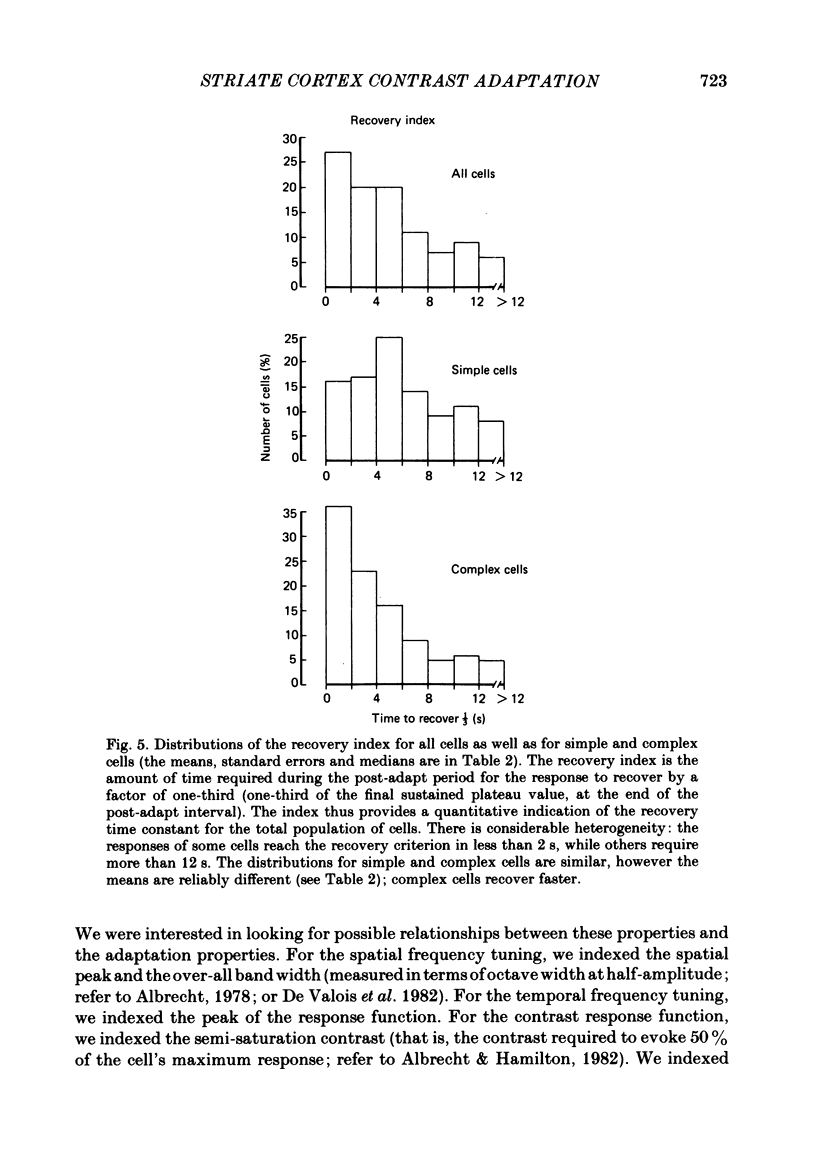
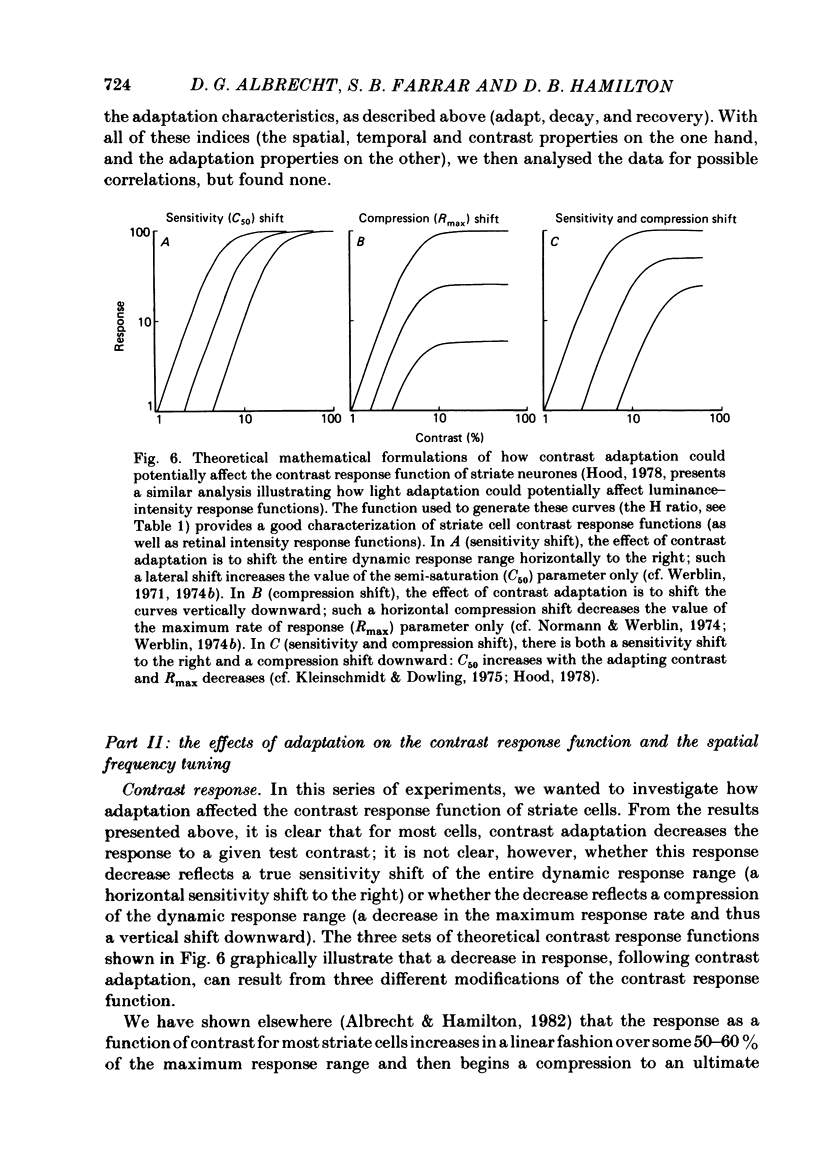
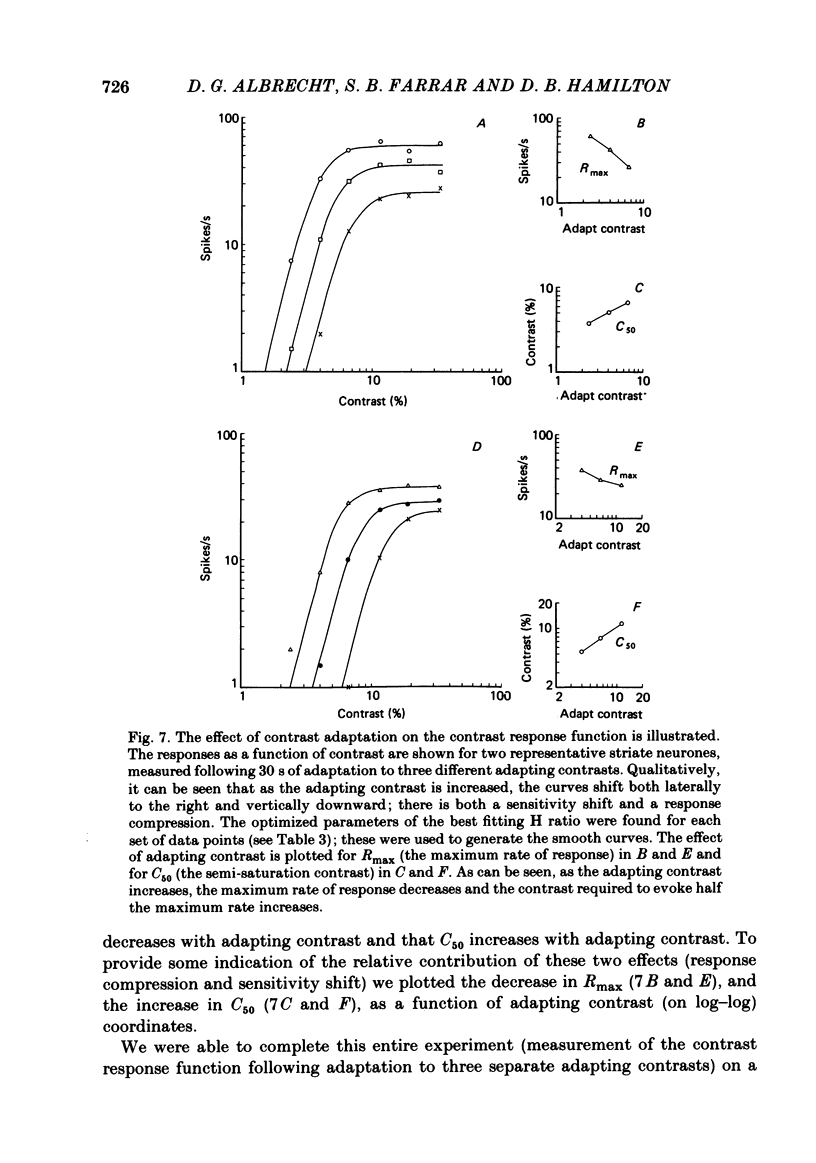
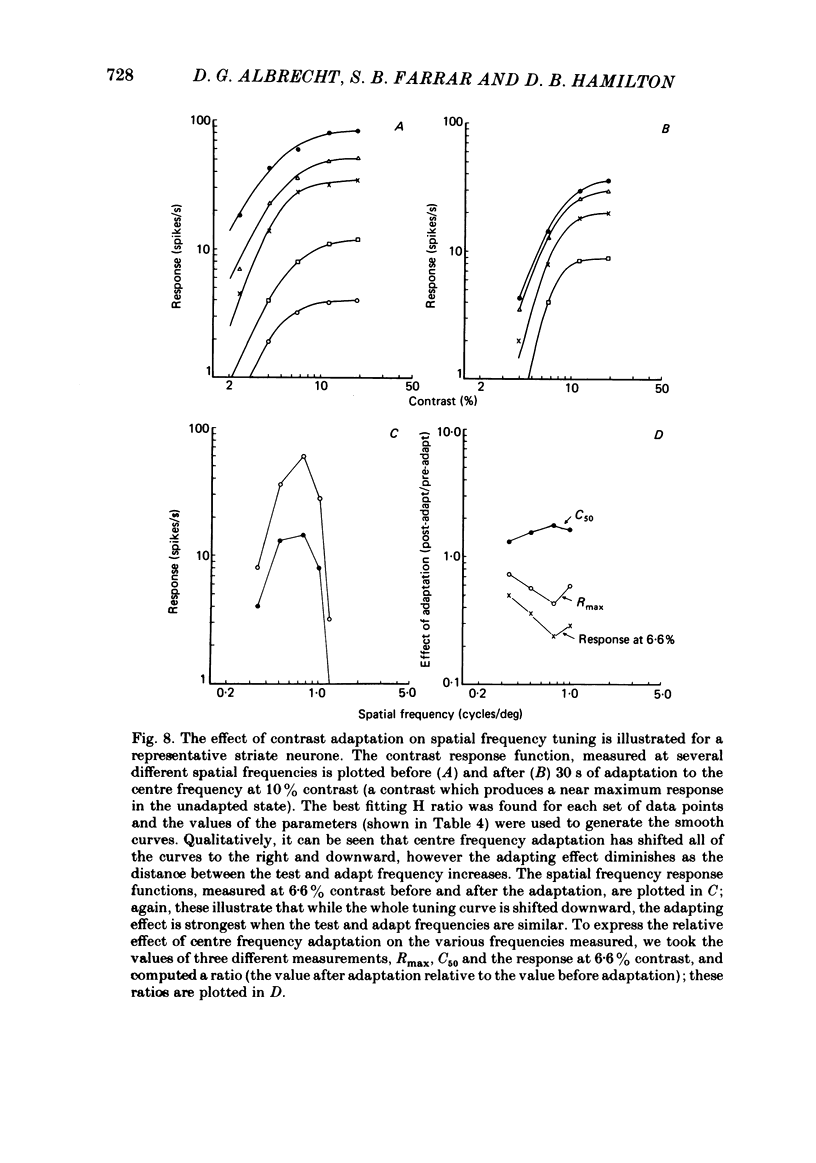
Abstract
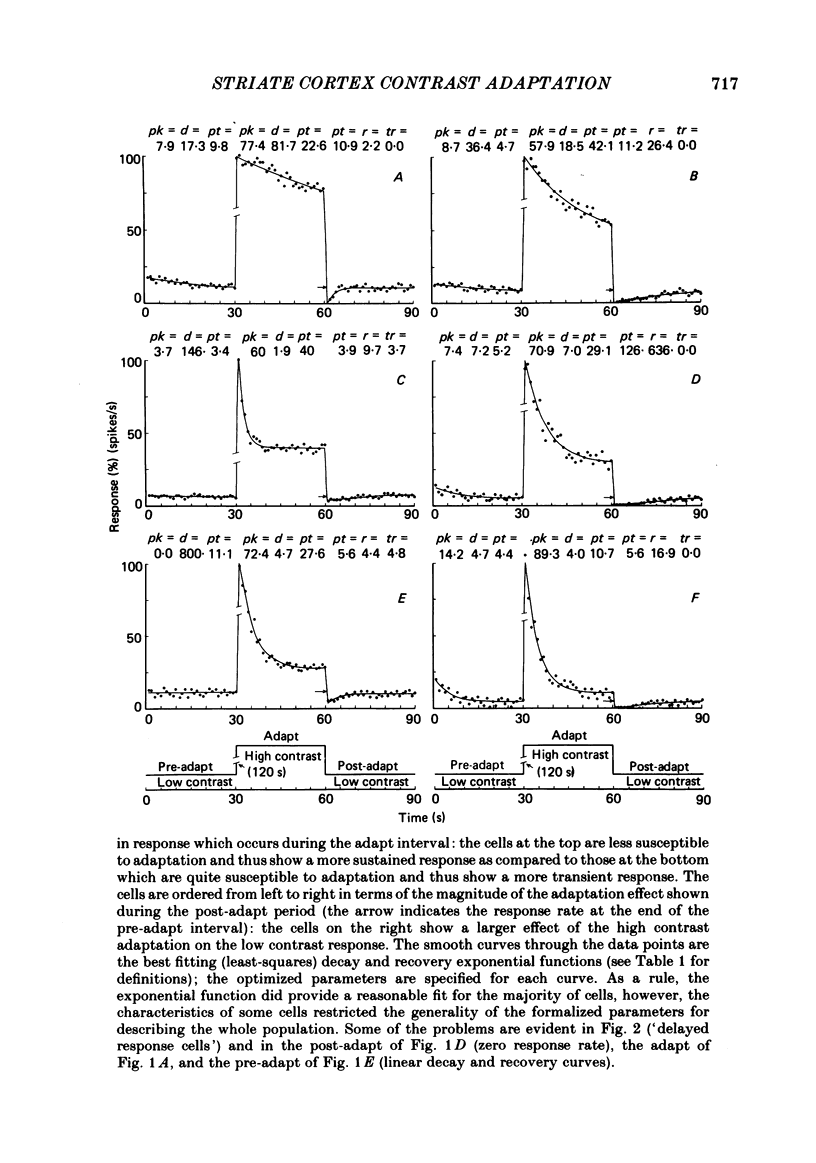
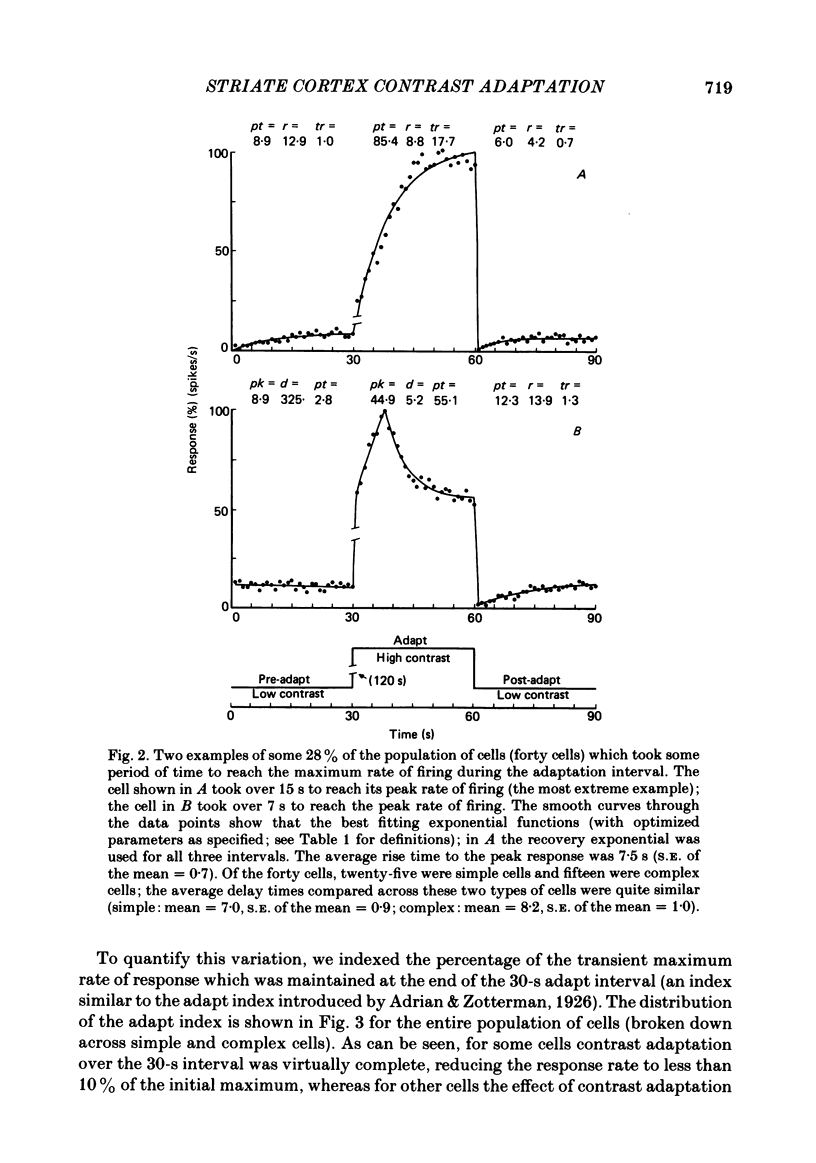
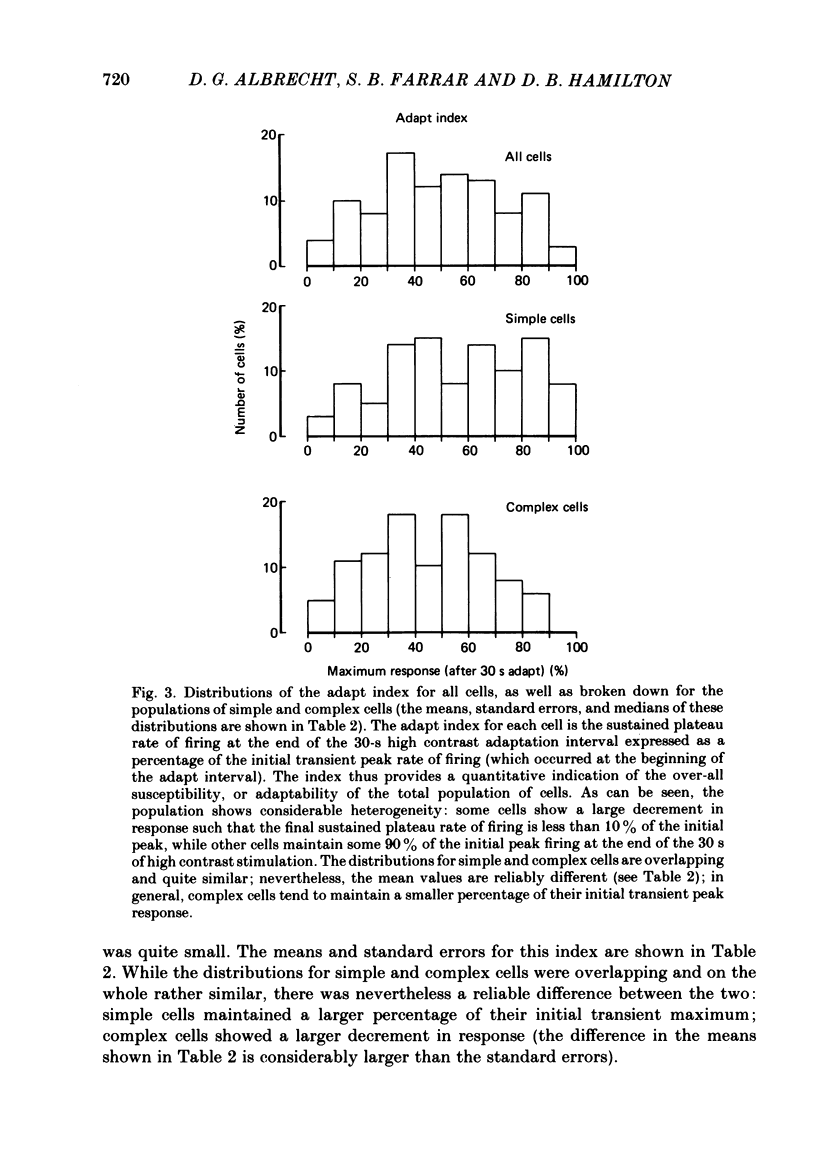
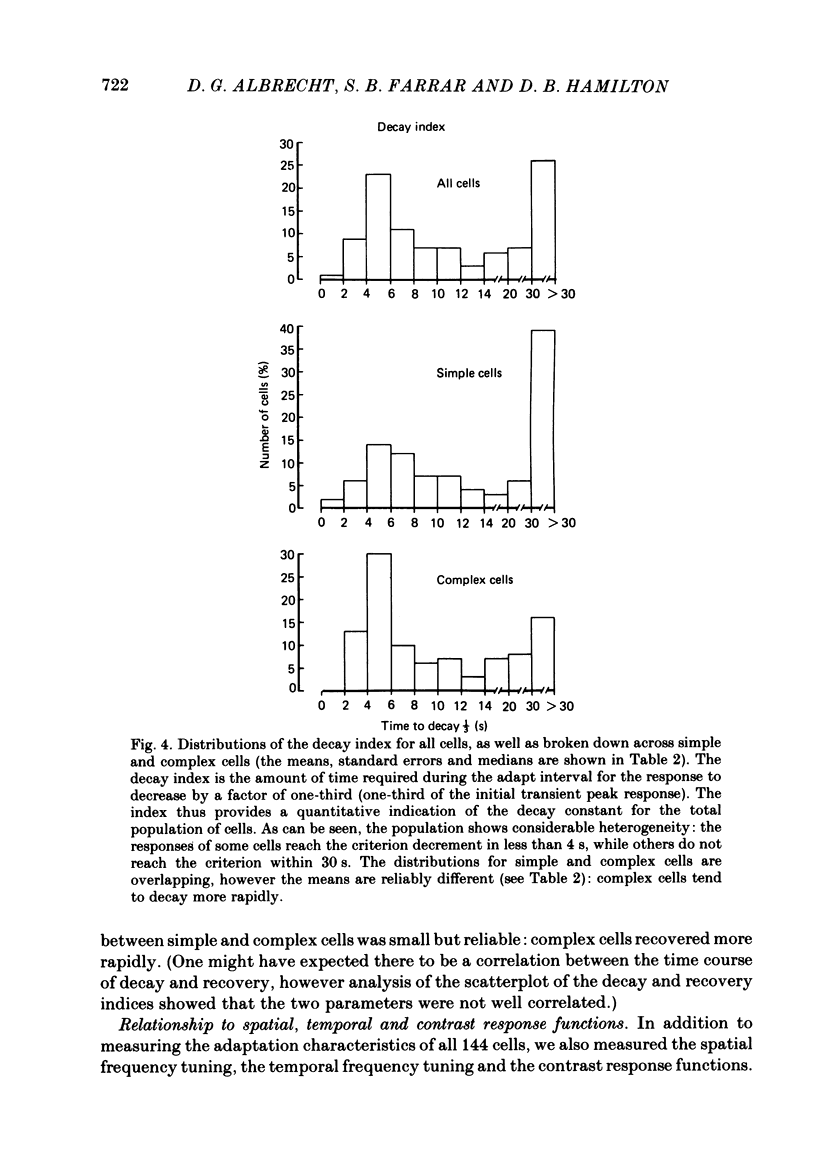
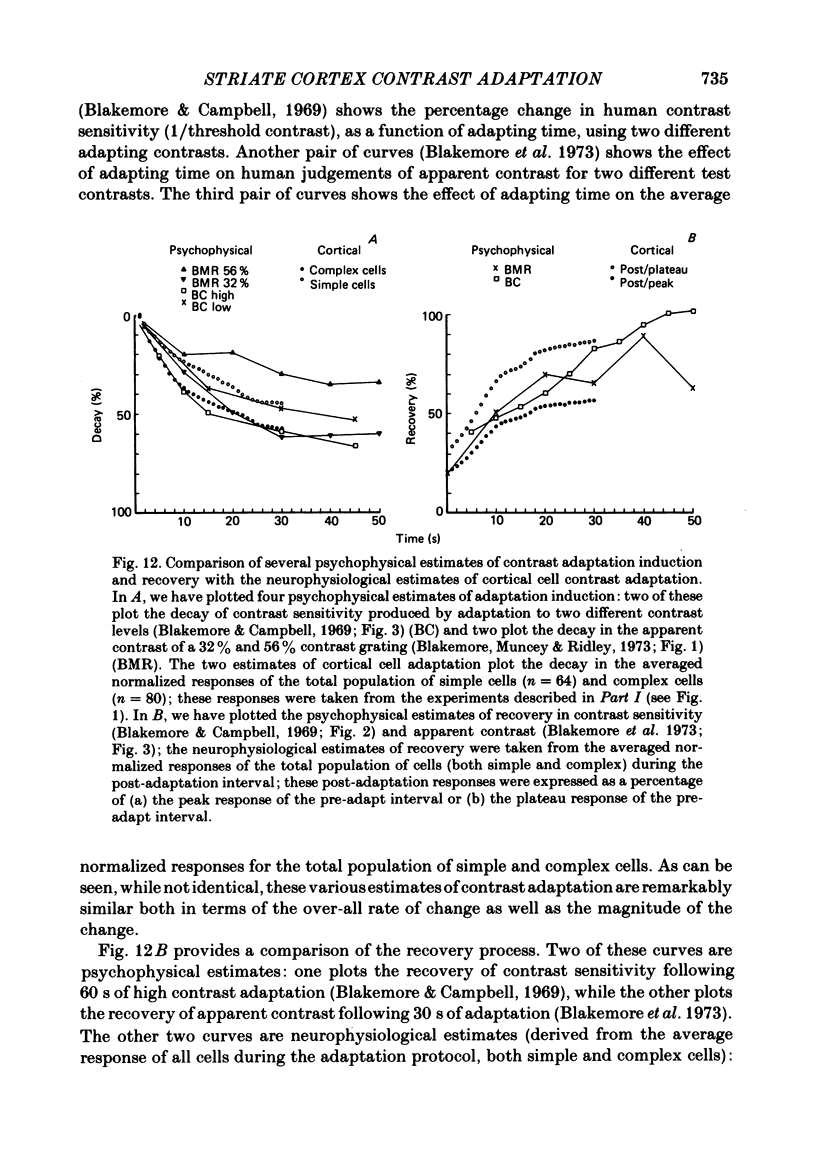
Spatial contrast adaptation, produced by prolonged exposure to high contrast grating patterns, has become an important psychophysical method for isolating spatial and orientation selective channels in the human visual system. It has been reasonably argued that this adaptation may be fundamentally dependent upon the activity of neurones in the striate cortex. To test the validity of this hypothesis, and several others, we measured the general adaptation characteristics of 144 striate neurones using a stimulus protocol comparable to the typical psychophysical methods. In general, during prolonged high contrast stimulation, the responses of most cells exponentially decayed from a transient peak response to a sustained plateau response; following adaptation, the responses to lower contrasts were depressed relative to the unadapted state but then gradually recovered from the transient depression to a sustained plateau. Such adaptation was a property common to both simple and complex cells (the