 2.0 microM by 6 days. This study has shown that lactose concentrations in the plasma vary according to the secretory activity of the mammary gland. Its plasma concentration provides an earlier temporal measure of lactogenesis in individual sows than is obtained either from observation or analysis of mammary secretion.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brew K. Secretion of alpha-lactalbumin into milk and its relevance to the organization and control of lactose synthetase. Nature. 1969 May 17;222(5194):671–672. doi: 10.1038/222671a0. [DOI] [PubMed] [Google Scholar]
- CHADWICK A. The onset of lactose synthesis after injection of prolactin. Biochem J. 1962 Dec;85:554–558. doi: 10.1042/bj0850554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROSS B. A., GOODWIN R. F., SILVER I. A. A histological and functional study of the mammary gland in normal and agalactic sows. J Endocrinol. 1958 May;17(1):63–74. doi: 10.1677/joe.0.0170063. [DOI] [PubMed] [Google Scholar]
- Fleet I. R., Peaker M. Mammary function and its control at the cessation of lactation in the goat. J Physiol. 1978 Jun;279:491–507. doi: 10.1113/jphysiol.1978.sp012358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUNTHER M., HAWKINS D. F., WHYLEY G. A. SOME OBSERVATIONS ON THE SODIUM AND POTASSIUM CONTENT OF HUMAN MILK. J Obstet Gynaecol Br Commonw. 1965 Feb;72:69–74. doi: 10.1111/j.1471-0528.1965.tb01375.x. [DOI] [PubMed] [Google Scholar]
- Gooneratne A., Hartmann P. E., McCauley I., Martin C. E. Control of parturition in the sow using progesterone and prostaglandin. Aust J Biol Sci. 1979 Dec;32(6):587–595. doi: 10.1071/bi9790587. [DOI] [PubMed] [Google Scholar]
- Hartmann P. E. Changes in the composition and yield of the mammary secretion of cows during the initiation of lactation. J Endocrinol. 1973 Nov;59(2):231–247. doi: 10.1677/joe.0.0590231. [DOI] [PubMed] [Google Scholar]
- Hartmann P. E., Kulski J. K. Changes in the composition of the mammary secretion of women after abrupt termination of breast feeding. J Physiol. 1978 Feb;275:1–11. doi: 10.1113/jphysiol.1978.sp012173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann P. E., Trevethan P., Shelton J. N. Progesterone and oestrogen and the initiation of lactation in ewes. J Endocrinol. 1973 Nov;59(2):249–259. doi: 10.1677/joe.0.0590249. [DOI] [PubMed] [Google Scholar]
- Kuhn N. J., Lowenstein J. M. Lactogenesis in the rat. Changes in metabolic parameters at parturition. Biochem J. 1967 Dec;105(3):995–1002. doi: 10.1042/bj1050995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn N. J. Progesterone withdrawal as the lactogenic trigger in the rat. J Endocrinol. 1969 May;44(1):39–54. doi: 10.1677/joe.0.0440039. [DOI] [PubMed] [Google Scholar]
- Kulski J. K., Buehring G. C. Microanalysis of lactose in tissue culture medium using an enzymatic-fluorometric method. Anal Biochem. 1982 Jan 15;119(2):341–350. doi: 10.1016/0003-2697(82)90596-6. [DOI] [PubMed] [Google Scholar]
- Kulski J. K., Hartmann P. E. Changes in human milk composition during the initiation of lactation. Aust J Exp Biol Med Sci. 1981 Feb;59(1):101–114. doi: 10.1038/icb.1981.6. [DOI] [PubMed] [Google Scholar]
- Kulski J. K., Smith M., Hartmann P. E. Perinatal concentrations of progesterone, lactose and alpha-lactalbumin in the mammary secretion of women. J Endocrinol. 1977 Sep;74(3):509–510. doi: 10.1677/joe.0.0740509. [DOI] [PubMed] [Google Scholar]
