Abstract
Although flavonoids manifest a diverse range of biological activities, including antitumor and antiviral effects, the molecular mechanisms underlying these activities await elucidation. We hypothesize that the flavonoid constituents of a proprietary grape seed extract (GSE) that contains procyandins exert significant antiviral and antitumor effects, by inducing production of the Th1-derived cytokine gamma interferon (IFN-γ) by peripheral blood mononuclear cells) from healthy donors. Our results show that GSE significantly induced the transcription of IFN-γ mRNA as demonstrated by reverse transcription-PCR but had no effect on the Th2-derived cytokine interleukin-6. The enhancing effect of GSE on IFN-γ expression was further supported by a concomitant increase in the number of cells with intracytoplasmic IFN-γ as well as the synthesis and secretion of IFN-γ. Our results demonstrate that the potentially beneficial immunostimulatory effects of GSE may be mediated through the induction of IFN-γ.
On average, the normal human diet contains 200 mg or more of mixed flavonoids per day. Flavonoids display a remarkable array of pharmacological and biochemical actions. Documented biological effects of dietary flavonoids include antiallergic, anticarcinogenic, anti-inflammatory, antimicrobial, antineoplastic, antithrombotic, antiviral, cardioprotective, and hepatoprotective effects, among others (7). Of these diverse pharmacological actions of the dietary flavonoids, their antitumor and antiviral effects stand out. The molecular mechanisms underlying these biological activities await elucidation. There is general acceptance that the Th1-derived cytokines such as interleukin-2 (IL-2), gamma interferon (IFN-γ), and IL-12 promote cell-mediated immunity (4). Conversely, the Th2-derived cytokines, such as IL-4, IL-5, and IL-6, exert negative immunoregulatory effects on cell-mediated immunity, while upregulating humoral immunity (5, 6, 8). We hypothesize that flavonoids exert significant immunomodulatory effects by differentially regulating the production of Th1- and Th2-derived cytokines. In the present studies, we assessed the effect of a proprietary grape seed extract (GSE) that contains oligomeric procyanidins on Th1 (IFN-γ)- and Th2 (IL-6)-derived cytokine production by normal peripheral blood mononuclear cells (PBMC) to test the above hypothesis.
MATERIALS AND METHODS
GSE.
A proprietary GSE (Vinox) was obtained from Polyphenolics, Canandaigua Brands, Inc., Madera, Calif. GSE, a hot water extract, essentially contains all flavonoids, predominantly those belonging to the class flavons and proanthocyanidins. Proanthocyanidins consist of flavonol units (10). GSE proanthocyanidins consisting of monomers, oligomers, and polymers belong to the subclass procyanidins since catechin is the constituent flavanol monomer present in them. The phenolic profile of the extract, GSE, used in the present study as determined by high-performance liquid chromatography was about 8% monomers, 70% oligomers, and 22% polymers.
Cell culture.
After obtaining informed consent, peripheral blood was drawn from healthy donors into a syringe containing heparin (20 U/ml). Subjects (5 males and 11 females, 20 to 40 years old) were free of any illness and were not taking medications known to affect immune functions, including nonsteroidal anti-inflammatory agents, histamine receptor antagonists, recreational drugs, or birth control pills. Total PBMC were separated by centrifugation over a discontinuous Ficoll-Hypaque cushion, and the cells were washed three times in Ca2+- and Mg2+-free Hanks' balanced salt solution. Two milliliters of PBMC (3 × 106 cells/ml) was cultured in 24-well plates (2-cm2 wells; Nunc, Naperville, Ill.) in RPMI 1640 medium containing 5% fetal bovine serum (GIBCO, Grand Island, N.Y.), fresh glutamine (300 μg/ml), and gentamicin (80 μg/ml) (complete medium) alone or with GSE at concentrations of 0.005, 0.01, 0.05, 0.1, and 0.5 mg/ml. Treated and control samples were incubated at 37°C for 24 to 72 h in a 5% CO2 and 95% air incubator.
RNA extraction.