distributions of the quantitative of adaptation were overlapping); there were however small but reliable differences. We compared the neurophysiological contrast adaptation with two psychophysical estimates of human contrast adaptation (threshold contrast elevation and apparent contrast reduction) and found that the time courses and the magnitudes were quite similar. The effect of contrast adaptation on the spatial frequency tuning was assessed by measuring the contrast response function at several different test spatial frequencies before and after adaptation at the optimum centre frequency. We found that the effect of adaptation decreased as the difference between test and adaptation frequency increased. Grating contrast adaptation has been alternatively described as 'constructive gain control' on the one hand and as 'deleterious fatigue' on the other. We tested the effect of contrast adaptation on the contrast response function and found (a) that adaptation shifts the curves vertically downward parallel to the response axis (thus reflecting a decrease in the maximum rate of firing and a deleterious compression of the response range) and (b) that adaptation shifts the curves horizontally to the right parallel to the contrast axis (thus reflecting a true sensitivity shift of the remaining response range for constructive maintenance of high differential sensitivity around the prevailing background level).(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF








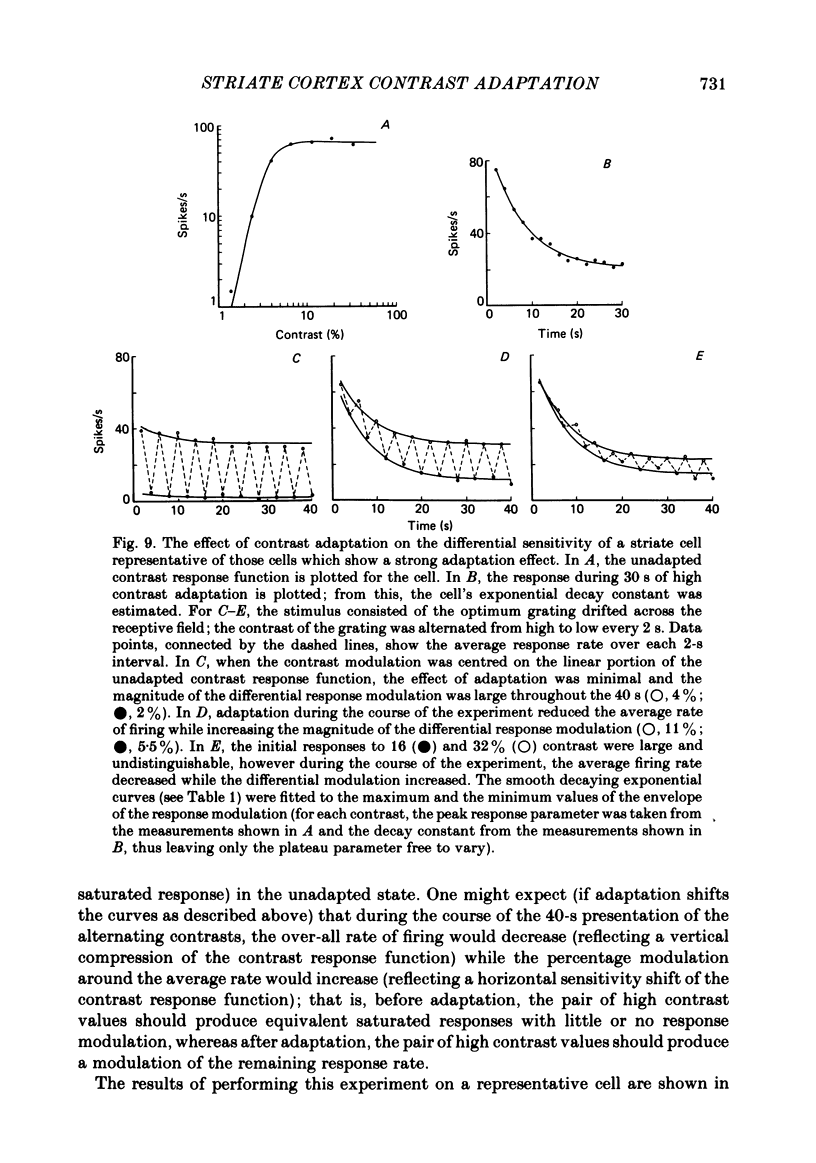

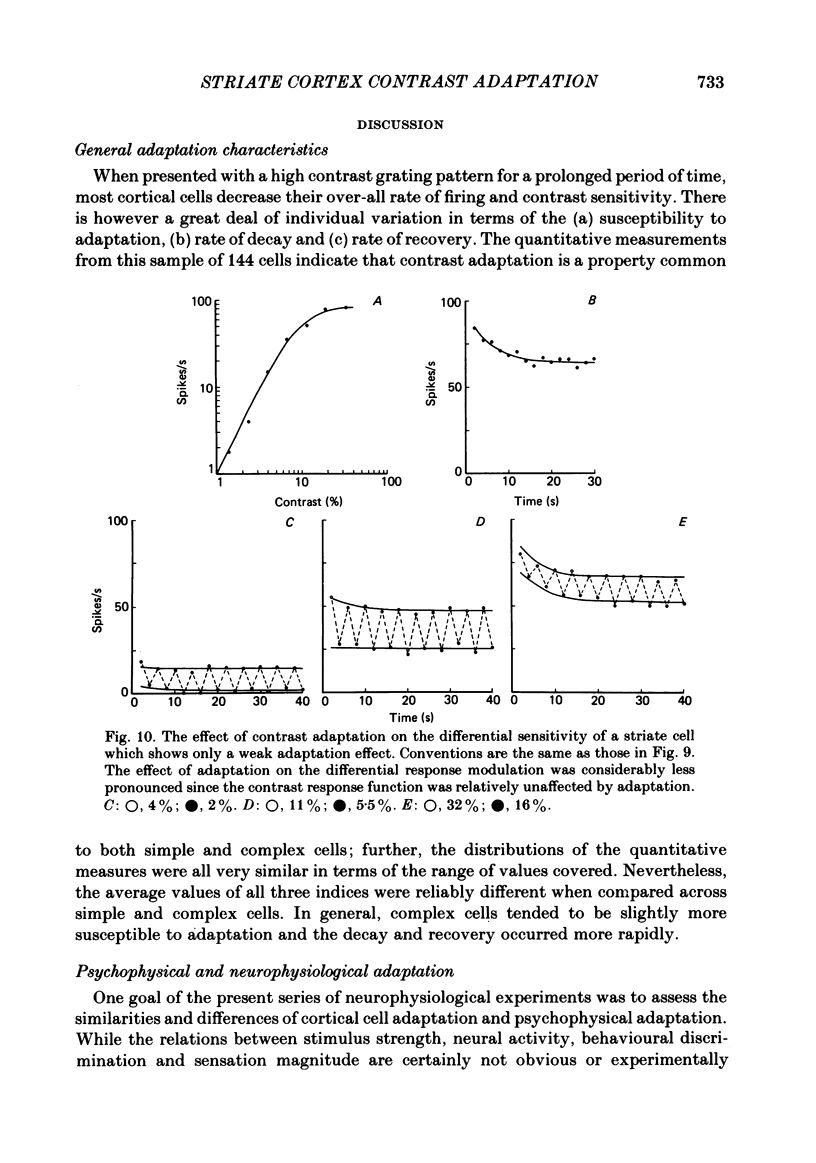
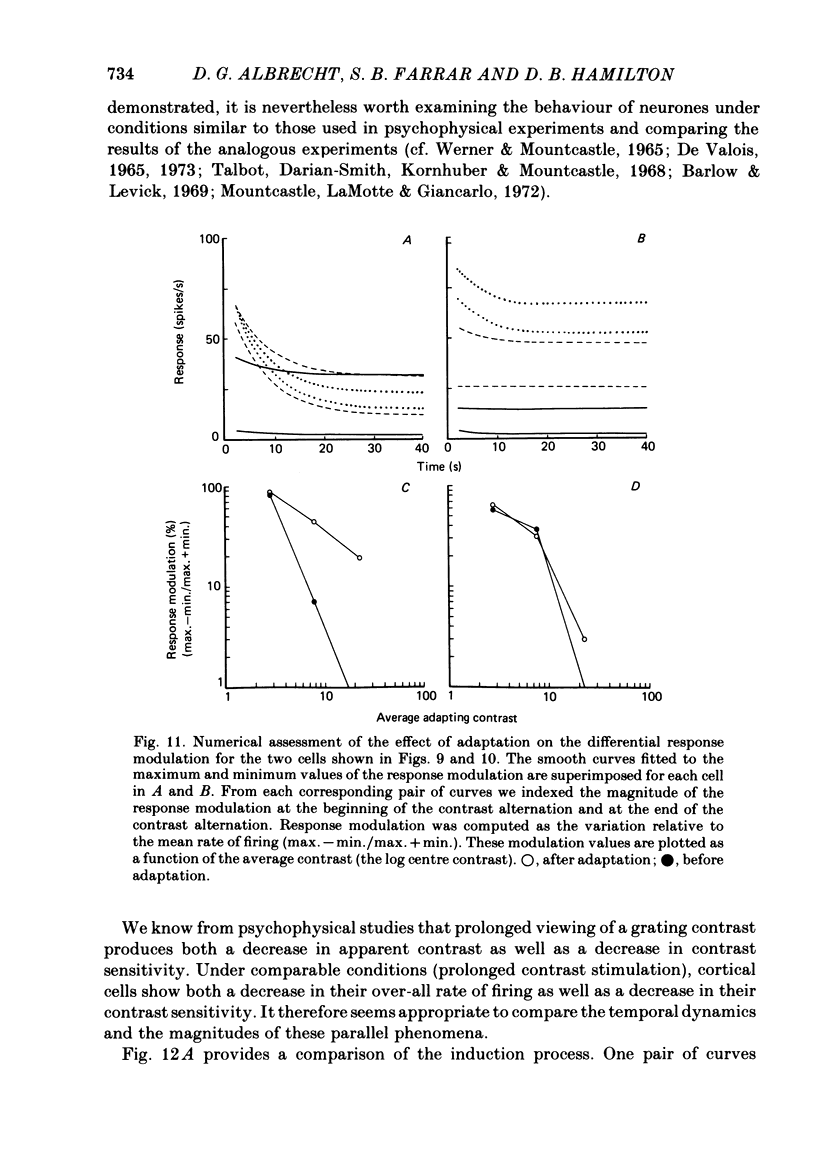




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian E. D., Zotterman Y. The impulses produced by sensory nerve-endings: Part II. The response of a Single End-Organ. J Physiol. 1926 Apr 23;61(2):151–171. doi: 10.1113/jphysiol.1926.sp002281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht D. G., De Valois R. L. Striate cortex responses to periodic patterns with and without the fundamental harmonics. J Physiol. 1981;319:497–514. doi: 10.1113/jphysiol.1981.sp013922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht D. G., Hamilton D. B. Striate cortex of monkey and cat: contrast response function. J Neurophysiol. 1982 Jul;48(1):217–237. doi: 10.1152/jn.1982.48.1.217. [DOI] [PubMed] [Google Scholar]
- Alpern M., Rushton W. A., Torii S. Signals from cones. J Physiol. 1970 Apr;207(2):463–475. doi: 10.1113/jphysiol.1970.sp009073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Levick W. R. Three factors limiting the reliable detection of light by retinal ganglion cells of the cat. J Physiol. 1969 Jan;200(1):1–24. doi: 10.1113/jphysiol.1969.sp008679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow H. B., Macleod D. I., van Meeteren A. Adaptation to gratings: no compensatory advantages found. Vision Res. 1976;16(10):1043–1045. doi: 10.1016/0042-6989(76)90241-8. [DOI] [PubMed] [Google Scholar]
