Abstract
O3-type layered oxide for sodium-ion batteries have attracted significant attention owing to their low cost and high energy density. However, their applications are restricted by rapid capacity decay during long-term cycling, with uneven Na+ distribution and microcrack formation being key contributing factors. In this study, a customized reconstruction layer integrating a fast ion conductor NaCaPO4 coating with gradient Ca2+ doping is developed to enhance the surface chemical and mechanical stability of the layered cathodes. The gradient Ca2+ doped interphase facilitates uniform phase transformation within the particles, minimizes lattice mismatch, ensures even Na+ distribution, and mitigates microcrack formation through a pinning effect. Consequently, the optimized sample exhibits improved electrochemical performance and robust reliability under high-voltage conditions and a broad temperature range (−10 to 50 °C). The practical feasibility of a pouch-type full cell paired with a hard carbon anode is demonstrated by a high capacity retention of 82.9% after 300 cycles at 0.5 C. This scalable interface modification strategy provides valuable insights into the development of advanced oxide cathode materials for sodium-ion batteries.
Subject terms: Batteries, Batteries
O3-type layered oxides are promising for sodium-ion batteries but suffer from rapid capacity decay. Here, the authors demonstrate that a NaCaPO4-derived gradient Ca2+-doped reconstruction layer enhances stability by mitigating phase transition-induced lattice stress and homogenizing Na-ion distribution.
Introduction
Sodium-ion batteries (SIBs), which share a similar electrochemical mechanism with lithium-ion batteries (LIBs), are considered an important supplement to LIB technology due to their low production cost, environmental sustainability, and high safety1. As a key component that influences the production cost and performance of SIBs, advanced cathode materials with a high energy density and long cycle life must be developed2. Among cathode materials, Na-layered oxides (NaxTMO2, where TM is a transition-metal ion) have emerged as commercially promising candidates owing to their numerous advantages3. Particular attention has been given to O3-type cathodes (NaxTMO2, 2/3 < x < 1), which are simple to synthesize, have a high theoretical specific capacity, and are compatible with hard carbon anodes4,5. However, for O3-type cathodes, achieving good cyclability, especially at high voltages, remains challenging, mainly due to detrimental phase transitions caused by an interlayer slip in deep desodiation states, lattice oxygen loss induced by anionic redox reactions, and microcracks formed by anisotropic changes in the lattice parameters6,7. Furthermore, residual alkali species on the surface react with H2O and CO2 in the air, leading to surface structure degradation and slurry gelation, which greatly increases transportation and processing costs8,9.
Conventional modification strategies include anion/cation doping to mitigate adverse phase transitions and enhance the stability of the layered framework structure5,10, surface coating to improve interfacial stability and prevent electrolyte corrosion11, and multiphase structure construction to reduce irreversible phase transitions12,13. Increasing research efforts have been directed toward interface modification strategies that integrate gradient doping and surface coating14–16. For instance, a surface ZrO2 coating and Zr4+ doping enhanced the Na storage performance of O3-Na[Mn1/3Fe1/3Ni1/3]O2 by improving the interfacial air/chemical stability, regulating the anion redox behavior, and optimizing the lattice structure17. In addition, a Na[Ni0.4Cu0.1Mn0.4Ti0.1]O2 cathode modified with a NaMgPO4 coating, serving as a good ion conductor, and Mg2+ doping, acting as a stabilizing agent, exhibited an extended high-voltage cycle life18.
Despite significant progress, previous studies have primarily focused on improvement mechanisms from the perspectives of physical confinement and structural optimization. However, lattice mismatch and internal stress accumulation caused by the uneven distribution of alkali-metal ions within particles are often overlooked19. The disparity in ion diffusion kinetics between the bulk and surface leads to uneven Na-ion concentration and phase structure, generating surface tensile stress and internal compressive stress, which drive the formation of microcracks—major contributors to mechanical failure20,21. Shortening the ion diffusion path, improving the ion diffusion kinetics, and stabilizing the layered framework can mitigate the internal stress caused by non-uniform phase transformations and ion diffusion, thereby improving cycling stability22. For example, uniform K-ion doping in the Na layer can enhance the reaction kinetics, suppress harmful high-voltage phase transitions, homogenize the Na-ion distribution, and reduce interlaminar stress23. In addition, the construction of high-entropy and multiphase structures can suppress interlayer slip and enhance Na+ migration through structural constraint, thereby preventing structural degradation caused by large Na+ concentration gradients24. However, homogeneous doping and multiphase structure designs struggle to fundamentally resolve this issue25. Given that the non-uniform distribution of Na-ions predominantly occurs in the near-surface/interface region of the particle, it is proposed to introduce appropriate dopants as pillars into the Na layer at the interface to regulate Na-ion diffusion and phase transition processes.
Building on these concepts, interface reconstruction induced by an epitaxially grown coating is proposed to enhance the Na storage performance of layered oxides. In this work, NaCaPO4, a fast ion conductor, was systematically investigated as a model coating. In situ spectroscopy and postmortem analysis revealed that the coating enhanced interfacial chemical stability, while gradient Ca2+ doping mitigated lattice mismatch-induced internal stress accumulation through a pinning effect, significantly improving the mechanical stability. Benefiting from this advanced customized interface structure design, the optimized sample exhibited enhanced Na storage performance and air/thermal stability. In particular, satisfactory cycling characteristics were achieved under extreme conditions, such as high voltage (4.5 V vs. Na+/Na), high temperature (50 °C), and low temperature (− 10 °C). The practicality of the optimized sample was validated in both Na-ion full cells and anode-free cells. This strategy, which integrates NaCaPO4 coating with gradient Ca2+ doping, is also applicable to other layered oxide cathodes, offering promising prospects for the development of advanced cathode materials for SIBs.