Cytoplasmic RNA was extracted by an acid guanidinium thiocyanate-phenol-chloroform method as described by Chomczynski and Sacchi (2). Cultured cells were pelleted by centrifugation and resuspended in a 4 M solution of guanidinium thiocyanate. Cells were agitated by repeated pipetting to lyse them and then phenol-chloroform extracted in the presence of sodium acetate. After centrifugation, RNA was precipitated from the aqueous layer by adding an equal volume of isopropanol, and the mixture was kept at −20°C for 1 h and then centrifuged to pellet the RNA. The RNA pellet was washed with 75% ethanol to remove any remaining traces of guanidinium. The final pellet was dried and resuspended in 0.1% diethyl pyrocarbonate in water, and the amount of RNA was determined using a spectrophotometer at 260 nm. Isolated RNA was kept at −70°C until used.
RT-PC.
The extracted RNA was used for reverse transcription (RT)-PCR as described using a Perkin-Elmer kit (catalog no. N808-0143). The RNA was reverse transcribed to make a DNA copy for use in PCR. Briefly 1 μg of RNA was added to a tube containing 5 mM MgCl2, a 1 mM concentration of each deoxynucleoside triphosphate (ATP, TTP, GTP, and CTP), 50 mM KCl, 10 mM pH 8.3 Tris buffer, 2.5 μM oligo(dT), 20 U of RNase inhibitor, and 50 U of murine leukemia virus reverse transcriptase. The mixture was incubated at 45°C for 35 min, heated to 95°C for 5 min, and placed on ice until used for PCR. The newly synthesized cDNA was then amplified by PCR using specific sense and antisense primers of the genes of interest along with a housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (G3PDH), or β-actin, as controls (Table 1). Briefly, to each tube a 10-μl sample of the RT product in a final concentration of 2 mM MgCl2-10 mM Tris (pH 8.3)-50 mM KCl plus a 0.02 μM concentration of both the 5′ and 3′ primers and 2.5 U of Taq polymerase was added. The mixture was placed in a thermocycler for 25 to 30 cycles of 95°C for 30 s, 60°C for 30 s, and 74°C for 1 min. PCR conditions were modified for housekeeping genes to prevent plateauing. Samples were separated by 1 to 1.2% agarose gel electrophoresis along with molecular weight markers for reference. Resultant bands were visualized with UV light and photographed, their sizes were determined, and they were quantitated using a scanning densitometer. All values were normalized to the constitutive expression of the housekeeping gene. Densitometric readings of the photographic negatives normalized to G3PDH are expressed as optical density (OD) units, and percent change or increase over control values is calculated as follows: % increase = (experimental OD/house keeping gene OD) × 100.
TABLE 1.
PCR primers used in this study
| Gene | Sequence | Fragment size (bp) |
|---|---|---|
| IFN-γ | 5′-GAGTGTGGAGACCATCAAGGAA-3′ (upstream) | 249 |
| 5′-GCAGGCAGGACAACCATTACTG-3′ (downstream) | ||
| IL-6 | 5′-CAGCCCACCCCTCAACACCTCT-3′ (upstream) | 488 |
| 5′-TCCCCCACTCAGCTCCCACTCT-3′ (downstream) | ||
| G3PDH | 5′-CATGTGGGCCATGAGGTCCACCAC-3′ (upstream) | 983 |
| 5′-TGAAGGTCGGAGTCAACGGATTTGGT-3′ (downstream) | ||
| β-Actin | 5′-GTGGGGCGCCCCAGGCACCA-3′ (upstream) | 548 |
| 5′-CTCCTTAATGTCACGCACGATTTC-3′ (downstream) |
FACS analysis.
The percentage of cells positive for intracytoplasmic IFN-γ using a carboxyfluorescein-conjugated antibody specific to IFN-γ (Becton-Dickinson, San Diego, Calif.) was quantitated by fluorescence-activated cell sorting (FACS) analysis as described by the manufacturer. Experimental and control cells were treated with the protein transport inhibitor Golgi Stop (B.D. Pharmingen, San Diego, Calif.) to enhance detection by concentrating cytokines in the endoplasmic reticulum. After cells were permeabilized with saponin, they were reacted with the labeled antibody. Cells positive for IFN-γ were expressed as a percentage of the total cells gated.
