Abstract
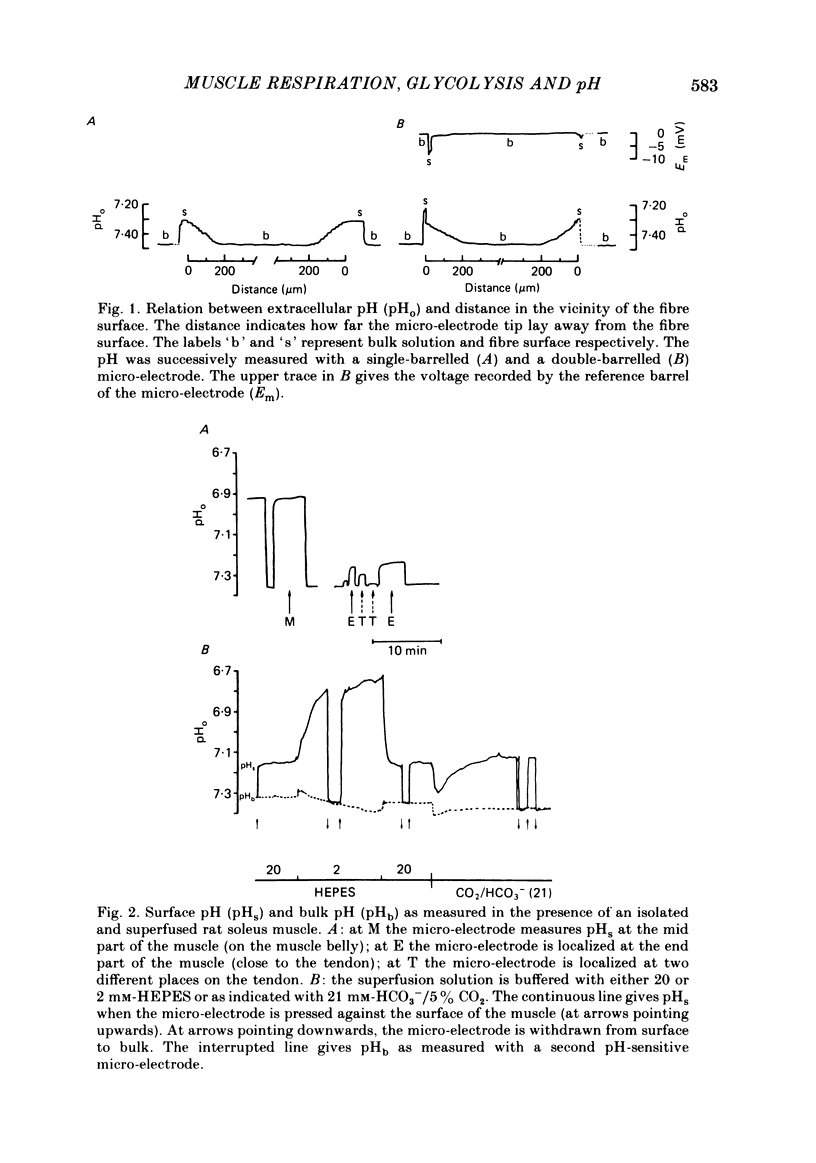
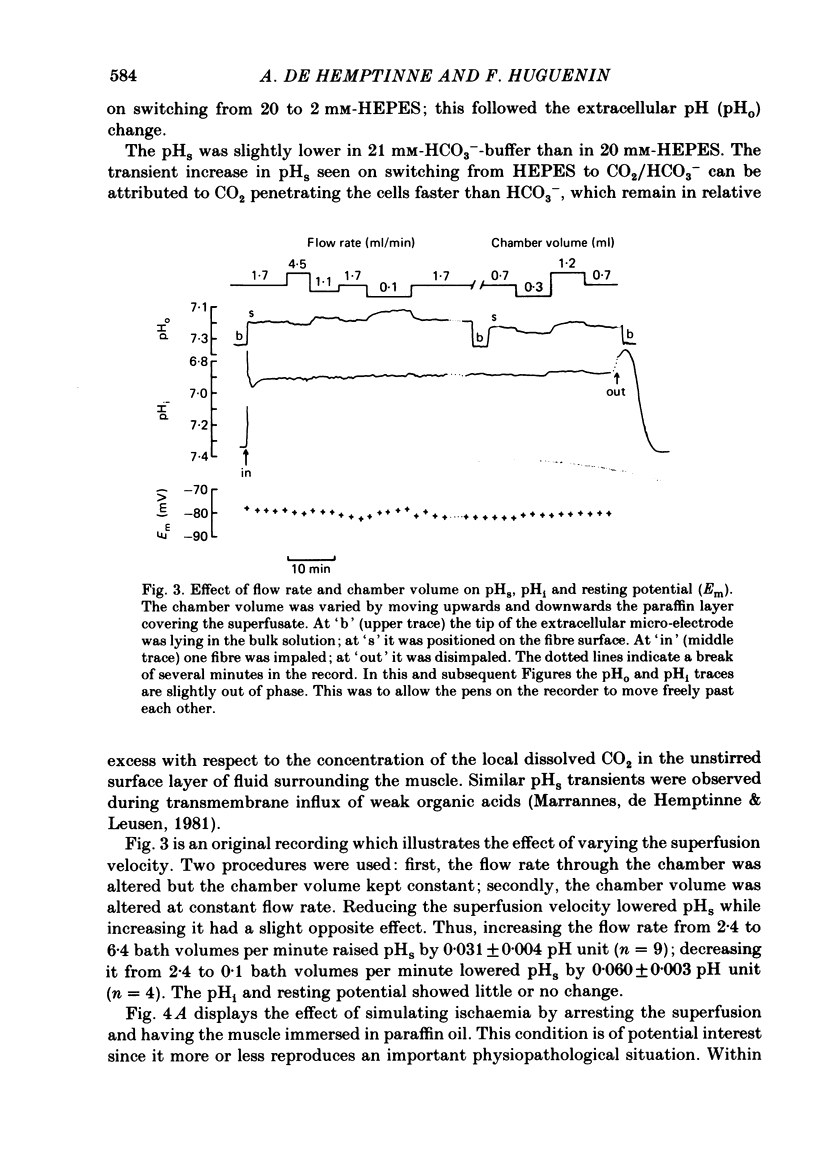
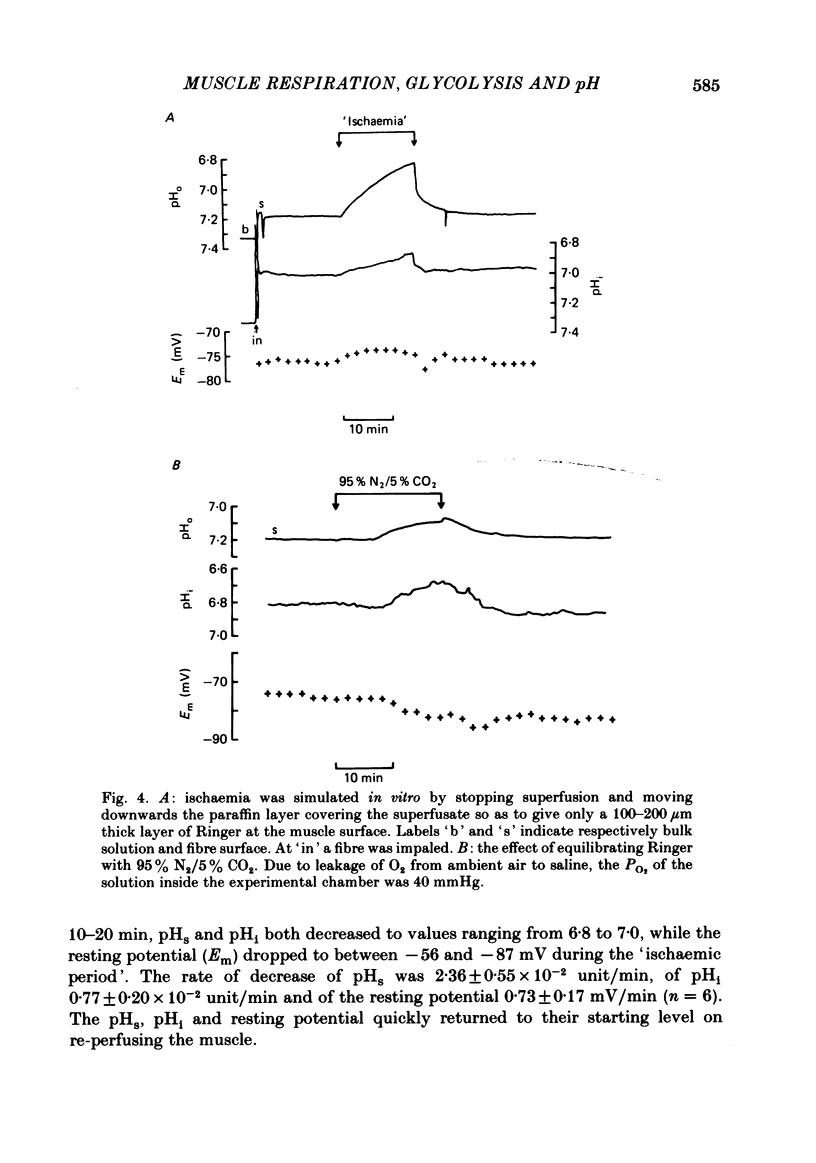
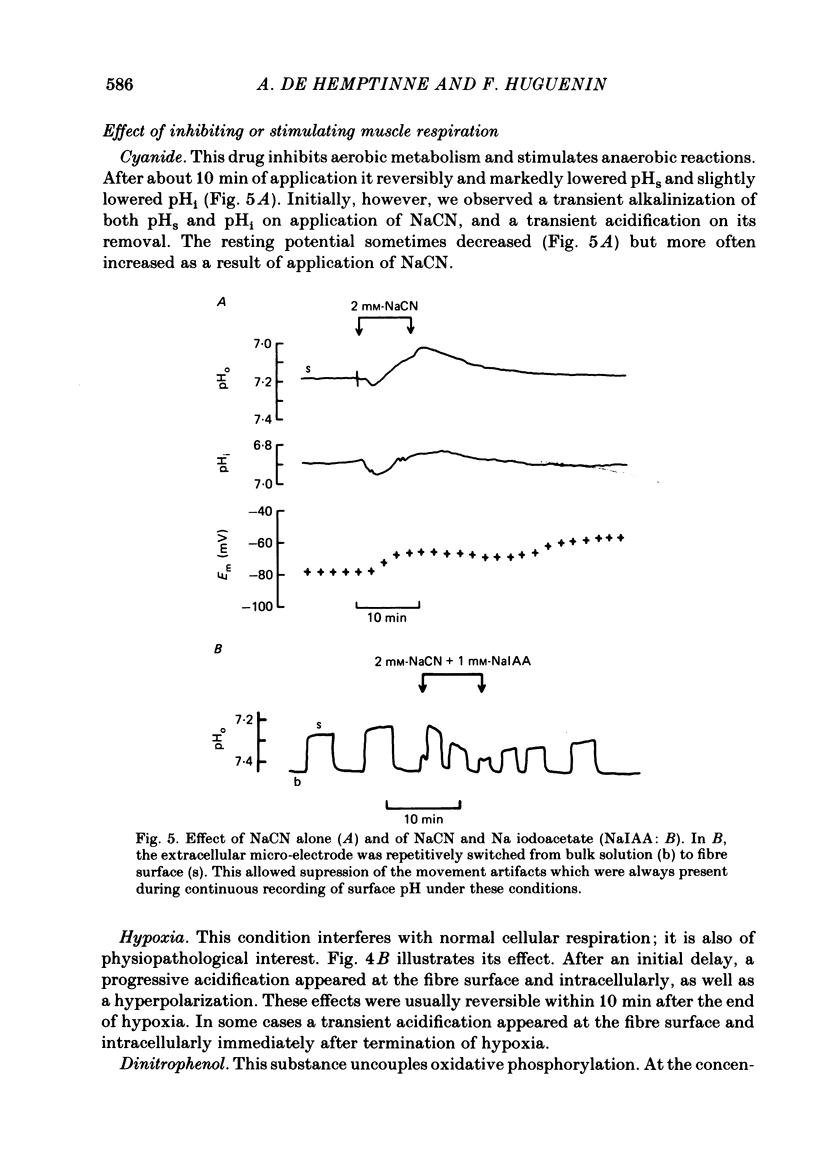
Extracellular pH (pHo) and intracellular pH (pHi) of superficial fibres of the rat soleus muscle were measured in vitro using pH-sensitive glass micro-electrodes. The origin of the pH gradient existing between the bulk phase of extracellular solution and the surface of muscle fibres was investigated. The pHo decreased almost linearly over a distance of 285 microns from bulk solution to fibre surface. The magnitude of the bulk-surface pH gradient is greater in the mid region of the muscle than close to the tendon. Decreasing the superfusate velocity increased the magnitude of the pH gradient. Reducing the buffer capacity of the superfusing solution had the same effect. Inhibiting the aerobic metabolism or stimulating it