- Baylor D. A., Fuortes M. G. Electrical responses of single cones in the retina of the turtle. J Physiol. 1970 Mar;207(1):77–92. doi: 10.1113/jphysiol.1970.sp009049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Campbell F. W. On the existence of neurones in the human visual system selectively sensitive to the orientation and size of retinal images. J Physiol. 1969 Jul;203(1):237–260. doi: 10.1113/jphysiol.1969.sp008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Muncey J. P., Ridley R. M. Perceptual fading of a stabilized cortical image. Nature. 1971 Sep 17;233(5316):204–205. doi: 10.1038/233204a0. [DOI] [PubMed] [Google Scholar]
- Blakemore C., Muncey J. P., Ridley R. M. Stimulus specificity in the human visual system. Vision Res. 1973 Oct;13(10):1915–1931. doi: 10.1016/0042-6989(73)90063-1. [DOI] [PubMed] [Google Scholar]
- Blakemore C., Nachmias J., Sutton P. The perceived spatial frequency shift: evidence for frequency-selective neurones in the human brain. J Physiol. 1970 Oct;210(3):727–750. doi: 10.1113/jphysiol.1970.sp009238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Nachmias J. The orientation specificity of two visual after-effects. J Physiol. 1971 Feb;213(1):157–174. doi: 10.1113/jphysiol.1971.sp009374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore C., Sutton P. Size adaptation: a new aftereffect. Science. 1969 Oct 10;166(3902):245–247. doi: 10.1126/science.166.3902.245. [DOI] [PubMed] [Google Scholar]
- Boynton R. M., Whitten D. N. Visual adaptation in monkey cones: recordings of late receptor potentials. Science. 1970 Dec 25;170(3965):1423–1426. doi: 10.1126/science.170.3965.1423. [DOI] [PubMed] [Google Scholar]
- Campbell F. W., Robson J. G. Application of Fourier analysis to the visibility of gratings. J Physiol. 1968 Aug;197(3):551–566. doi: 10.1113/jphysiol.1968.sp008574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik K. J. The effect of adaptation on differential brightness discrimination. J Physiol. 1938 May 14;92(4):406–421. doi: 10.1113/jphysiol.1938.sp003612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Valois R. L., Albrecht D. G., Thorell L. G. Spatial frequency selectivity of cells in macaque visual cortex. Vision Res. 1982;22(5):545–559. doi: 10.1016/0042-6989(82)90113-4. [DOI] [PubMed] [Google Scholar]
- De Valois R. L. Analysis and coding of color vision in the primate visual system. Cold Spring Harb Symp Quant Biol. 1965;30:567–579. doi: 10.1101/sqb.1965.030.01.055. [DOI] [PubMed] [Google Scholar]
- De Valois R. L., De Valois K. K. Spatial vision. Annu Rev Psychol. 1980;31:309–341. doi: 10.1146/annurev.ps.31.020180.001521. [DOI] [PubMed] [Google Scholar]
