Abstract
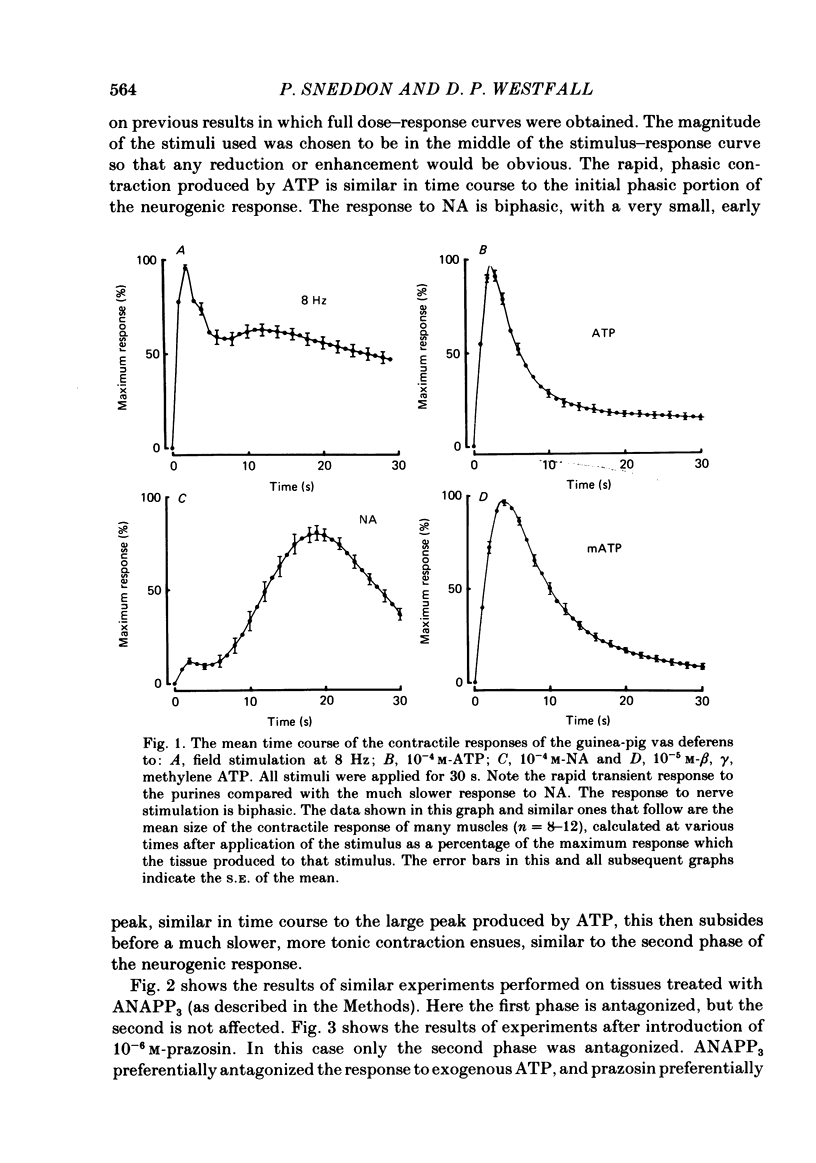
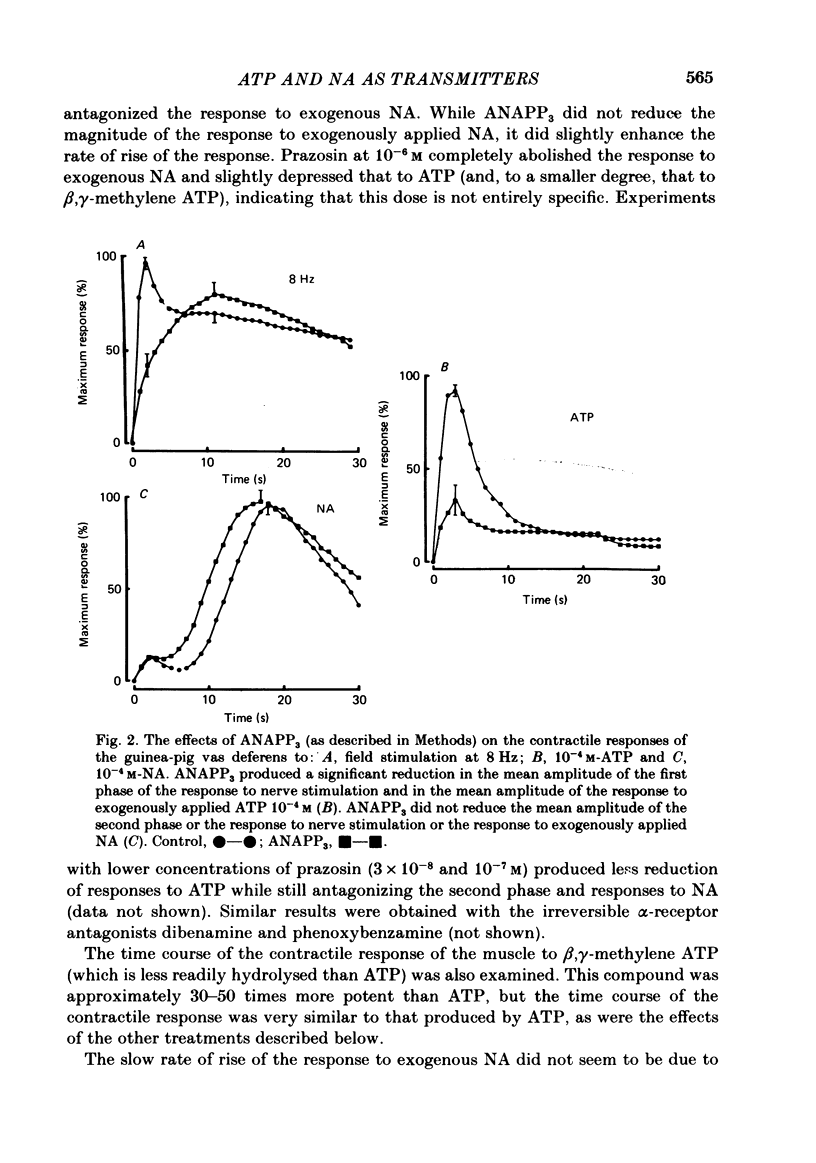
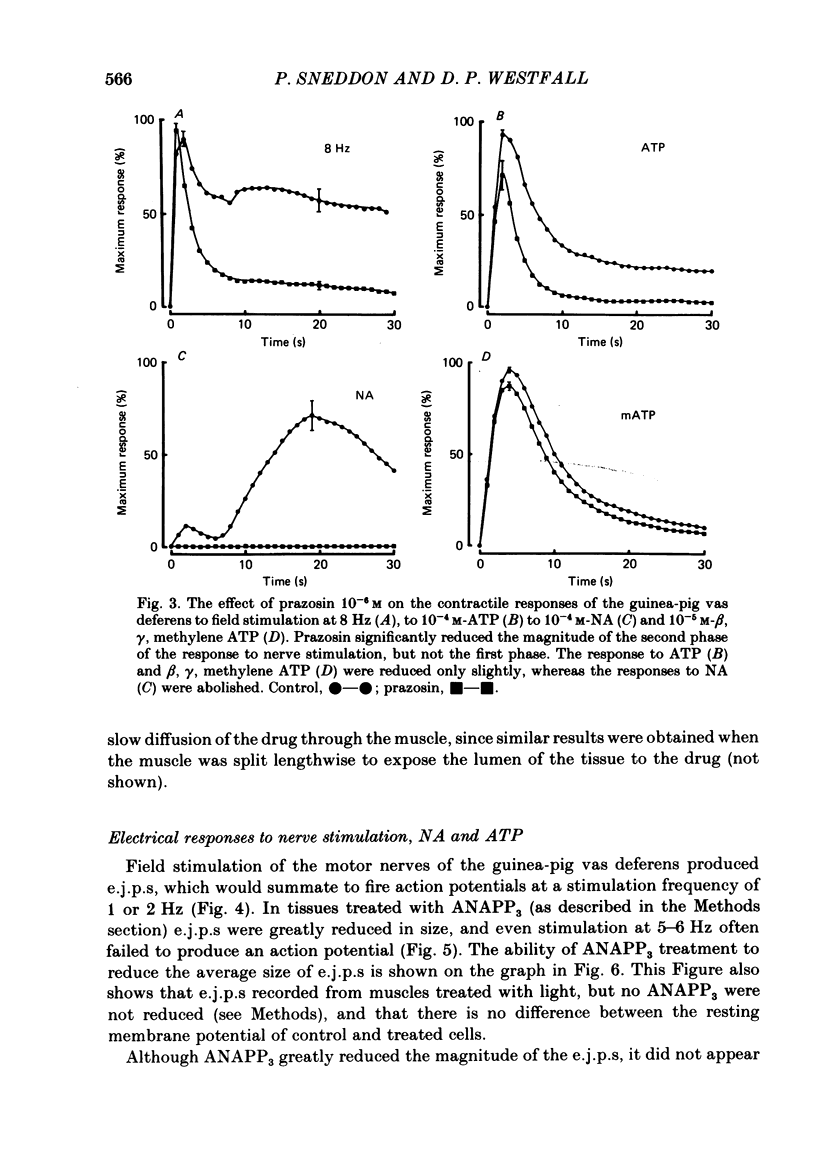
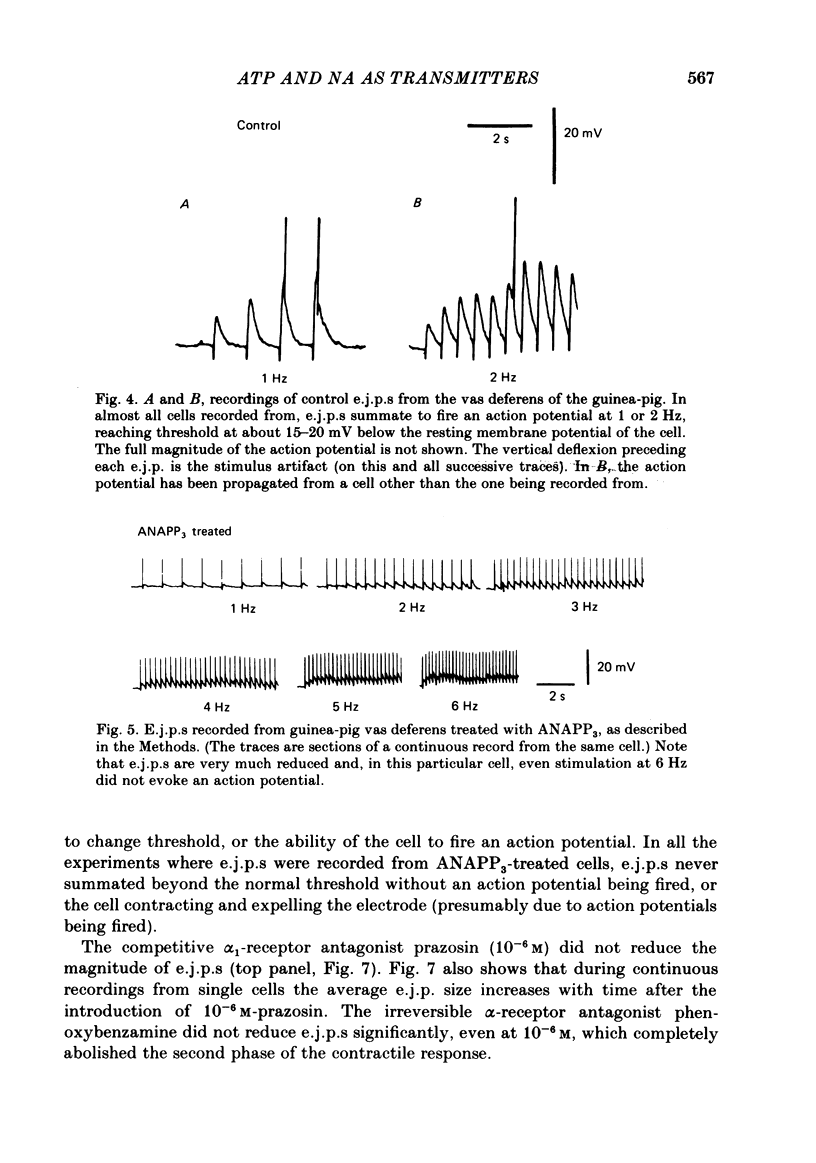
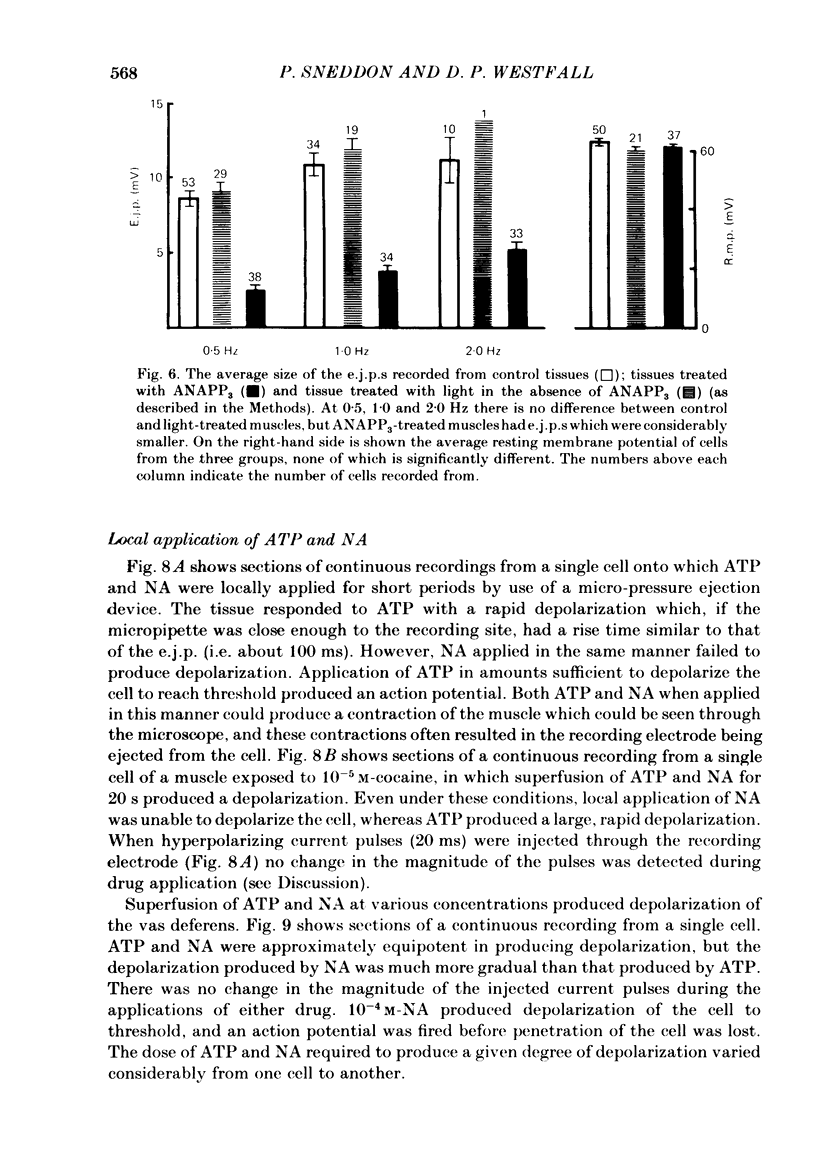
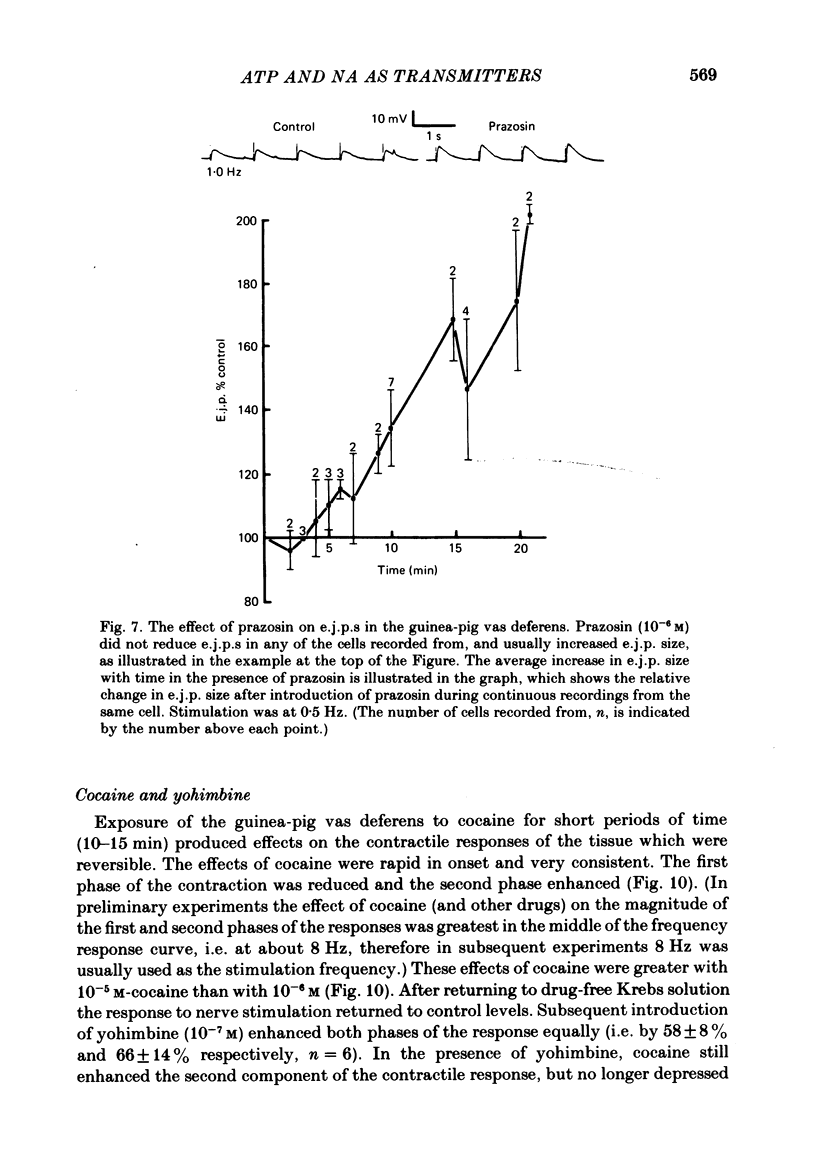
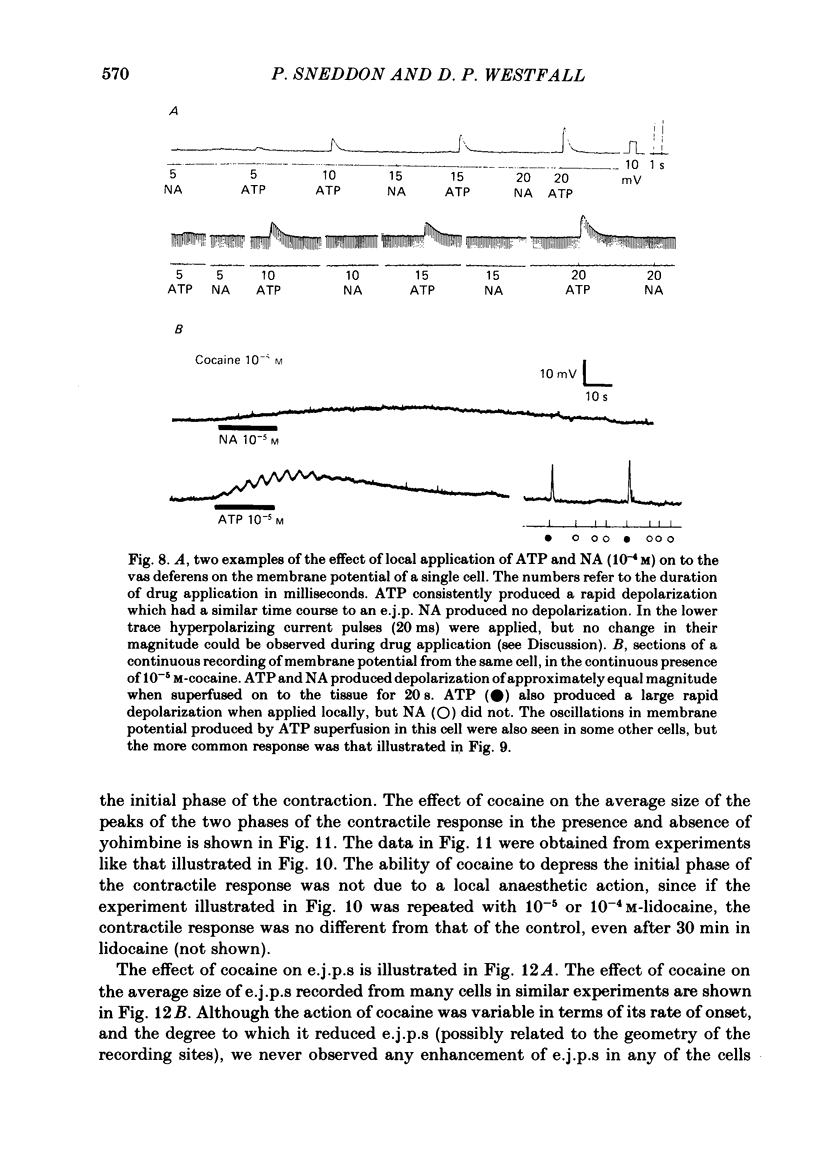
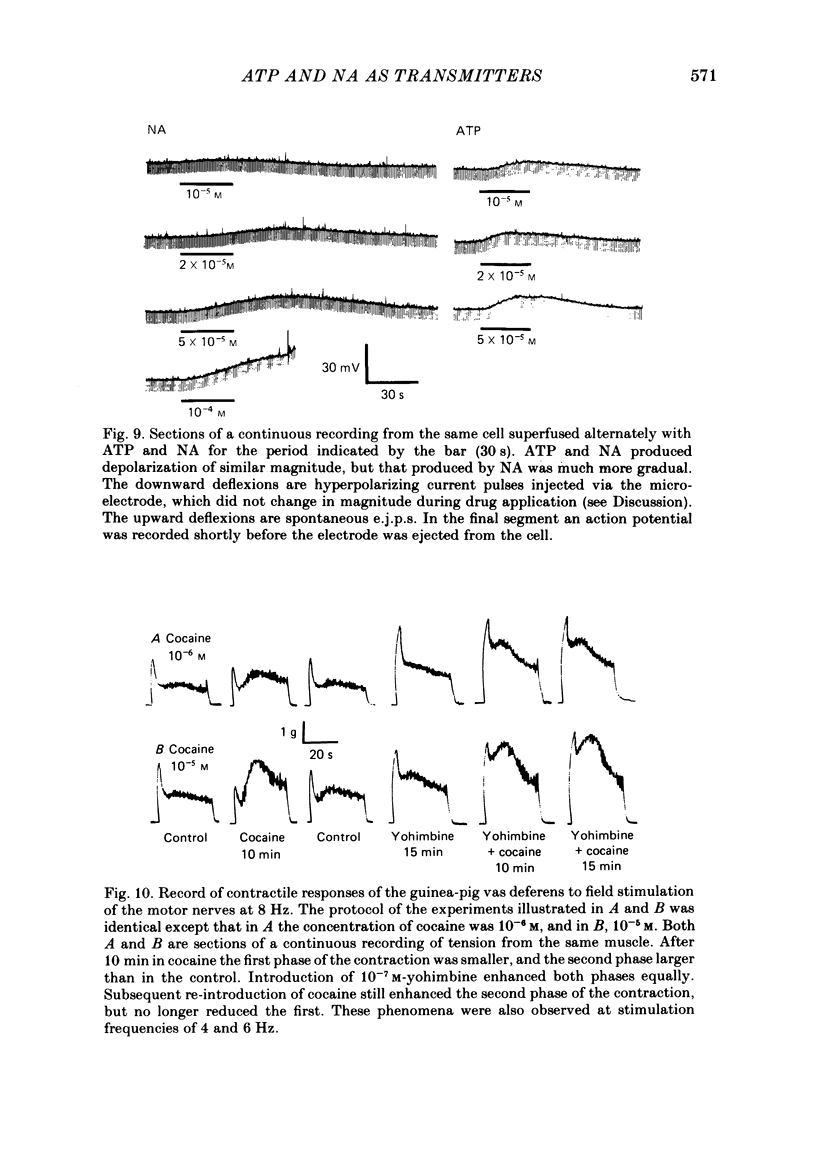
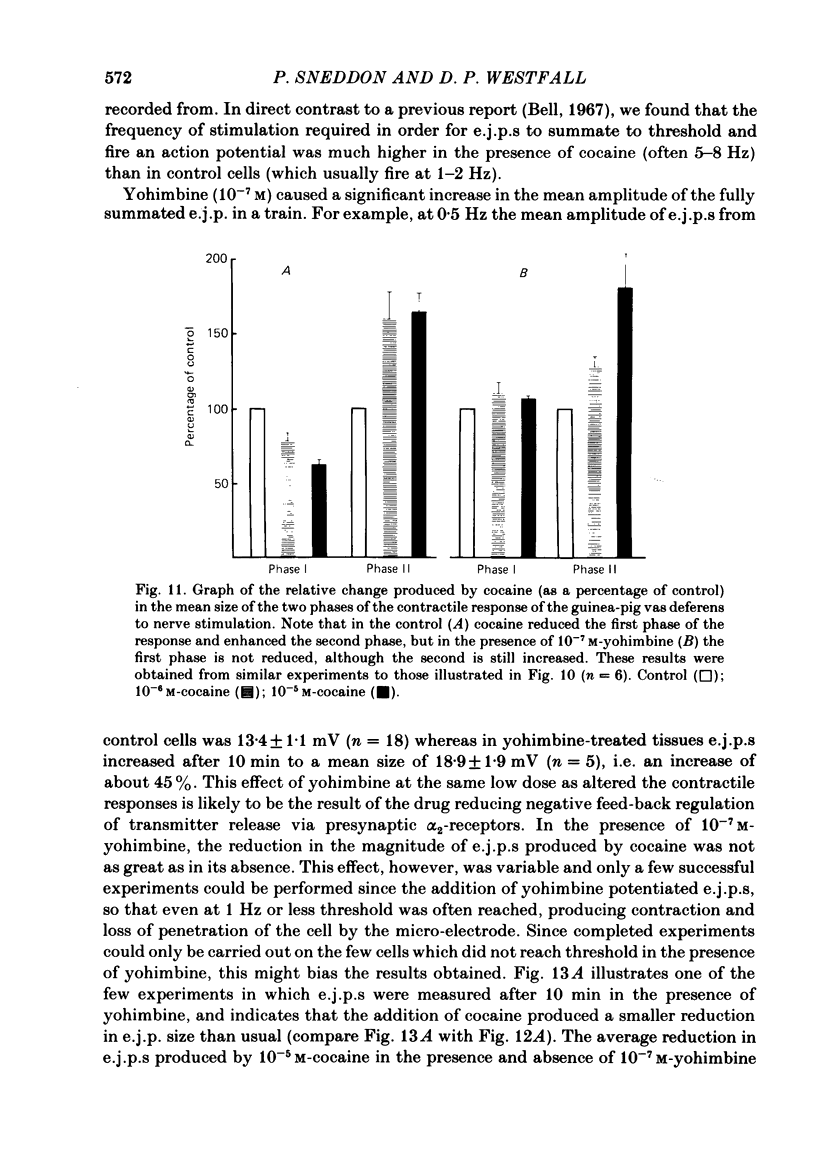
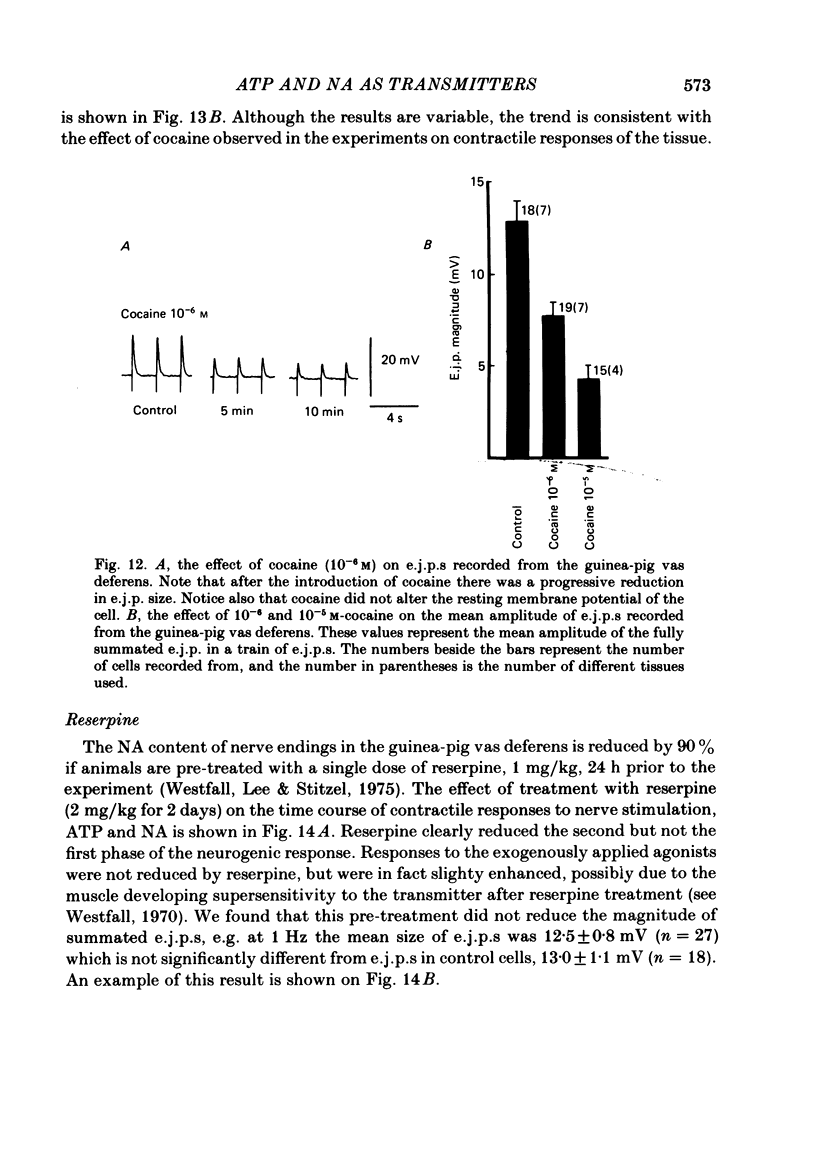
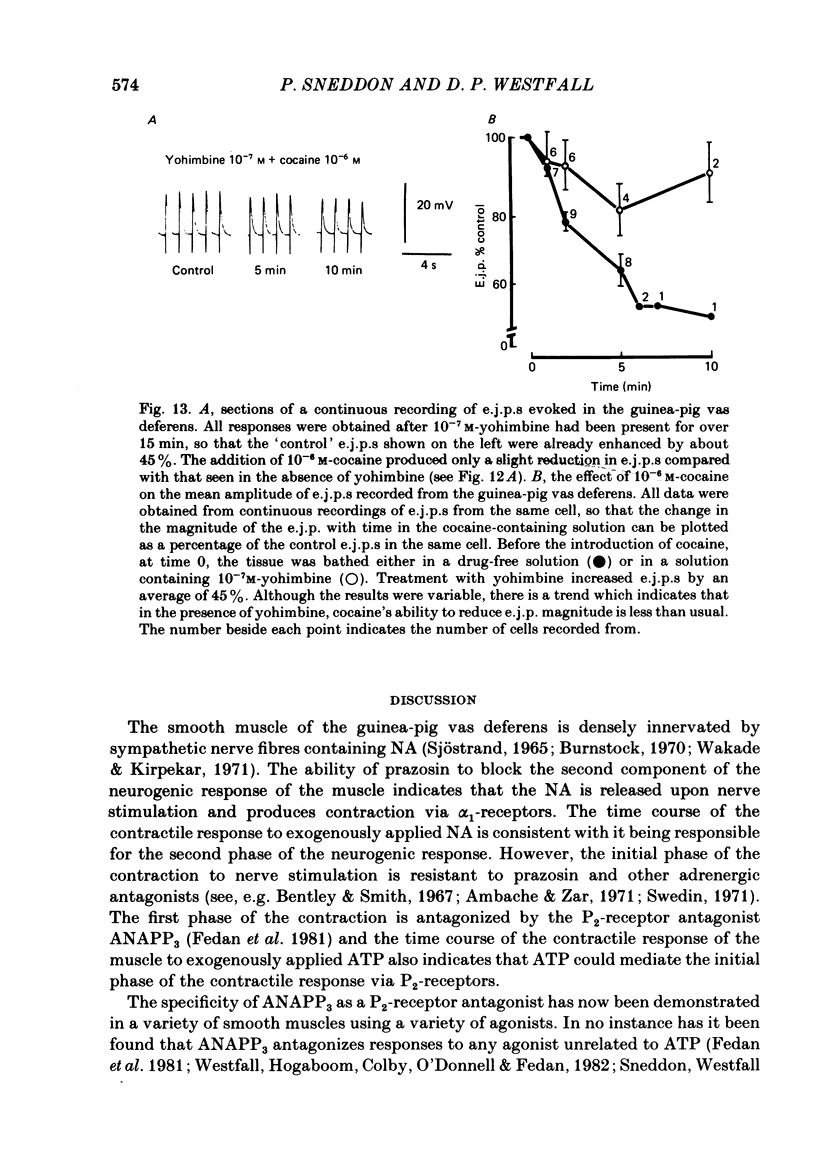
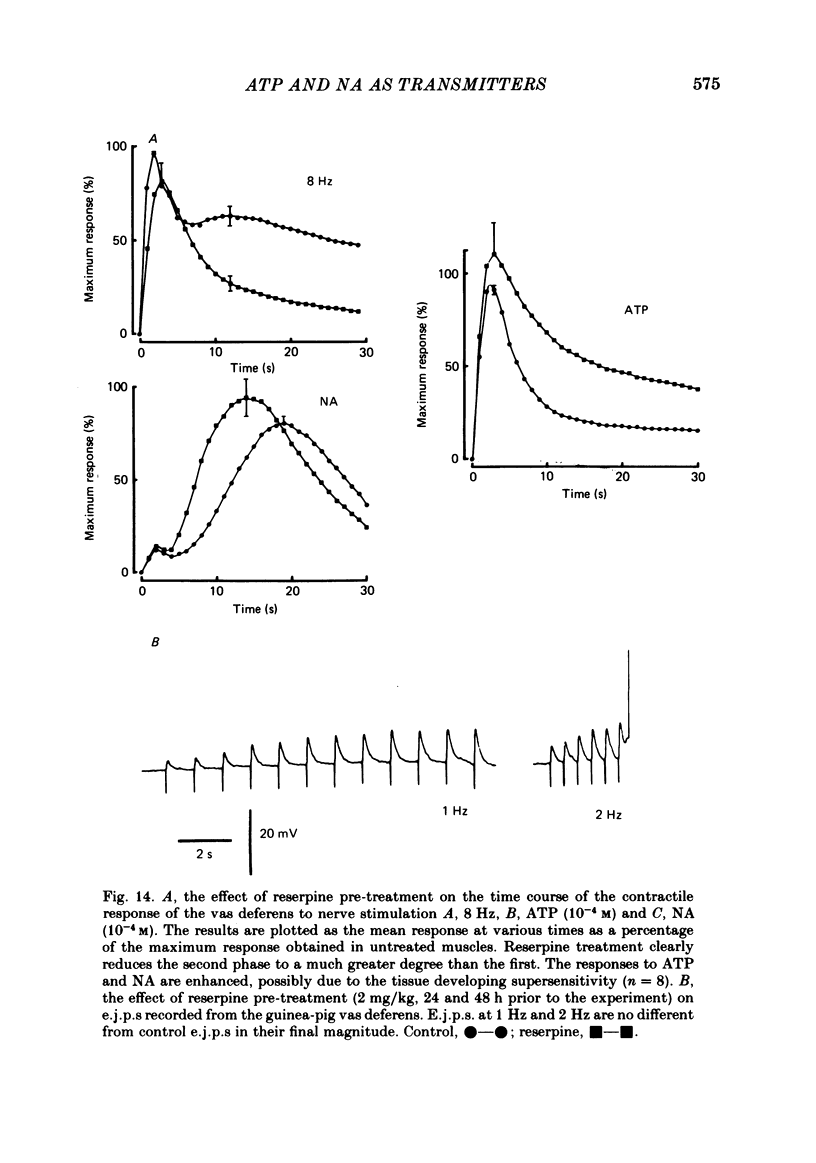
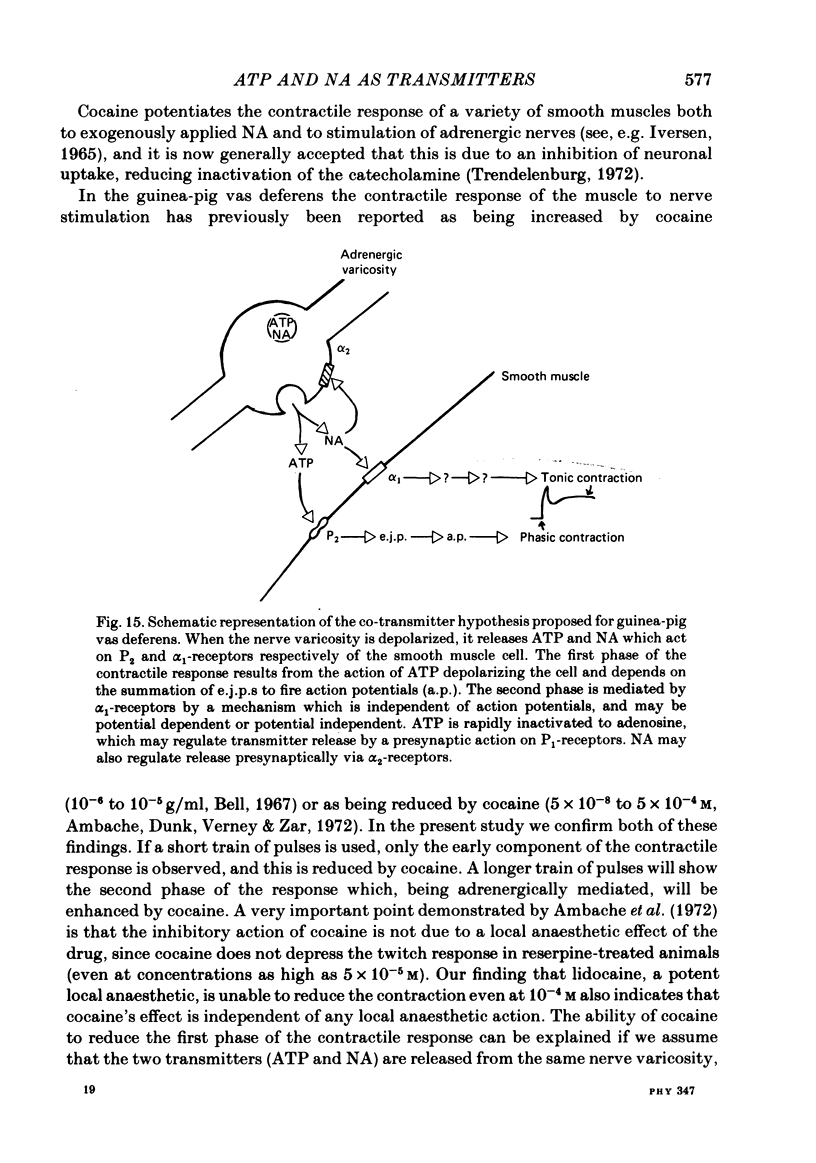
The contractile response of the guinea-pig vas deferens to tetanic nerve stimulation was biphasic. The first phase was mimicked by exogenously applied ATP. The second more tonic phase was mimicked by exogenously applied noradrenaline (NA). Intracellular micro-electrodes were used to record the electrical response of the vas deferens to nerve stimulation and to exogenously applied ATP and