ELISA.
Treated and control cell cultures were centrifuged at 900 × g for 10 min at 4°C, and the supernatants were diluted 1:1 with RPMI medium supplemented with 1.0% fetal calf serum and were stored at −20°C until assayed for IFN-γ and IL-6 by a quantitative sandwich enzyme-linked immunosorbent assay (ELISA) technique using the Quantikine kit (R & D Systems, Minneapolis, Minn.) as described by the manufacturer. The minimum doses of IFN-γ and IL-6 detectable by ELISA were <4 and <0.1 pg/ml, respectively. Any out of a range of samples subsequently were diluted at least to 1:10 or more and used in the ELISA.
Statistical analysis.
Results are expressed as mean values ± standard deviations (SD). The quantitation of changes in IFN-γ and IL-6 gene expression, respectively, in Fig. 1C and 4C were calculated by Student's t test. The percent change in the densitometry reading of photographic negatives after normalization with the corresponding housekeeping G3PDH values was quantitated. The treated groups were compared to the untreated control group, and the significance of differences between control and experimental values was determined by a paired Student t test formula.
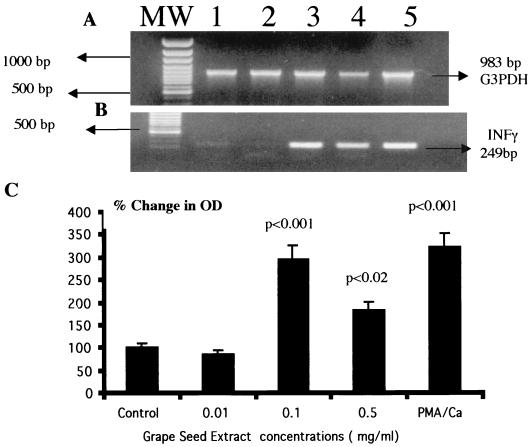
FIG. 1.
GSE induces IFN-γ gene expression by PBMC as measured by RT-PCR. PBMC (3 × 106 cells/ml) were cultured in RPMI 1640 medium alone or with GSE at concentrations of 0.01 (lanes 2), 0.1 (lanes 3), and 0.5 (lanes 4) mg/ml for 48 h. Cytoplasmic RNA was extracted, reverse transcribed, and amplified with specific primers for housekeeping gene G3PDH (A) and IFN-γ (B). Additional lanes: MW, molecular weight markers; 1, control; 5, PMA plus ionophore (50 ng/ml each). (C) Quantitation of changes in IFN-γ gene expression. The statistical significance of the difference between control and treated samples was calculated by Student's t test. These data represent the means ± SD (error bars) of three experiments using PBMC from three different subjects.
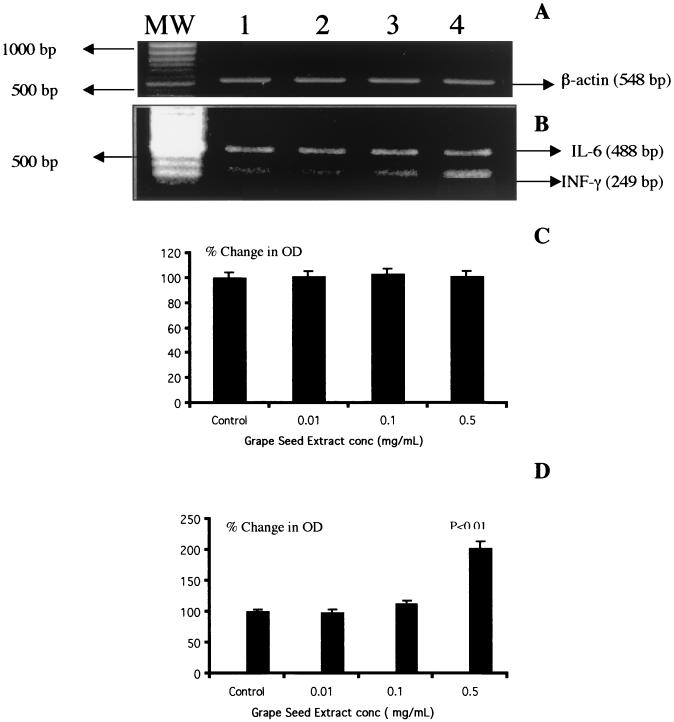
FIG. 4.
