Abstract
Seroprevalence of human herpesvirus 6 (HHV-6) and HHV-7 infections is very high throughout the world, and almost all people are exposed first to HHV-6 and second to HHV-7 in their childhood. However, it is not clear whether the neutralizing (NT) antibody response between each virus is cross-reactive or not. To elucidate the NT antibody response between each virus, 55 serum samples from an adult group (subjects 22 to 88 years old) and 60 serum samples from a young group (subjects 2 to 18 years old) were examined by a dot blot method for detecting viral late antigen. Thirty-nine serum samples obtained from cord bloods and a few serum samples obtained from pediatric patients with exanthem subitum were also examined to assess the maternal transferred NT antibodies against each virus. The NT antibody titers against HHV-7 in the adult group remained high throughout all the individuals, and none were negative. Those against HHV-6 were high values in the young group but low values, including negative values (three samples), in the adult group. These results suggested that the NT antibody response to either HHV-6 or HHV-7 in each individual was specific to each virus and did not cross-react with each other. In the adult group, the NT antibody response to HHV-6 decreased, while that to HHV-7 remained high throughout all the individuals. Maternal transferred NT antibody titers against HHV-7 were higher and remained longer after birth than those of HHV-6, and these findings were in accord with the clinical observation that HHV-6 infection usually occurs earlier than HHV-7 infection.
Human herpesvirus 6 (HHV-6) (19) and HHV-7 (9) have recently been discovered as etiologic agents of exanthem subitum (roseola). HHV-6 and HHV-7 are T-lymphotropic viruses and have been classified as betaherpesviruses. HHV-6 was first isolated from the peripheral blood lymphocytes of patients with AIDS (19) and has been divided into two variants, HHV-6A and HHV-6B (1, 2). HHV-7 was first isolated from the peripheral blood lymphocytes (9) and the saliva of healthy adults (5, 10, 12, 23, 27). Clinically, HHV-6B and HHV-7 are the common etiologic agents of exanthem subitum (roseola) (24, 29), but diseases caused by HHV-6A are less apparent. While diseases caused by primary infection of either HHV-6 or HHV-7 in childhood are usually not fatal, HHV-6 and HHV-7, as well as the other members of the herpesviruses, are thought to establish latent, life-long infection. It has been reported that HHV-6 may contribute to life-threatening diseases in immunosuppressed conditions such as organ transplant and AIDS (3, 4, 6, 7, 16) and to drug-induced hypersensitivity syndrome (8, 21, 22, 25). Several investigators have reported that HHV-7 is easily isolated from the saliva of individuals who have antibodies to HHV-7 (10, 23). However, it is unknown which diseases can be caused by reactivated HHV-7.
Serologic studies showed that seroprevalence of HHV-6 and HHV-7 infections are very high throughout the world and that almost all people are exposed first to HHV-6 and second to HHV-7 in their childhood (17). Several serologic studies for detection of antibodies to either HHV-6 or HHV-7 were performed by indirect immunofluorescent (IF) antibody assay (IFA), enzyme-linked immunosorbent assay (ELISA), neutralization, radioimmunoprecipitation, and Western blotting (11, 17, 28). The neutralizing (NT) antibody response is thought to be important in preventing infection from these viruses. However, there have been few comparative studies among these reports on the humoral antibody response between HHV-6 and HHV-7, and none has reported on the cross-reactive response based on the NT antibodies between HHV-6 and HHV-7 in individuals. These facts prompted us to investigate the cross-reactive response of NT antibodies between each virus and to assess the maternal transferred NT antibodies. In this report, we thought that it was important to determine the degree of immunological cross-reactivity between HHV-6 and HHV-7 based on the NT antibodies, which have taken an important role in the prevention of infection. In order to assess the antibody response to each virus, we established a dot blot method for detecting the NT antibody (26, 28) and an ELISA method for detecting the immunoglobulin G (IgG) and IgM antibodies (32). Here, we describe the following. (i) NT antibody responses between HHV-6 and HHV-7 are specific and do not cross-react to each other. (ii) NT antibody response to HHV-6 decreases with aging, while that to HHV-7 is maintained highly throughout all individuals of all ages. (iii) Maternal transferred NT antibodies against HHV-6 and HHV-7 contribute to the sequential infection between each virus.
MATERIALS AND METHODS
Serum samples.