acidified the fibre surface. Inhibiting glycolysis alone, or both aerobic metabolism and glycolysis, alkalinized the fibre surface. Inhibiting the membrane ionic exchange process involved in pHi regulation had no effect on surface pH. Changing the rate of aerobic or anaerobic metabolism quickly modified pHi in most cases. In conclusion the bulk-surface pH gradient seems to result mainly from diffusion of CO2 and lactic acid across an unstirred layer of fluid covering the surface of muscle fibres.
Full text
PDF

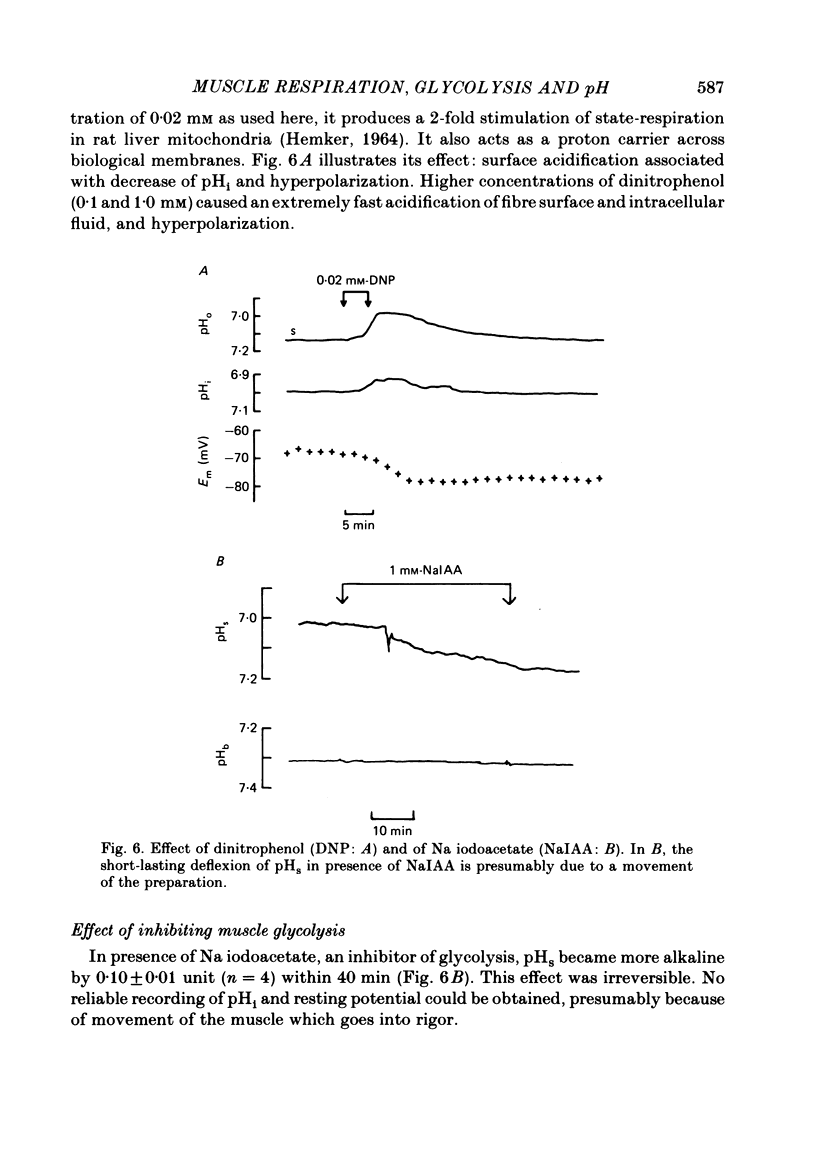
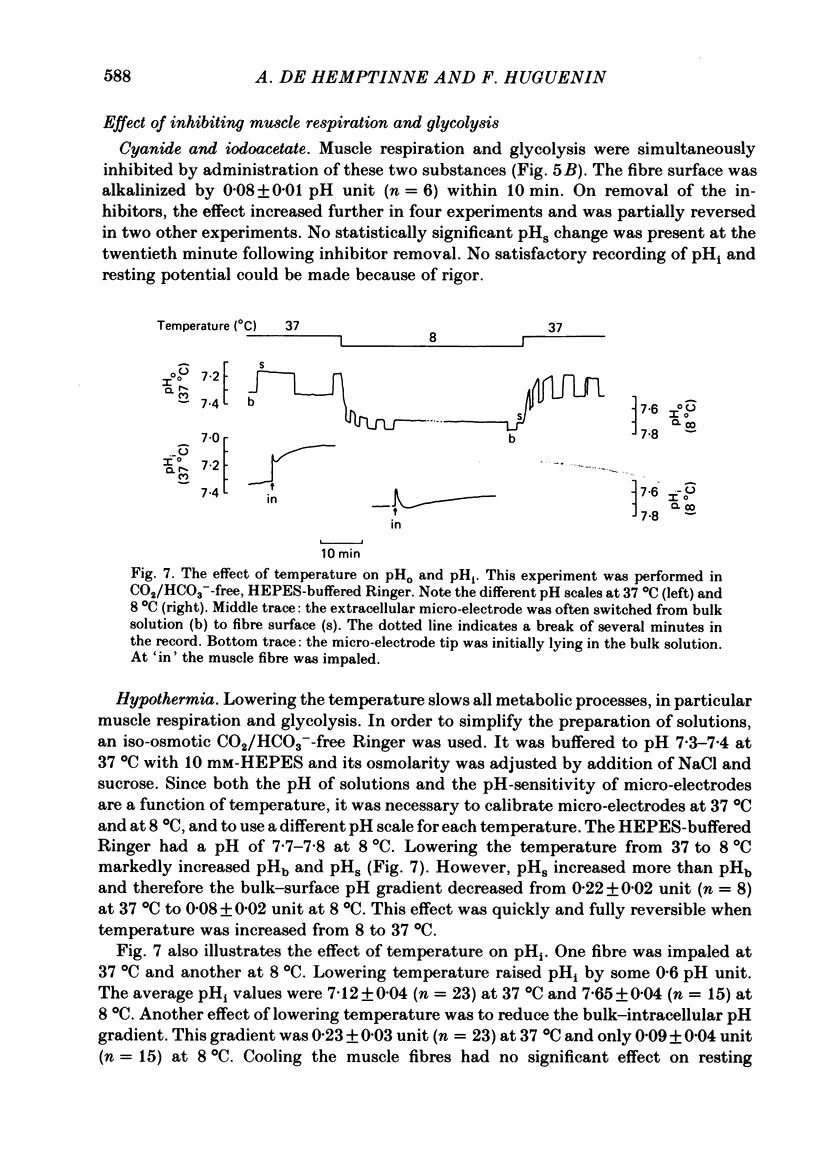




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler S. The interrelationship of metabolism and pH heterogeneity in muscle cells. J Lab Clin Med. 1972 Sep;80(3):351–363. [PubMed] [Google Scholar]