GSE does not modulate IL-6-specific gene expression by normal PBMC. PBMC (3 × 106 cells/ml) were cultured alone or with different concentrations of GSE (0.01 [lanes 2], 0.1 [lanes 3], and 0.5 [lanes 4] mg/ml) for 48 h. Cytoplasmic RNA was extracted, reverse transcribed, and amplified with specific primers for housekeeping gene β-actin (A) and IL-6 and IFN-γ (B). Additional lanes: MW, molecular weight markers; 1, control. Also shown are quantitation of changes in IL-6 (C) and IFN-γ (D) gene expression. Data are means ± SD (error bars) of four experiments performed in triplicate using PBMC from four different subjects. Results showed that GSE upregulated IFN-γ gene expression, while IL-6 gene expression was not modulated in the same RNA samples. The statistical significance of the differences between control and treated samples was calculated by Student's t test.
RESULTS
To examine whether GSE has a toxic effect on PBMC during culture periods, we examined the viability of PBMC cultured with GSE for 72 h. At 72 h of incubation, PBMC cultured with GSE at concentrations of 0.005, 0.01, 0.05, 0.1, and 0.5 mg/ml showed 92, 90, 92, 86, and 85% viability, respectively, compared to 90% viability for the control culture as determined by the trypan blue vital dye exclusion assay. This demonstrates that GSE was not toxic to the cells. For each of the following experiments both gene expression and protein synthesis data were obtained from the same culture. The results presented are the mean values ± SD of four separate experiments.
GSE induces IFN-γ gene expression by PBMC.
Since flavonoids manifest significant antiviral effects and IFN-γ is known to exert significant antiviral effects, we studied the effects of GSE on IFN-γ gene expression by PBMC. Data presented in Fig. 1 show the effect of GSE on IFN-γ-specific gene expression. GSE at any concentration did not affect the expression of the housekeeping gene, G3PDH (Fig. 1A, lanes 2 to 5). GSE at a lower concentration of 0.01 mg/ml (Fig. 1B, lane 2 [OD = 0.16]) showed a level of IFN-γ-specific mRNA similar to that in the control culture (lane 1 [OD = 0.17]). However, GSE at 0.1 and 0.5 mg/ml (Fig. 1B, lane 3 [OD = 0.52] and lane 4 [OD = 0.32], respectively) significantly upregulated IFN-γ gene expression (P < 0.001 and P < 0.02, respectively) compared to the untreated control culture (Fig. 1B, lane 1 [OD = 0.17]). Phorbol-12-myristate 13-acetate (PMA) plus calcium ionophore (50 ng/ml each) used as a positive control significantly upregulated IFN-γ gene expression (Fig. 1B, lane 5 [OD= 0.57]; P < 0.001) compared to that in the unstimulated control culture (Fig. 1B, lane 1 [OD = 0.17]). Data presented in Fig. 1C show the percent change in the OD of the IFN-γ signals in GSE-treated cultures in comparison with that in the control culture after normalization with the corresponding G3PDH gene signals. Cultures treated with GSE at 0.1 (197% increase) and 0.5 mg/ml (83% increase) significantly upregulated IFN-γ gene expression compared to that in the control culture. Cultures treated with a known stimulant, PMA plus calcium ionophore, also upregulated IFN-γ gene expression (224% increase) compared to the control culture.
GSE upregulates the number of IFN-γ positive cells.