Sixty serum samples were selected from healthy individuals in different age groups from 2 to 18 years old (as the young group) who had had a medical examination within the 3 months from February to May in 1998 at Shingu Municipal Hospital, Shingu, Japan. Fifty-five serum samples were also selected from healthy individuals in different age groups from 22 to 89 years old (as the adult group) who had had a medical examination within the 3 months from February to May in 1996 at Tsukazaki Hospital, Himeji, Japan. Thirty-nine serum samples obtained from cord blood specimens were selected to evaluate the maternal NT antibody, which was collected in January 2001 at Sun Clinic, Okayama, Japan under informed consent from the mothers. Sequential serum samples were obtained from five pediatric patients with exanthem subitum who were 1 month to 1.5 years old and who had had a medical examination within the 4 years from 1992 to 1996 at Shingu Municipal Hospital under informed consent from their mothers. Thirty serum samples from pediatric patients from 5 months to 1.7 years old who were negative for either anti-HHV-6 or -7 antibody described below were selected as negative reference samples for determining the cutoff values for the ELISA for IgG [ELISA(IgG)] and ELISA(IgM). These samples were also collected at Shingu Municipal Hospital under informed consent from the mothers. All of the serum samples were stored at −20°C before use.
Host cells.
Fresh cord blood mononuclear cells (CBMCs) were prepared by centrifugation through a Ficoll-Conray gradient from heparinized samples and cultured for 3 days in RPMI 1640 medium containing 10% heat inactivated fetal bovine serum, recombinant human interleukin-2 (0.1 U/ml; GIBCO BRL Life Technology Inc., Grand Island, N.Y.), and phytohemagglutinin (5 μg/ml; Sigma Chemical Co., St. Louis, Mo.) at 37°C in a 5% CO2 incubator. After 3 days, the CBMCs were infected with the virus and cultured for different experiments in RPMI 1640 medium containing the above reagents.
Preparation of virus stocks.
The Z29 strain of HHV-6B and the SB strain of HHV-7 were used throughout this study and prepared as described elsewhere (26, 31). The cells infected with each virus were cocultivated with uninfected cells at a ratio of 1:5 for 7 days. Virus stocks were prepared by centrifugation of the culture fluids at 2,000 × g for 10 min and stored at −80°C. Titration of the virus stocks was performed by an end point dilution method using a dot blot assay (30, 31). In brief, 25 μl of the CBMCs (adjusted to 5 × 106 cells/ml) was divided into each well of a 96-well microtiter U-bottom plate. The CBMCs were then infected with 25 μl of the virus preparation (in 10-fold dilution series) and incubated for 7 days at 37°C in a 5% CO2 incubator. After incubation, the supernatant medium was removed and the cells were washed with phosphate-buffered saline (PBS) (pH 7.4). A dot blot assay to detect the viral antigens is described below. After treatment with Lumi-Phos 530, the membrane was exposed to Fuji RX-U film. The 50% cell culture infectious dose was calculated according to the method of Reed and Muench (18).
IFA.
An IFA described elsewhere (30) was performed to determine the titer of either anti-HHV-6 or -7 antibody in human serum samples. In brief, HHV-6- or -7-infected CBMCs were mounted on a 14-well slide, dried, and kept at −20°C in a freezer before use. Each serum was diluted 1:10 in PBS to prevent nonspecific reactions, and then twofold serial dilutions of the 1:10-diluted samples were made. The diluted samples were applied to each well of the slide fixed with acetone. The slides were incubated at 37°C for 30 min and washed in PBS for 15 min three times. The fluorescein isothiocyanate-conjugated goat anti-human IgG F(ab′)2 antibody (Cappel, West Chester, Pa.) was diluted 1:100 in PBS and added to each well of the slide. After 30 min of incubation at 37°C, the slides were washed and examined with a fluorescence microscope. The end point of the positive fluorescence was determined visually at a low magnification, and the IFA antibody titers were calculated as the reciprocal of the serum dilution.
Dot blot method for determining the NT antibody titers to HHV-6 and HHV-7.
A dot blot method described elsewhere (26, 32) was performed to determine the titers of NT antibody to HHV-6 and -7. In brief, 25 μl of serial twofold serum dilutions or medium without sera (control) were prepared on each well of a 96-well microtiter U-bottom plate and mixed with equal volumes of virus strains of HHV-6 or HHV-7 containing 2 × 104 50% tissue culture infectious doses/ml. After the microtiter plate was incubated for 1 h at 37°C in a CO2 incubator, 50 μl of CBMCs (2.5 × 106 cells/ml) was added to each well and centrifuged at 800 × g for 1 h. For removing the added sera, 100 μl of medium was added to each well of the plate and centrifuged at 800 × g for 5 min, and about 180 μl of the supernatant was aspirated. Two hundred microliters of medium was added to each well of the plate, and the microtiter plate was incubated for 7days at 37°C in a CO2 incubator.
To monitor the virus growth in each well, a dot blot antigen detection was performed as described previously (26, 30). In brief, after 7 days of incubation the microtiter plate was centrifuged at 500 × g for 10 min. The supernatant medium was removed and the cells were washed with PBS including Mg2+ and Ca2+ [PBS(+)] (pH 7.4). One hundred and eighty microliters of lysis buffer (20 mM Tris, 0.5 M NaCl, and 0.5% Nonidet P-40 [pH 7.5]) was added to each well. The cell lysates were spotted onto a nylon membrane (Boehringer Mannheim Biochemica, Indianapolis, Ind.), washed with blocking solution, and incubated with one of the monoclonal antibodies (MAb) described below overnight at 4°C. The membrane was washed, incubated with anti-mouse IgG-alkaline phosphatase conjugate (Sigma Chemical Co.), treated with Lumi-Phos 530 (Boehringer Mannheim Corporation), and exposed to Fuji RX-U film.