NA. Local application of ATP (10(-5) to 10(-3)M), by pressure ejection from a micropipette, produced a depolarization similar in magnitude and time course to the excitatory junction potential (e.j.p.). NA produced no such response. Superfusion of the vas deferens with ATP and NA (10(-6) to 10(-4)M) produced a depolarization. The depolarization produced by NA was more gradual than that produced by the same concentration of ATP. The ATP-receptor antagonist ANAPP3 (arylazido aminopropionyl-ATP) preferentially antagonized the first component of the neurogenic contractile response and also antagonized the e.j.p. The alpha-receptor antagonist prazosin preferentially antagonized the second phase of the neurogenic contractile response and enhanced the e.j.p. Similar results were obtained using the irreversible alpha-receptor antagonists phenoxybenzamine and dibenamine. Cocaine (10(-6) and 10(-5)M) enhanced the second phase of the contractile response to nerve stimulation, but reduced the first phase. Lidocaine (10(-5) and 10(-4)M) had no such effect. Cocaine (10(-6) and 10(-5)M) reduced the magnitude of e.j.p.s. at all stimulation frequencies from 1 to 8 Hz. In the presence of the selective alpha 2-receptor antagonist yohimbine (10(-7)M), both phases of the contractile response to nerve stimulation were enhanced to the same degree. This concentration of yohimbine also increased the magnitude of e.j.p.s. In the presence of 10(-7) M-yohimbine, cocaine (10(-6) and 10(-5)M) still enhanced the second phase of the contractile response, but no longer reduced the initial phase of the contraction or e.j.p.s to the same degree. In vas deferens from animals pre-treated with reserpine (2 mg/kg.day), the second phase of the contractile response to nerve stimulation was reduced but neither the first phase of the contraction nor the e.j.p.s was blocked. These