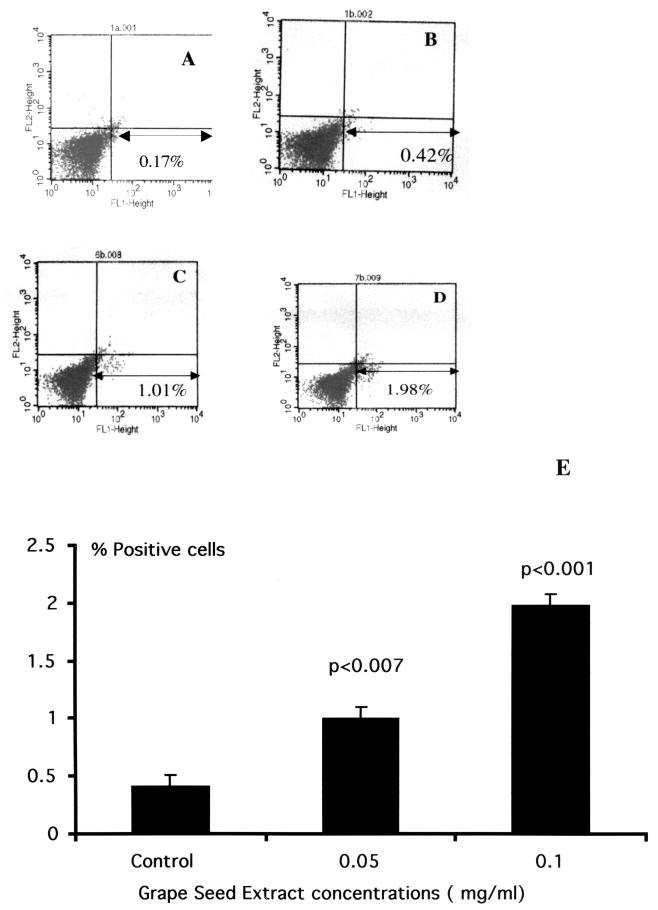
In order to examine whether the gene expression results as demonstrated by RT-PCR correlate with the quantitation and identification of cells expressing the intracytoplasmic IFN-γ, we performed immunofluorescent staining by flow cytometry analysis. Data presented in Fig. 2 show the flow cytometric profile of GSE-treated cultures for intracytoplasmic IFN-γ, using carboxyfluorescein-conjugated anti-IFN-γ antibody. Lymphocyte population was gated by labeling the population with CD45 conjugated to cytochrome c. Isotype antibody (immunoglobulin G2b) staining was used as a control to set the quadrant statistics (Fig. 2A, FL1 axis). Figure 2C and D show a significant increase in the number of IFN-γ-positive cells in cultures treated with GSE at 0.05 mg/ml (1.01%) and 0.1 mg/ml (1.98%) compared to 0.42% IFN-γ-positive cells demonstrated in the untreated cultures (Fig. 2B). The data presented in Fig. 2E show the means ± SD from three different experiments performed. The number of IFN-γ positive cells in 0.05 mg/ml (1.24 ± 0.22; P < 0.007) and 0.1 mg/ml (2.12 ± 0.12; P < 0.001) of GSE-treated cultures were significantly higher in comparison to that in the untreated cultures (0.53 ± 0.09).
FIG. 2.
FACS analysis of PBMC treated with GSE. Percentage of cells positive for intracytoplasmic IFN-γ determined by using a carboxyfluorescein-conjugated antibody specific to IFN-γ. The lymphocyte population was gated by labeling the population with CD45 conjugated to cytochrome c. (A) Isotype control. Immunoglobulin G2b was used as a negative control to set the quadrants. (B) Untreated control. The FL1 axis represents FITC-labeled IFN-γ positive cells. Cells treated with GSE at concentrations of 0.05 (C) and 0.1 (D) mg/ml, respectively. (E) Data are presented as percentages (means ± SD [error bars]) of IFN-γ-positive cells from three separate experimental results.
GSE induces the production of IFN-γ by PBMC.