MAb.
MAb to HHV-6 and HHV-7 were established in our laboratory (26) and characterized by radioimmunoprecipitation. For monitoring HHV-6 replication, MAb TK-2, which recognizes a 135-kDa late polypeptide, was used. MAb TK-2 reacts to both variants of HHV-6. For HHV-7, MAb IK-3, which recognizes a 125-kDa corresponding polypeptide, was used. MAb IK-3 is specific to HHV-7.
Antigen preparation for ELISA.
The CBMCs infected with each virus of HHV-6 (Z29 strain) or HHV-7 (SB strain) were cultured for 5 to 7 days and harvested in 15-ml conical tubes at the period when the numbers of IF positive cells had reached almost 80%. The infected cells were pelletted down by centrifugation at 800 × g for 10 min, washed with PBS(+) (pH 7.4), and then centrifuged. The lysis buffer (20 mM Tris, 0.5 M NaCl, and 0.5% Nonidet P-40 [pH 7.5]) was added to each pellet at four times the pellet volume. The uninfected CBMCs were cultured for 6 days and prepared for control antigen the same way as described above.
ELISA.
Wells of a microplate (Nunc Immunoplate II; Nunc, Aarhus, Denmark) were coated with 50 μl of appropriate dilutions in PBS(+) of each virus or control antigen overnight at 4°C. After coating, the wells were washed and blocked for 30 min at room temperature by adding 200 μl of the blocking solution [PBS(+) containing 5% skim milk]. After discarding the blocking solution, 50 μl of serum diluted in the blocking solution (1:100 for IgG and 1:25 for IgM) was added to wells of the plate and incubated at 37°C for 2 h. The plate was washed five times with 200 μl of the blocking solution. Then, 50 μl of alkaline phosphatase-conjugated goat anti-human IgG (γ-chain specific) F(ab′)2 fragment (Sigma Chemical Company) or alkaline phosphatase-conjugated goat anti-human IgM (μ-chain specific) F(ab′)2 fragment (Sigma Chemical Company) diluted in the blocking solution (1:1,000) was added to each well of the plate. After 2 h of incubation at 37°C, the plate was washed five times with 200 μl of the blocking solution and twice with PBS(+). Seventy-five microliters of substrate, p-nitrophenylphosphate in diethanolamine buffer (alkaline phosphatase substrate kit [Bio-Rad]) was added to each well of the plate, and the plate was incubated at 37°C for 1 h. Then, the reaction was stopped by adding 75 μl of 0.4 M NaOH, and the absorbance of each well at 405 nm was read with the microplate reader (model 550; Bio-Rad). The results (net absorbance) were expressed as the absorbency reading of each of the viral-antigen-coated wells minus the absorbency reading for the wells coated with control antigen.
For ELISA(IgG) antibody, the absorbance readings (mean ± standard deviation [SD]) for HHV-6 and HHV-7 obtained from about 30 negative reference serum samples of pediatric patients were 0.052 ± 0.067 and 0.027 ± 0.034, respectively. All of the samples studied were negative for IF and NT antibody titers against HHV-6. These samples were also negative for IF antibody against HHV-7, and for eight of these samples the titer of NT antibody against HHV-7 was under 1:8. Studied samples with net absorbance readings of ≥3 SD above the mean absorbance of the negative reference sera were considered reactive for anti-HHV-6 or HHV-7 antibody. The cutoff values for HHV-6 and -7 were determined with the absorbance readings of 0.26 and 0.13, respectively. For ELISA(IgM) antibody, about 20 negative reference serum samples which were negative for IF, NT, and ELISA(IgG) antibodies against HHV-6 and HHV-7 were selected from reference sera for ELISA(IgG) antibody. The absorbance readings (mean ± SD) for HHV-6 and HHV-7 obtained from these sera were 0.049 ± 0.030 and 0.033 ± 0.027, respectively, and the cutoff values for HHV-6 and -7 were determined with the absorbance readings of 0.14 and 0.12, respectively.
RESULTS
NT antibody responses against HHV-6 and HHV-7 do not cross-react with each other.