- Aickin C. C., Thomas R. C. An investigation of the ionic mechanism of intracellular pH regulation in mouse soleus muscle fibres. J Physiol. 1977 Dec;273(1):295–316. doi: 10.1113/jphysiol.1977.sp012095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALDWELL P. C. Studies on the internal pH of large muscle and nerve fibres. J Physiol. 1958 Jun 18;142(1):22–62. doi: 10.1113/jphysiol.1958.sp005998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cechetto D., Mainwood G. W. Carbon dioxide and acid base balance in the isolated rat diaphragm. Pflugers Arch. 1978 Sep 29;376(3):251–258. doi: 10.1007/BF00584959. [DOI] [PubMed] [Google Scholar]
- Gros G., Moll W., Hoppe H., Gros H. Proton transport by phosphate diffusion--a mechanism of facilitated CO2 transfer. J Gen Physiol. 1976 Jun;67(6):773–790. doi: 10.1085/jgp.67.6.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutknecht J., Bisson M. A., Tosteson F. C. Diffusion of carbon dioxide through lipid bilayer membranes: effects of carbonic anhydrase, bicarbonate, and unstirred layers. J Gen Physiol. 1977 Jun;69(6):779–794. doi: 10.1085/jgp.69.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutknecht J., Tosteson D. C. Diffusion of weak acids across lipid bilayer membranes: effects of chemical reactions in the unstirred layers. Science. 1973 Dec 21;182(4118):1258–1261. doi: 10.1126/science.182.4118.1258. [DOI] [PubMed] [Google Scholar]
- Kost G. J. Muscle surface pH: measurement technique and responses to acidosis and alkalosis. J Appl Physiol Respir Environ Exerc Physiol. 1982 Jan;52(1):85–90. doi: 10.1152/jappl.1982.52.1.85. [DOI] [PubMed] [Google Scholar]
- Marrannes R., de Hemptinne A., Leusen I. pH aspects of transient changes in conduction velocity in isolated heart fibers after partial replacement of chloride with organic anions. Pflugers Arch. 1981 Mar;389(3):199–209. doi: 10.1007/BF00584780. [DOI] [PubMed] [Google Scholar]