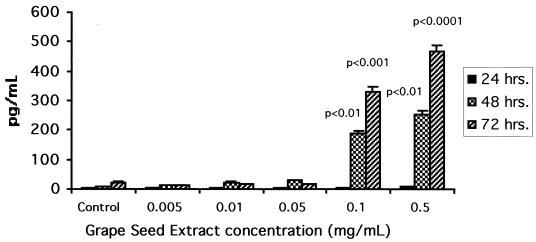
Since GSE significantly upregulates the IFN-γ specific gene expression by PBMC as quantitated by RT-PCR, we also examined whether GSE exerts its effects at the IFN-γ protein transcription or synthesis level. Supernatants from GSE-treated cultures were quantitated for IFN-γ protein by ELISA. Data presented in Fig. 3 depict the effect of GSE on the endogenous production of IFN-γ by normal PBMC. PBMC cultured with GSE for 24, 48, and 72 h at 0. 005, 0.01, and 0.05 mg/ml produced negligible levels of IFN-γ, and these levels were similar to those produced by the control culture. Similarly, PBMC cultured with higher concentrations of GSE (0.1 and 0.5 mg/ml) for 24 h also produced negligible levels of IFN-γ comparable to those produced in the control culture. However, PBMC cultured with GSE at these higher concentrations for 48 h produced significantly increased levels of IFN-γ (presented as means ± SD): 186.6 ± 18.2 (P < 0.01) and 251.2 ± 15.9 pg/ml (P < 0.01), respectively, compared to 7.9 ± 2.4 pg/ml produced by the control culture. PBMC cultured with GSE at 0.1 and 0.5 mg/ml for 72 h produced even higher levels of IFN-γ, with values being 331.8 ± 13.8 (P < 0.001) and 466.8 ± 29.8 pg/ml (P < 0.0001, respectively, compared to 22.5 ± 11.6 pg/ml produced by the control culture.
FIG. 3.
GSE induces IFN-γ synthesis and secretion by normal PBMC. Supernatants from GSE-treated and control cultures (24 to 72 h) were assayed for IFN-γ by a quantitative sandwich ELISA technique using the Quantikine kit (R & D Systems) as described by the manufacturer. Data are means ± SD (error bars) of four experiments performed in triplicate using PBMC from four different healthy subjects. The statistical significance of the differences between control and GSE-treated cultures was evaluated by Student's t test. The cultures treated with PMA plus ionophore (50 ng/ml each) used as a positive stimulant produced a mean of 2,780 ± 189 pg of IFN-γ/ml.
GSE did not affect IL-6 gene expression or production by PBMC.
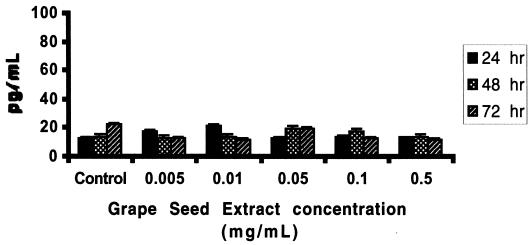
Since GSE significantly upregulated Th1-derived cytokine IFN-γ gene expression and production by PBMC, we also examined whether GSE can exert a modulatory effect on the gene expression and production of Th2-derived cytokine IL-6. Data presented in Fig. 4 show the effect of GSE on IL-6-specific gene expression. GSE at all concentrations did not affect the expression of the housekeeping gene, β-actin (Fig. 4A, lanes 2 to 4), and GSE-treated cultures were comparable to the control cultures. GSE at concentrations of 0.01 mg/ml (lane 2 [OD = 0.907]), 0.1 mg/ml (lane 3 [OD = 0.924), and 0.5 mg/ml (lane 4 [OD = 0.906]) showed similar levels of IL-6-specific mRNA compared to control culture (lane 1 [OD = 0.903]). We also examined the IFN-γ gene expression in the same sample which did not show upregulation of IL-6 gene expression. Data presented in Fig. 4B show that GSE at a concentration of 0.5 mg/ml produced significant upregulation of IFN-γ-specific gene expression (lane 4 [OD = 0.92]; P < 0.01) compared to control culture (lane 1 [OD = 0.45]). Data presented in Fig. 4C show the percent change in the OD of the IL-6 signals in GSE-treated cultures in comparison with that in the control culture after normalization with the corresponding β-actin gene signals. Cultures treated with GSE at 0.01 (2% increase) and 0.1 mg/ml (5% increase) and 0.5 mg/ml (5% decrease) showed mRNA expression which was comparable to that of the control culture. Data presented in Fig. 4D show the percent change in the OD of the IFN-γ signals in GSE-treated cultures in comparison with that in the control culture after normalization with the corresponding β-actin gene. Cultures treated with GSE at 0.5 mg/ml significantly (103% increase; P < 0.01) upregulated IFN-γ gene expression compared to that in the control culture. Data presented in Fig. 5 show that PBMC cultured with GSE at various concentrations for 24 to 72 h did not induce any significant level of the Th2-derived cytokine, IL-6, compared to the control culture, in contrast to the significant levels of IFN-γ induced by GSE at the same concentrations (Fig. 3).