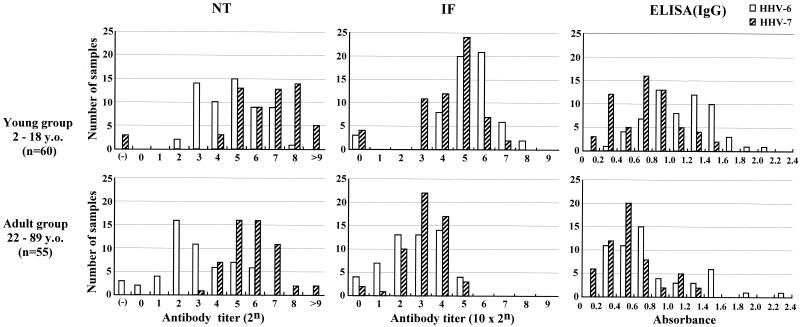
First, we compared the NT, IF, and ELISA(IgG) antibody titers against HHV-6 and HHV-7 in 115 serum samples, including 55 serum samples from the adult group and 60 serum samples from the young group. A histogram based on each antibody titer against these viruses is shown in Fig. 1. The NT antibody responses against HHV-6 and HHV-7 showed different distributions between each virus. The NT antibody titers against HHV-7 were high throughout individuals of all ages, ranging from 1:8 to 1:>512 (geometric mean titer = 25.64). A few samples were negative for those antibodies in the young group, but none were negative in the adult group. In contrast, the NT antibody titers against HHV-6 in the young group (geometric mean titer = 24.52) were high and comparable to those of HHV-7 in the young group (geometric mean titers = 25.63), while significantly low titers, including negative titers (ranging from 1:<1 to 1:64), were observed in the adult group (geometric mean titer = 22.64). No significant difference in distribution patterns of either IF antibody or ELISA(IgG) antibody titers was observed between HHV-6 and HHV-7, although these distribution patterns were generally higher in the young group than in the adult group.
FIG. 1.
Comparative study of NT antibody titers, IF antibody titers, and ELISA(IgG) antibody titers against HHV-6 and HHV-7. A histogram based on the titers of each antibody against these viruses were illustrated for 60 serum samples from the young group (subjects 2 to 18 years old [y.o.]) and 55 serum samples from the adult group (subjects 22 to 88 y.o.), respectively. A histogram based on ELISA(IgG) antibody titers was plotted by intervals of 0.2 optical density units. Secondary antibody used for IF antibody detection was directed against whole IgG (heavy plus light chain) of human immunoglobulins. Secondary antibody used for ELISA(IgG) antibody detection was directed against γ-chain specific IgG of human immunoglobulins.
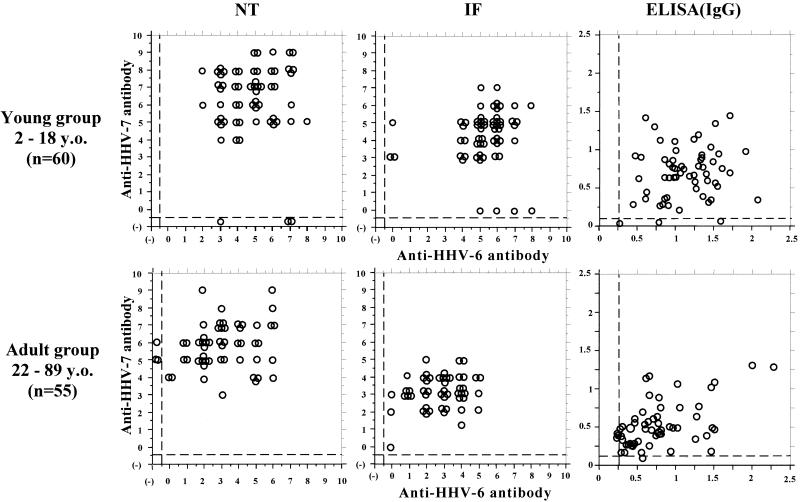
Second, to further elucidate the humoral antibody responses between HHV-6 and HHV-7 in each individual, we analyzed the scattergrams based on the NT, IF, and ELISA(IgG) antibodies between each virus (Fig. 2). The NT antibody responses between each virus were distinct in each individual. The sera with high titers of anti-HHV-7 antibodies do not always have high titers of anti-HHV-6 antibodies, while some sera negative for anti-HHV-6 NT antibodies have high titers for anti-HHV-7 antibodies. Namely, the NT antibodies between HHV-6 and HHV-7 did not correlate in each individual (Wilcoxon signed rank test, P < 0.01; Spearman rank correlation, P > 0.01). Figure 2 shows that the IF and ELISA(IgG) antibody titers in each individual did not apparently correlate either.
FIG. 2.
Comparative study of titers of NT antibody, IF antibody, and ELISA(IgG) antibody against HHV-6 and HHV-7 in each individual. Scattergrams based on each antibody titer between each virus were illustrated for 60 serum samples from the young group (subjects 2 to 18 years old [y.o.]) and 55 serum samples from the adult group (subjects 22 to 88 y.o.), respectively. Negative values are shown under the dashed line. Secondary antibody used for IF antibody detection was directed against whole IgG (heavy plus light chain) of human immunoglobulins. Secondary antibody used for ELISA(IgG) antibody detection was directed against γ-chain-specific IgG of human immunoglobulins.