- Micro-electrode measurement of the intracellular pH and buffering power of mouse soleus muscle fibres. J Physiol. 1977 Jun;267(3):791–810. doi: 10.1113/jphysiol.1977.sp011838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves R. B., Malan A. Model studies of intracellular acid-base temperature responses in ectotherms. Respir Physiol. 1976 Oct;28(1):49–63. doi: 10.1016/0034-5687(76)90084-0. [DOI] [PubMed] [Google Scholar]
- Roos A. Intracellular pH and distribution of weak acids across cell membranes. A study of D- and L-lactate and of DMO in rat diaphragm. J Physiol. 1975 Jul;249(1):1–25. doi: 10.1113/jphysiol.1975.sp011000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEGEMANN J. DER EINFLUSS VON KOHLENDIOXYDDRUCKEN AUF DAS INTERSTITIELLE PH DES ISOLIERTEN RATTENDIAPHRAGMAS. Pflugers Arch Gesamte Physiol Menschen Tiere. 1964 Mar 12;279:36–49. [PubMed] [Google Scholar]
- Steinhagen C., Hirche H. J., Nestle H. W., Bovenkamp U., Hosselmann I. The interstitial pH of the working gastrocnemius muscle of the dog. Pflugers Arch. 1976 Dec 28;367(2):151–156. doi: 10.1007/BF00585151. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Comparison of the mechanisms controlling intracellular pH and sodium in snail neurones. Respir Physiol. 1978 Apr;33(1):63–73. doi: 10.1016/0034-5687(78)90085-3. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Intracellular pH of snail neurones measured with a new pH-sensitive glass mirco-electrode. J Physiol. 1974 Apr;238(1):159–180. doi: 10.1113/jphysiol.1974.sp010516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Hemptinne A. Intracellular pH and surface pH in skeletal and cardiac muscle measured with a double-barrelled pH microelectrode. Pflugers Arch. 1980 Jul;386(2):121–126. doi: 10.1007/BF00584198. [DOI] [PubMed] [Google Scholar]