FIG. 5.
GSE does not induce IL-6 synthesis and secretion by normal PBMC. Supernatants from GSE-treated and control cultures (24 to 72 h) were assayed for IL-6 by a quantitative sandwich ELISA technique using the Quantikine kit (R & D Systems) as described by the manufacturer. Data are means ± SD (error bars) of four experiments performed in triplicate using PBMC from four different healthy subjects. Statistical significance of the differences between control and GSE-treated cultures was evaluated by Student's t test. These same culture supernatants were also quantitated for IFN-γ levels, and GSE at 0.1 and 0.5 mg/ml produced significant levels of IFN-γ compared to untreated control cultures, similar to results reported in Fig. 3 (these data are not included in Fig. 5 for clarity).
DISCUSSION
The juices of red grapes and red wine have been proposed to have a variety of beneficial effects on health due in part to the presence of flavonoids in these beverages (7, 11). The GSE used in the present study is an extract from unfermented seeds of California grapes. GSE flavonoids consisting of monomers, oligomers, and polymers belong to the chemical subclass procyanidins since catechin is the constituent flavanol monomer present. The phenolic profile of the extract used in the present study, as determined by high-performance liquid chromatography, was approximately 8% monomers, 70% oligomers, and 22% polymers. Operationally, the oligomers have chain lengths of 2 to 7 (dimers to heptamers). Polymers represent components with chain lengths greater than 7 (above heptamers). The extract contains a high degree of oligomers, in contrast with some commercially available GSEs that possess a higher percentage of polymers. We believe that a product in which oligomers predominate would have significant biologic activity. Recent work with experimental animals and in vitro systems shows the above trend (4, 9, 10).The concentration of polyphenols in GSE is 35% higher than that of pycnogenol, a procyanidin extracted from the bark of the pine tree Pinus maritima (11), which is popular as an herbal food supplement. The quality of these polyphenols, in terms of their antioxidant properties, was 3 times greater than that of vitamin C, 5 times greater than that of vitamin E, 1.6 times greater than that of whole grapes, and 1.5 times greater than that of grape juice (11).
It is currently recognized that helper T (Th) lymphocytes may be divided into two functional subclasses, Th1 and Th2 cells, based upon the cytokines that they produce and their effects on cell-mediated and humoral immunity. Th1 cells produce IL-2 and IFN-γ and enhance cell-mediated immunity. Th2 cells produce IL-4, IL-5, and IL-6 and upregulate humoral immunity. Th2 cells also can inhibit cell-mediated immunity. In our studies, the effect of GSE on the production of IFN-γ, a Th1-associated cytokine, by normal PBMC was evident after 48 h of incubation. GSE further increased the production of IFN-γ at 72 h. The differentiation of precursor Th0 cells into Th1 and Th2 subsets depends upon the presence of IFN-γ during this process (1). Our present studies show that GSE can upregulate the transcription of the Th1-associated IFN-γ gene in PBMC. We also demonstrated that this effect is biologically significant, as GSE can induce an increase in lymphocytes with intracytoplasmic IFN-γ as well as the synthesis and secretion of IFN-γ. However, GSE did not modulate either gene expression or protein secretion of IL-6, a Th2-associated cytokine, by PBMC. These effects do not appear to be due to selective cytotoxicity of GSE, as PBMC treated with GSE at up to 0.5 mg/ml showed viability comparable to that of untreated control cultures. Although GSE did not affect IL-6 gene expression (Fig. 4) and protein synthesis and secretion (Fig. 5), it has been shown that IFN-γ can downregulate the expression of other Th2-derived cytokines such as IL-4 (3). Thus, it is possible that GSE can indirectly inhibit the expression of Th2-derived cytokines such as IL-6, through the induction of IFN-γ. However, the effect of GSE on other Th2-derived anti-inflammatory cytokines remains to be examined. Furthermore, the phenolic contents in GSE vary from monomers to oligomers, and it is possible that these constituents may show varying or differential effects on IFN-γ production. This question, however, remains to be resolved. Nevertheless, our observations clearly demonstrate that GSE selectively induces production of the Th1-derived cytokine IFN-γ by PBMC from healthy donors. Evaluation of the molecular mechanisms underlying the immunomodulatory activities mediated by GSE may be useful in the development of new flavonoid-based pharmaceutical agents.