These results revealed that the NT antibody response to either HHV-6 or HHV-7 in each individual was specific to each virus and that they did not cross-react with each other.
Maternal transferred NT antibodies against HHV-6 and HHV-7 contribute to the sequential infection between each virus.
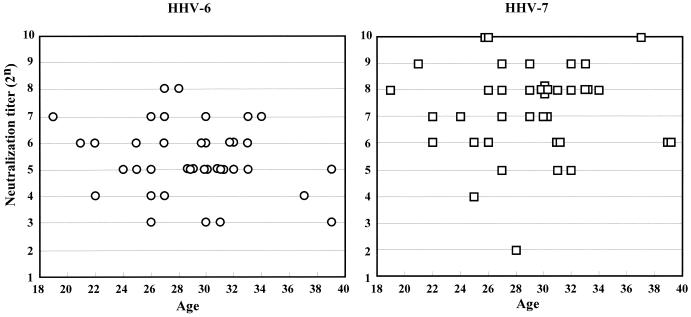
First, 39 serum samples obtained from cord blood specimens were used to evaluate the NT antibody titers against HHV-6 and HHV-7 transferred from mothers to children (Fig. 3). The transferred NT antibody titers against HHV-7 (geometric mean titer = 27.03) were higher than those against HHV-6 (geometric mean titer = 25.22) among almost all the samples. The ELISA(IgG) antibody titers of antibodies to HHV-6 and HHV-7 did not represent a significant difference (data not shown). These agree well with the results described above.
FIG. 3.
Maternal transferred NT antibody titers against HHV-6 and HHV-7 were compared in serum obtained from cord blood specimens.
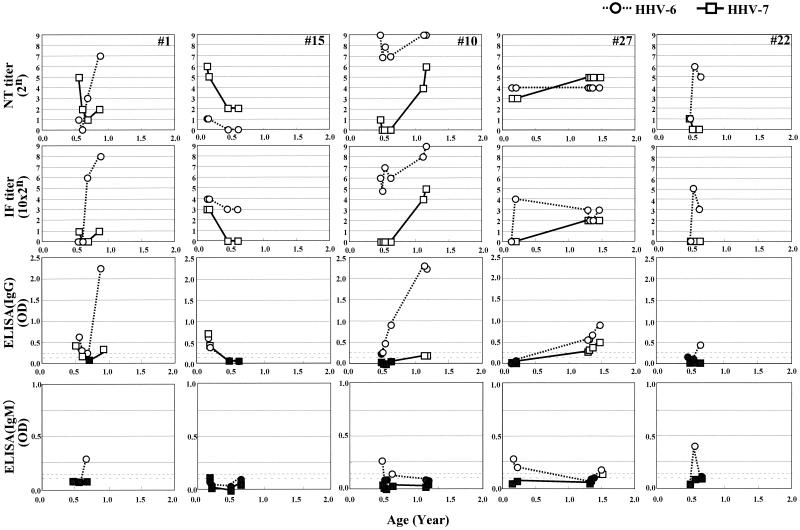
Second, we monitored the NT antibody titers against these viruses in five children with exanthem subitum. The ELISA(IgM) antibodies, as well as the IF antibody and ELISA(IgG) antibodies, were also examined to determine the primary infection of these viruses, and the results are shown in Fig. 4. The titers of NT antibody against HHV-7 in early time point samples obtained sequentially were maintained higher than those of HHV-6 among three samples (samples 1, 15, and 22, shown in Fig. 4). Among them, two samples (samples 1 and 22) represented seroconversion against HHV-6 after the NT antibody titers decreased to almost negative values. The ELISA(IgM) antibody response against HHV-6 was detected temporally at the same time of elevation of the NT and IF antibodies and just before elevation of the ELISA(IgG) antibodies. One sample (sample 15, shown in Fig. 4) revealed reversion of antibody titers between the NT and IF antibodies. The titers of NT antibody against HHV-6 were lower than those of HHV-7 and almost negative, while the titers of IF antibody against HHV-6 were higher than those against HHV-7. These data suggest that the IF antibody response is not useful to elucidate the transferred antibody that prevent infection with these viruses. In the case of two samples (samples 10 and 27, shown in Fig. 4), the NT antibodies against HHV-6 were maintained higher than those of HHV-7. It was difficult to determine which was the transferred antibody from the mother or the primary infection of HHV-6. However, these two samples revealed an apparent seroconversion against HHV-6, because the ELISA(IgM) antibodies were found in the first point of the sequentially obtained samples. In the case of sample 10, the patient was diagnosed with exanthem subitum twice. These data revealed that this patient experienced exanthem subitum caused first by HHV-6 infection and second by HHV-7 infection.
FIG. 4.
Changes of NT antibody titers, IF antibody titers, and ELISA(IgG) and ELISA(IgM) antibody titers against HHV-6 and HHV-7 in five infants diagnosed with exanthem subitum. Shaded symbols represent negative values for ELISA(IgG) and ELISA(IgM) antibody titers for each virus. Dotted lines with short intervals represent the cutoff value for HHV-6, while dotted lines with long intervals represent the cutoff value for HHV-7.