- Linzell J. L., Peaker M. Changes in colostrum composition and in the permeability of the mammary epithelium at about the time of parturition in the goat. J Physiol. 1974 Nov;243(1):129–151. doi: 10.1113/jphysiol.1974.sp010746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzell J. L., Peaker M., Taylor J. C. The effects of prolactin and oxytocin on milk secretion and on the permeability of the mammary epithelium in the rabbit. J Physiol. 1975 Dec;253(2):547–563. doi: 10.1113/jphysiol.1975.sp011206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzell J. L., Peaker M. The effects of oxytocin and milk removal on milk secretion in the goat. J Physiol. 1971 Aug;216(3):717–734. doi: 10.1113/jphysiol.1971.sp009549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C. E., Hartmann P. E., Gooneratne A. Progesterone and corticosteroids in the initiation of lactation in the sow. Aust J Biol Sci. 1978 Oct;31(5):517–525. doi: 10.1071/bi9780517. [DOI] [PubMed] [Google Scholar]
- Morgan G., Wooding F. B. A freeze-fracture study of tight junction structure in sheep mammary gland epithelium during pregnancy and lactation. J Dairy Res. 1982 Feb;49(1):1–11. doi: 10.1017/s002202990002207x. [DOI] [PubMed] [Google Scholar]
- Nara B. S., First N. L. Effect of indomethacin and prostaglandin F2 alpha on parturition in swine. J Anim Sci. 1981 Jun;52(6):1360–1370. doi: 10.2527/jas1981.5261360x. [DOI] [PubMed] [Google Scholar]
- Nara B. S., Welk F. A., Rutherford J. E., Sherwood O. D., First N. L. Effect of relaxin on parturition and frequency of live births in pigs. J Reprod Fertil. 1982 Sep;66(1):359–365. doi: 10.1530/jrf.0.0660359. [DOI] [PubMed] [Google Scholar]
- Nicholas K. R., Hartmann P. E. Progesterone control of the initiation of lactose synthesis in the rat. Aust J Biol Sci. 1981;34(4):435–443. doi: 10.1071/bi9810435. [DOI] [PubMed] [Google Scholar]
- Peaker M. Mechanism of milk secretion: milk composition in relation to potential difference across the mammary epithelium. J Physiol. 1977 Sep;270(2):489–505. doi: 10.1113/jphysiol.1977.sp011964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peaker M. Recent advances in the study of monovalent ions movements across the mammary epithelium: relation to onset of lactation. J Dairy Sci. 1975 Jul;58(7):1042–1047. doi: 10.3168/jds.s0022-0302(75)84677-7. [DOI] [PubMed] [Google Scholar]
- Peaker M. The effect of raised intramammary pressure on mammary function in the goat in relation to the cessation of lactation. J Physiol. 1980 Apr;301:415–428. doi: 10.1113/jphysiol.1980.sp013214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitelka D. R., Hamamoto S. T., Duafala J. G., Nemanic M. K. Cell contacts in the mouse mammary gland. I. Normal gland in postnatal development and the secretory cycle. J Cell Biol. 1973 Mar;56(3):797–818. doi: 10.1083/jcb.56.3.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHINDE Y., OTA K., YOKOYAMA A. LACTOSE CONTENT OF MAMMARY GLANDS OF PREGNANT RATS NEAR TERM: EFFECT OF REMOVAL OF OVARY, PLACENTA AND FOETUS. J Endocrinol. 1965 Jan;31:105–114. doi: 10.1677/joe.0.0310105. [DOI] [PubMed] [Google Scholar]
- Vitek V., Vitek K., Cowley R. A. Lactosuria - a new metabolic feature of severe cerebrocranial trauma. Clin Chim Acta. 1975 Jan 20;58(2):109–119. doi: 10.1016/s0009-8981(75)80003-9. [DOI] [PubMed] [Google Scholar]
- Weser E., Sleisenger M. H. Metabolism of circulating disaccharides in man and the rat. J Clin Invest. 1967 Apr;46(4):499–505. doi: 10.1172/JCI105552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcox D. L., Arthur P. G., Hartmann P. E., Whitely J. L. Perinatal changes in plasma oestradiol-17 beta, cortisol and progesterone and the initiation of lactation in sows. Aust J Biol Sci. 1983;36(2):173–181. doi: 10.1071/bi9830173. [DOI] [PubMed] [Google Scholar]