Acknowledgments
We thank Mohan Sopori of the Lovelace Respiratory Research Institute, Albuquerque, N.Mex., and Viswanath P. Kurup of the Medical College of Wisconsin, Milwaukee, for valuable suggestions and critical reading of the manuscript. We also express our appreciation to Gerry Sobkowiak and Carol Sperry for their excellent secretarial assistance.
This work was supported in part by NIH grants R03 DA11119, R01 DA10632, R01 DA14218, R01 DA12366, and R01 MH 47225; the Margaret Duffy and Robert Cameron Troup Memorial Fund for Cancer Research of the Kaleida Health System; and the Buffalo General Foundation.
REFERENCES
- 1.Belardelli, F. 1995. Role of interferons and other cytokines in the regulation of the immune responses. APMIS 103:161-176. [DOI] [PubMed] [Google Scholar]
- 2.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Ann. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 3.Dickensheets, H. L., and R. P. Donnelly. 1999. Inhibition of IL-4-inducible gene expression in human monocytes by type I and type II interferons. J. Leukoc. Biol. 65:307-312. [DOI] [PubMed] [Google Scholar]
- 4.Kappagoda, C. T., M. Karim, K. McCormick, and C. K. Kandaswami. 2000. Unravelling the French paradox. C. Chem. Innov. 30:26-31. [Google Scholar]
- 5.Lucey, D. R. 1999. Evolution of the type-1 (Th1)-type-2 (Th2) cytokine paradigm. Infect. Dis. Clin. N. Am. 13:1-9. [DOI] [PubMed] [Google Scholar]
- 6.Lucey, D. R., M. Clerici, and G. M. Shearer. 1996. Type 1 and type 2 cytokine dysregulation in human infectious, neoplastic, and inflammatory diseases. Clin. Microbiol. Rev. 4:532-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Middleton, E., Jr., C. K. Kandaswami, and T. C. Theoharidis. 2000. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 52:673-751. [PubMed] [Google Scholar]
- 8.Norbiato, G., M. Bevilacqua, T. Vago, and M. Clerici. 1997. Glucocorticoids and Th-1, Th2 type cytokines in rheumatoid arthritis, osteoarthritis asthma, atopic dermatitis and AIDS. Clin. Exp. Rheumatol. 15:315-323. [PubMed] [Google Scholar]
- 9.Palma, M., L. T. Taylor, R. M. Varela, S. J. Cutler, and H. G. Cutler. 1999. Fractional extraction of compounds from grape seeds by supercritical fluid extraction and analysis for antimicrobial and agrochemical activities. J. Agric. Food Chem. 47:5044-5048. [DOI] [PubMed] [Google Scholar]
- 10.Porter, L. J. 1994. Flavans and proanthocyanidins, p. 23-55 In J. B. Harborne (ed.) The flavonoids: advances in research since 1986. Chapman & Hall/CRC, Boca Raton, Fla.
- 11.Vinson, J. A., and J. Proch. 1999. Antioxidant and clinical studies with grapeseed extract, p. 65-66. In Polyphenols, wine and health communications. Group Polyphenols International Symposium proceedings, vol. 1. Group Polyphenols and the Phytochemical Society of Europe, Bordeaux, France. [Google Scholar]