It is not easy to collect serum samples from subjects within 6 months after birth. In this work, only 15 serum samples were obtained from eight patients in this periods. We have calculated the geometric mean titers of these samples. Among them, 7 serum samples were apparently negative for HHV-6 and 11 serum samples were also negative for HHV-7. The geometric mean titers of antibodies against HHV-6 and HHV-7 were 21.10 and 22.14, respectively. We also selected serum samples within 3 months after birth which were apparently negative for both viruses and calculated the geometric mean titers. The geometric mean titers were 21.26 and 24.04 for HHV-6 (three serum samples) and HHV-7 (five serum samples), respectively. Within 6 months after birth, two patients experienced exanthem subitum caused by HHV-6. In case of one patient (data not shown), it occurred within 3 months, and HHV-6 was isolated from peripheral blood mononuclear cells. Although sample numbers were not enough to conclude that the protective effect of HHV-7 maternal transferred NT antibody plays a role, these results suggested that the average titer of NT antibodies against HHV-7 in cord blood was approximately 2 logs higher than those against HHV-6 and that HHV-7 maternal transferred NT antibodies were maintained higher than those of HHV-6 within 6 months after birth.
These results suggested that the maternal transferred NT antibody response to HHV-7 was maintained at a high level from just after birth and was delayed in decreasing, while those to HHV-6 were maintained at considerably lower levels from just after birth and decreased faster than those to HHV-7. These findings were in accord with the clinical observation that HHV-6 infection usually occurs earlier than HHV-7 infection.
DISCUSSION
Seroepidemiology of HHV-6 and HHV-7 has been based on the antibody titers determined by the IFA or ELISA methods. There have been some reports on the NT antibody assay for HHV-6, conventionally determining the end points by cytopathic changes or IF. A few of these were comparative studies of the titers of NT antibodies against HHV-6 and HHV-7 in each individual (26, 32). In the present study, we performed a dot blot method for viral late antigen detection to assess the NT antibodies described elsewhere (26, 32), because this method takes advantage of a reliable, reproducible and visible end point. This report precisely demonstrated the humoral immune responses based on the NT antibodies between HHV-6 and HHV-7 in each individual. The results suggest that the immunological cross-reactivity between HHV-6 and HHV-7 is not exhibited in the NT antibodies and that the neutralizing epitopes of these two viruses are apparently distinct (14, 20). Serologic studies also showed that almost all individuals are exposed first to HHV-6 and second to HHV-7 in their childhood, acquire the NT antibodies against these viruses, and keep them at high levels for 2 or 3 decades after primary infection (11, 13, 17, 28, 32). However, an interesting result in the present report was that the NT antibody response to HHV-6 was kept at significantly lower level than that to HHV-7. The continuation at high levels of anti-HHV-7 NT antibody titers may contribute to the finding that it was easy to isolate HHV-7 from adult saliva (10). In contrast, it was difficult to isolate HHV-6 from any source in healthy adults, while HHV-6 DNA was detected in adult saliva by PCR as well as HHV-7. That is, these results suggested that the continuous reactivation of HHV-7 might have an important role for keeping the NT antibody response at a high level. Serological investigation based on the NT antibody against HHV-6 will become a useful tool to determine the reactivation of HHV-6, because of the lower levels of anti-HHV-6 NT antibody response. Recently, drug hypersensitivity syndrome (DHS), which is a severe idiosyncratic reaction associated with drug therapy, has focused on the relationship with the reactivation of HHV-6 (8, 21, 22, 25). We plan a retrospective study to investigate the reactivation of HHV-6 associated with DHS using sera obtained from DHS patients.
In this report, we demonstrated that maternal transferred NT antibodies have an important role in preventing infection with each virus and that the levels of these transferred-NT antibody titers contributed to the sequential infection with each virus; namely, HHV-6 infects first and HHV-7 infects second. We also used the sera obtained from cord blood specimens to examine the maternal transferred NT antibody against HHV-6 and HHV-7 and demonstrated that maternal transferred NT antibodies against HHV-7 are maintained at much higher levels than those against HHV-6. These data are in accord with the results in paired sera obtained from mothers and cord blood specimens based on the IFA (15). These facts support the notion that infants after birth are protected from infection with these viruses by maternal transferred NT antibodies and acquire the primary infection after a decrease in these transferred NT antibodies.
Acknowledgments
We thank T. Yamagata and colleagues for helpful support and discussion.
Part of this work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Science, and Technology of Japan.