results suggest that the first phase of the neurogenic contractile response of the vas deferens and the e.j.p. are mediated by ATP acting on P2-purinoreceptors, whereas NA mediates phase two, via alpha 1-adrenoceptors. The results also suggest that release of ATP and NA is influenced by a negative feed-back mechanism involving presynaptic alpha 2-adrenoceptors.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambache N., Dunk L. P., Verney J., Zar M. A. Inhibition of post-ganglionic motor transmission in vas deferens by indirectly acting sympathomimetic drugs. J Physiol. 1972 Dec;227(2):433–456. doi: 10.1113/jphysiol.1972.sp010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambache N., Zar M. A. Evidence against adrenergic motor transmission in the guinea-pig vas deferens. J Physiol. 1971 Jul;216(2):359–389. doi: 10.1113/jphysiol.1971.sp009530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., HOLMAN M. E., KURIYAMA H. FACILITATION OF TRANSMISSION FROM AUTONOMIC NERVE TO SMOOTH MUSCLE OF GUINEA-PIG VAS DEFERENS. J Physiol. 1964 Jul;172:31–49. doi: 10.1113/jphysiol.1964.sp007401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakeley A. G., Brown D. A., Cunnane T. C., French A. M., McGrath J. C., Scott N. C. Effects of nifedipine on electrical and mechanical responses of rat and guinea pig vas deferens. Nature. 1981 Dec 24;294(5843):759–761. doi: 10.1038/294759a0. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Do some nerve cells release more than one transmitter? Neuroscience. 1976 Aug;1(4):239–248. doi: 10.1016/0306-4522(76)90054-3. [DOI] [PubMed] [Google Scholar]
- Fedan J. S., Hogaboom G. K., O'Donnell J. P., Colby J., Westfall D. P. Contribution by purines to the neurogenic response of the vas deferens of the guinea pig. Eur J Pharmacol. 1981 Jan 5;69(1):41–53. doi: 10.1016/0014-2999(81)90600-2. [DOI] [PubMed] [Google Scholar]