- De Valois R. L., Yund E. W., Hepler N. The orientation and direction selectivity of cells in macaque visual cortex. Vision Res. 1982;22(5):531–544. doi: 10.1016/0042-6989(82)90112-2. [DOI] [PubMed] [Google Scholar]
- Dowling J. E., Ripps H. Adaptation in skate photoreceptors. J Gen Physiol. 1972 Dec;60(6):698–719. doi: 10.1085/jgp.60.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fain G. L., Dowling J. E. Intracellular recordings from single rods and cones in the mudpuppy retina. Science. 1973 Jun 15;180(4091):1178–1181. doi: 10.1126/science.180.4091.1178. [DOI] [PubMed] [Google Scholar]
- Geisler W. S. Effects of bleaching and backgrounds on the flash response of the cone system. J Physiol. 1981 Mar;312:413–434. doi: 10.1113/jphysiol.1981.sp013635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUBEL D. H., WIESEL T. N. Receptive fields, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962 Jan;160:106–154. doi: 10.1113/jphysiol.1962.sp006837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemilä S. Background adaptation in the rods of the frog's retina. J Physiol. 1977 Mar;265(3):721–741. doi: 10.1113/jphysiol.1977.sp011740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood D. C., Ilves T., Maurer E., Wandell B., Buckingham E. Human cone saturation as a function of