REFERENCES
- 1.Ablashi, D. V., H. Agut, Z. Berneman, G. Campadelli-Fiume, D. Carrigan, L. Ceccerini-Nelli, B. Chandran, S. Chou, H. Collandre, R. Cone, T. Dambaugh, S. Dewhurst, D. Diluca, L. Foa-Tomasi, B. Fleckenstein, N. Frenkel, R. Gallo, U. Gompels, C. Hall, M. Jomes, G. Lawrence, M. Martin, L. Montagnier, F. Neipel, J. Nicholas, P. Pellet, A. Razzaque, G. Torrelli, B. Thomson, S. Salahuddin, L. Wyatt, and K. Yamanishi. 1993. Human herpesvirus-6 strain groups: a nomenclature. Arch. Virol. 129:363-366.8385923 [Google Scholar]
- 2.Ablashi, D. V., N. Balachandran, S. F. Josephs, C. L. Hung, G. R. Krueger, B. Kramarsky, S. Z. Salahuddin, and R. C. Gallo. 1991. Genomic polymorphism, growth properties, and immunologic variations in human herpesvirus-6 isolates. Virology 184:545-552. [DOI] [PubMed] [Google Scholar]
- 3.Akashi, K., Y. Eizuru, Y. Sumiyoshi, T. Minematsu, S. Hara, M. Harada, M. Kikuchi, Y. Niho, and Y. Minamishima. 1993. Brief report: severe infectious mononucleosis-like syndrome and primary human herpesvirus 6 infection in an adult. N. Engl. J. Med. 329:168-171. [DOI] [PubMed] [Google Scholar]
- 4.Asano, Y., T. Yoshikawa, S. Suga, T. Yazaki, K. Kondo, and K. Yamanishi. 1990. Fatal fulminant hepatitis in an infant with human herpesvirus-6 infection. Lancet 335:862-863. [DOI] [PubMed] [Google Scholar]
- 5.Black, J. B., N. Inoue, K. Kite-Powell, S. Zaki, and P. E. Pellett. 1993. Frequent isolation of human herpesvirus 7 from saliva. Virus Res. 29:91-98. [DOI] [PubMed] [Google Scholar]
- 6.Buchwald, D., P. R. Cheney, D. L. Peterson, B. Henry, S. B. Wormsley, A. Geiger, D. V. Ablashi, S. Z. Salahuddin, C. Saxinger, R. Biddle, et al. 1992. A chronic illness characterized by fatigue, neurologic and immunologic disorders, and active human herpesvirus type 6 infection. Ann. Intern. Med. 116:103-113. [DOI] [PubMed] [Google Scholar]
- 7.Challoner, P. B., K. T. Smith, J. D. Parker, D. L. MacLeod, S. N. Coulter, T. M. Rose, E. R. Schultz, J. L. Bennett, R. L. Garber, M. Chang, P. A. Schad, P. M. Stewart, R. C. Nowinski, J. P. Brown, and G. C. Burmer. 1995. Plaque-associated expression of human herpesvirus 6 in multiple sclerosis. Proc. Natl. Acad. Sci. USA 92:7440-7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Descamps, V., F. Bouscarat, S. Laglenne, E. Aslangul, B. Veber, D. Descamps, J. L. Saraux, M. J. Grange, M. Grossin, E. Navratil, B. Crickx, and S. Belaich. 1997. Human herpesvirus 6 infection associated with anticonvulsant hypersensitivity syndrome and reactive haemophagocytic syndrome. Br. J. Dermatol. 137:605-608. [DOI] [PubMed] [Google Scholar]
- 9.Frenkel, N., E. C. Schirmer, L. S. Wyatt, G. Katsafanas, E. Roffman, R. M. Danovich, and C. H. June. 1990. Isolation of a new herpesvirus from human CD4+ T cells. Proc. Natl. Acad. Sci. USA 87:748-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujiwara, N., H. Namba, R. Ohuchi, H. Isomura, F. Uno, M. Yoshida, S. Nii, and M. Yamada. 2000. Monitoring of human herpesvirus-6 and -7 genomes in saliva samples of healthy adults by competitive quantitative PCR. J. Med. Virol. 61:208-213. [DOI] [PubMed] [Google Scholar]
- 11.Grose, C. 1996. Childhood infections with human herpesviruses types 6, 7, and 8. Adv. Pediatr. Infect. Dis. 12:181-208. [PubMed] [Google Scholar]
- 12.Hidaka, Y., Y. Liu, M. Yamamoto, R. Mori, C. Miyazaki, K. Kusuhara, K. Okada, and K. Ueda. 1993. Frequent isolation of human herpesvirus 7 from saliva samples. J. Med. Virol. 40:343-346. [DOI] [PubMed] [Google Scholar]
- 13.Levy, J. A. 1997. Three new human herpesviruses (HHV6, 7, and 8). Lancet 349:558-563. [DOI] [PubMed] [Google Scholar]
- 14.Lusso, P., P. Secchiero, R. W. Crowley, A. Garzino-Demo, Z. N. Berneman, and R. C. Gallo. 1994. CD4 is a critical component of the receptor for human herpesvirus 7: interference with human immunodeficiency virus. Proc. Natl. Acad. Sci. USA 91:3872-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohashi, M., M. Ihira, K. Suzuki, S. Suga, Y. Asano, T. Yoshikawa, Y. Saito, and H. Sakui. 2001. Transfer of human herpesvirus 6 and 7 antibodies from mothers to their offspring. Pediatr. Infect. Dis. J. 20:449-450. [DOI] [PubMed] [Google Scholar]