- French A. M., Scott N. C. Evidence to support the hypothesis that ATP is a co-transmitter in rat vas deferens. Experientia. 1983 Mar 15;39(3):264–266. doi: 10.1007/BF01955295. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Neild T. O. Evidence for two populations of excitatory receptors for noradrenaline on arteriolar smooth muscle. Nature. 1980 Feb 21;283(5749):767–768. doi: 10.1038/283767a0. [DOI] [PubMed] [Google Scholar]
- Hogaboom G. K., O'Donnell J. P., Fedan J. S. Purinergic receptors: photoaffinity analog of adenosine triphosphate is a specific adenosine triphosphate antagonist. Science. 1980 Jun 13;208(4449):1273–1276. doi: 10.1126/science.6103581. [DOI] [PubMed] [Google Scholar]
- Iversen L. L. The inhibition of noradrenaline uptake by drugs. Adv Drug Res. 1965;2:1–46. [PubMed] [Google Scholar]
- Jeng S. J., Guillory R. J. The use of aryl azido ATP analogs as photoaffinity labels for myosin ATPase. J Supramol Struct. 1975;3(5-6):448–468. doi: 10.1002/jss.400030506. [DOI] [PubMed] [Google Scholar]
- McGrath J. C. Adrenergic and 'non-adrenergic' components in the contractile response of the vas deferens to a single indirect stimulus. J Physiol. 1978 Oct;283:23–39. doi: 10.1113/jphysiol.1978.sp012486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon P., Westfall D. P., Fedan J. S. Cotransmitters in the motor nerves of the guinea pig vas deferens: electrophysiological evidence. Science. 1982 Nov 12;218(4573):693–695. doi: 10.1126/science.6291151. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Westfall D. P., Fedan J. S. Investigation of relaxations of the rabbit anococcygeus muscle by nerve stimulation and ATP using the ATP antagonist ANAPP3. Eur J Pharmacol. 1982 May 7;80(1):93–98. doi: 10.1016/0014-2999(82)90181-9. [DOI] [PubMed] [Google Scholar]
- Wakade A. R., Kirpekar S. M. Chemical and histochemical studies on the sympathetic innervation of the vas deferens and seminal vesicle of the guinea pig. J Pharmacol Exp Ther. 1971 Sep;178(3):432–441. [PubMed] [Google Scholar]
- Westfall D. P., Hogaboom G. K., Colby J., O'Donnell J. P., Fedan J. S. Direct evidence against a role of ATP as the nonadrenergic, noncholinergic inhibitory neurotransmitter in guinea pig tenia coli. Proc Natl Acad Sci U S A. 1982 Nov;79(22):7041–7045. doi: 10.1073/pnas.79.22.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall D. P., Lee T. J., Stitzel R. E. Morphological and biochemical changes in supersensitive smooth muscle. Fed Proc. 1975 Sep;34(10):1985–1989. [PubMed] [Google Scholar]
- Westfall D. P. Nonspecific supersensitivity of the guinea-pig vas deferens produced by decentralization and reserpine treatment. Br J Pharmacol. 1970 May;39(1):110–120. doi: 10.1111/j.1476-5381.1970.tb09560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