ambient intensity: a test of models of shifts in the dynamic range. Vision Res. 1978;18(8):983–993. doi: 10.1016/0042-6989(78)90026-3. [DOI] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Receptive fields and functional architecture of monkey striate cortex. J Physiol. 1968 Mar;195(1):215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt J., Dowling J. E. Intracellular recordings from gecko photoreceptors during light and dark adaptation. J Gen Physiol. 1975 Nov;66(5):617–648. doi: 10.1085/jgp.66.5.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei L., Fiorentini A., Bisti S. Neural correlate of perceptual adaptation to gratings. Science. 1973 Dec 7;182(4116):1036–1038. doi: 10.1126/science.182.4116.1036. [DOI] [PubMed] [Google Scholar]
- Mountcastle V. B., LaMotte R. H., Carli G. Detection thresholds for stimuli in humans and monkeys: comparison with threshold events in mechanoreceptive afferent nerve fibers innervating the monkey hand. J Neurophysiol. 1972 Jan;35(1):122–136. doi: 10.1152/jn.1972.35.1.122. [DOI] [PubMed] [Google Scholar]
- Movshon J. A., Blakemore C. Orientation specificity and spatial selectivity in human vision. Perception. 1973;2(1):53–60. doi: 10.1068/p020053. [DOI] [PubMed] [Google Scholar]
- Movshon J. A., Lennie P. Pattern-selective adaptation in visual cortical neurones. Nature. 1979 Apr 26;278(5707):850–852. doi: 10.1038/278850a0. [DOI] [PubMed] [Google Scholar]