- 16.Okuno, T., K. Higashi, K. Shiraki, K. Yamanishi, M. Takahashi, Y. Kokado, M. Ishibashi, S. Takahara, T. Sonoda, K. Tanaka, and et al. 1990. Human herpesvirus 6 infection in renal transplantation. Transplantation 49:519-522. [DOI] [PubMed] [Google Scholar]
- 17.Pellet, P. E., and J. B. Black. 1996. Human herpesvirus 6, p. 2587-2608. In B. N. Field, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed., vol. 2. Lippincott-Raven Publishers, Philadelphia, Pa. [Google Scholar]
- 18.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg 27:493-497. [Google Scholar]
- 19.Salahuddin, S. Z., D. V. Ablashi, P. D. Markham, S. F. Josephs, S. Sturzenegger, M. Kaplan, G. Halligan, P. Biberfeld, F. Wong-Staal, B. Kramarsky, et al. 1986. Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science 234:596-601. [DOI] [PubMed] [Google Scholar]
- 20.Santoro, F., P. E. Kennedy, G. Locatelli, M. S. Malnati, E. A. Berger, and P. Lusso. 1999. CD46 is a cellular receptor for human herpesvirus 6. Cell 99:817-827. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan, J. R., and N. H. Shear. 2001. The drug hypersensitivity syndrome: what is the pathogenesis? Arch. Dermatol. 137:357-364. [PubMed] [Google Scholar]
- 22.Suzuki, Y., R. Inagi, T. Aono, K. Yamanishi, and T. Shiohara. 1998. Human herpesvirus 6 infection as a risk factor for the development of severe drug-induced hypersensitivity syndrome. Arch. Dermatol. 134:1108-1112. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi, Y., M. Yamada, J. Nakamura, T. Tsukazaki, J. Padilla, T. Kitamura, M. Yoshida, and S. Nii. 1997. Transmission of human herpesvirus 7 through multigenerational families in the same household. Pediatr. Infect. Dis. J. 16:975-978. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka, K., T. Kondo, S. Torigoe, S. Okada, T. Mukai, and K. Yamanishi. 1994. Human herpesvirus 7: another causal agent for roseola (exanthem subitum). J. Pediatr. 125:1-5. [DOI] [PubMed] [Google Scholar]
- 25.Tohyama, M., Y. Yahata, M. Yasukawa, R. Inagi, Y. Urano, K. Yamanishi, and K. Hashimoto. 1998. Severe hypersensitivity syndrome due to sulfasalazine associated with reactivation of human herpesvirus 6. Arch. Dermatol. 134:1113-1117. [DOI] [PubMed] [Google Scholar]
- 26.Tsukazaki, T., M. Yoshida, H. Namba, M. Yamada, N. Shimizu, and S. Nii. 1998. Development of a dot blot neutralizing assay for HHV-6 and HHV-7 using specific monoclonal antibodies. J. Virol. Methods 73:141-149. [DOI] [PubMed] [Google Scholar]
- 27.Wyatt, L. S., and N. Frenkel. 1992. Human herpesvirus 7 is a constitutive inhabitant of adult human saliva. J. Virol. 66:3206-3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamada, M. 2001. Human herpesviruses 6 and 7: effects on hematopoiesis and mode of transmission. Jpn. J. Infect. Dis. 54:47-54. [PubMed] [Google Scholar]
- 29.Yamanishi, K., T. Okuno, K. Shiraki, M. Takahashi, T. Kondo, Y. Asano, and T. Kurata. 1988. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet i:1065-1067. [DOI] [PubMed] [Google Scholar]
- 30.Yoshida, M., M. Yamada, S. Chatterjee, F. Lakeman, S. Nii, and R. J. Whitley. 1996. A method for detection of HHV-6 antigens and its use for evaluating antiviral drugs. J. Virol. Methods 58:137-143. [DOI] [PubMed] [Google Scholar]
- 31.Yoshida, M., M. Yamada, T. Tsukazaki, S. Chatterjee, F. D. Lakeman, S. Nii, and R. J. Whitley. 1998. Comparison of antiviral compounds against human herpesvirus 6 and 7. Antivir. Res. 40:73-84. [DOI] [PubMed] [Google Scholar]
- 32.Yoshida, M., S. Torigoe, and M. Yamada. 2002. Elucidation of the cross-reactive immunoglobulin M response to human herpesviruses 6 and 7 on the basis of neutralizing antibodies. Clin. Diagn. Lab. Immunol. 9:394-402. [DOI] [PMC free article] [PubMed]