- Naka K. I., Rushton W. A. S-potentials from colour units in the retina of fish (Cyprinidae). J Physiol. 1966 Aug;185(3):536–555. doi: 10.1113/jphysiol.1966.sp008001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normann R. A., Werblin F. S. Control of retinal sensitivity. I. Light and dark adaptation of vertebrate rods and cones. J Gen Physiol. 1974 Jan;63(1):37–61. doi: 10.1085/jgp.63.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekuler R. Spatial vision. Annu Rev Psychol. 1974;25:195–232. doi: 10.1146/annurev.ps.25.020174.001211. [DOI] [PubMed] [Google Scholar]
- Talbot W. H., Darian-Smith I., Kornhuber H. H., Mountcastle V. B. The sense of flutter-vibration: comparison of the human capacity with response patterns of mechanoreceptive afferents from the monkey hand. J Neurophysiol. 1968 Mar;31(2):301–334. doi: 10.1152/jn.1968.31.2.301. [DOI] [PubMed] [Google Scholar]
- Vautin R. G., Berkley M. A. Responses of single cells in cat visual cortex to prolonged stimulus movement: neural correlates of visual aftereffects. J Neurophysiol. 1977 Sep;40(5):1051–1065. doi: 10.1152/jn.1977.40.5.1051. [DOI] [PubMed] [Google Scholar]
- Werblin F. S. Adaptation in a vertebrate retina: intracellular recording in Necturus. J Neurophysiol. 1971 Mar;34(2):228–241. doi: 10.1152/jn.1971.34.2.228. [DOI] [PubMed] [Google Scholar]