Results
Formation of interface reconstruction layer
In this study, Na[Ni0.425Fe0.15Mn0.425]O2 (NFM), a low-cost, high-capacity material with commercial application potential, was used as a model. The Na storage performance was enhanced by bulk doping with Mg and Ti (Na[Ni0.4Fe0.1Mn0.4Mg0.05Ti0.05]O2; NFMMT) to mitigate adverse high-voltage phase transitions and improve the stability of the layered framework structure (Supplementary Fig. 1)5,14. The powder X-ray diffraction (XRD) results indicate that the Ca3(PO4)2 precoating reacted with surface residual Na species at elevated temperatures (650 °C) to form the target NaCaPO4 (space group: Pnam)-coated NFMMT (NFMMT/NaCaPO4) cathode (Fig. 1a and Supplementary Fig. 2)26. To demonstrate the enhanced performance of the reconstructed surface, a cathode with homogeneous Ca doping in the Na layer ([Na0.98Ca0.01][Ni0.4Fe0.1Mn0.4Mg0.05Ti0.05]O2; NFMMT-Ca doped) was prepared as a control16.
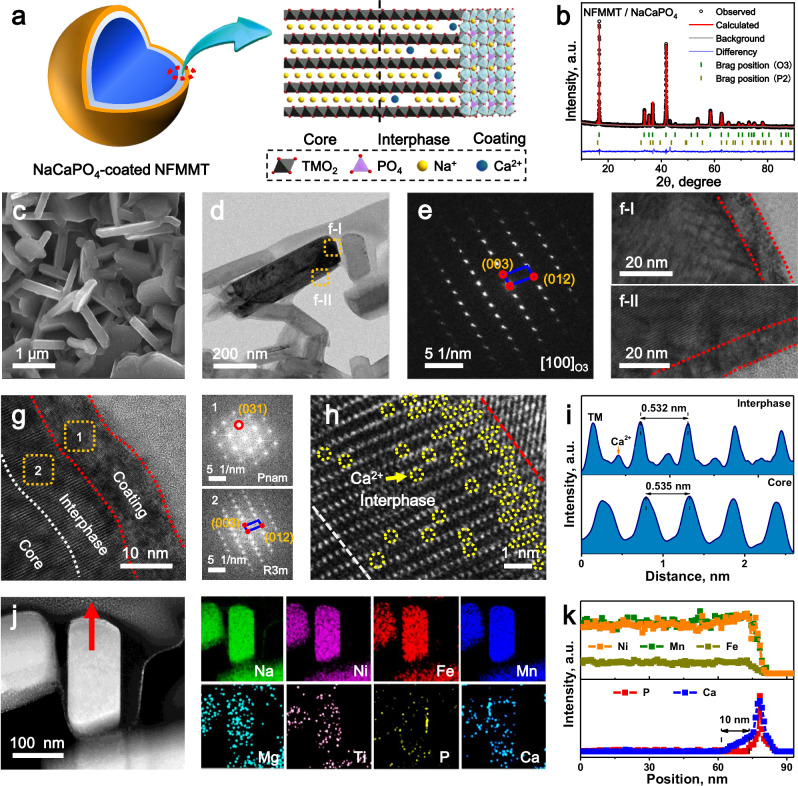
Fig. 1. Schematic diagram and characterization of NFMMT/NaCaPO4.
a Schematic of the strategy of surface NaCaPO4 coating combined with gradient Ca2+ doping. b Rietveld-refined profiles of NFMMT/NaCaPO4. c SEM image of NFMMT/NaCaPO4. d Low-magnification STEM images and (e) corresponding SAED pattern of NFMMT/NaCaPO4; f, g HRTEM image on the particle surface and corresponding FFT pattern. h HRTEM images of the interlayer Ca2+ intercalation structures along the [100] projection in the interface region. i Intensity line profile extracted from the interphase (top) and core (bottom) regions. j TEM-EDS mapping images of Na, Ni, Fe, Mn, Mg, Ti, P, and Ca in NFMMT/NaCaPO4. k EDS line scan profiles of Ni, Fe, Mn, P, and Ca along the arrow in (j).
The chemical composition was verified using inductively coupled plasma optical emission spectrometry (ICP-OES). The calculated normalized ratio is similar to the design value, confirming the successful synthesis of the cathode material (Supplementary Table 1). As shown in Supplementary Fig. 3a, all the synthesized cathodes can be indexed to the R3̅m space group, indicating a typical O3-type structure5,11. The Rietveld refinement results indicate that the doped samples have similar lattice parameters, suggesting that the introduction of low-concentration dopants does not significantly alter the phase structure or interlayer spacing (Supplementary Fig. 4 and Supplementary Table 2)27. According to previous reports, under a sufficient thermodynamic driving force, ion exchange can occur to form a functional gradient-doped interphase28. As Ca–O and Na–O bonds are weaker than TM–O bonds, Na/Ca exchange near the interphase may occur under thermodynamic control and kinetic constraints16. After high-temperature calcination, a small amount of the P2 phase (space group: P63/mmc) was detected in NFMMT/NaCaPO4, and the (003) diffraction peak shifted toward a lower angle (Supplementary Fig. 5), indicating a decrease in the Na content of the bulk phase and an expansion of the Na interlayer spacing29. Thus, the coating formation process consumes surface residual Na species and some Na ions from the bulk phase, suggesting that Na/Ca ion exchange occurs at the interface. The corresponding Rietveld-refined XRD pattern reveals lattice parameters of a = 2.967 Å, c = 16.009 Å, and V = 122.069 Å3 for NFMMT/NaCaPO4, with a structure consisting of 98.6% O3 phase and 1.4% P2-type phase (Fig. 1b). This P2/O3 intergrown structure further enhances the interface stability through biphasic interlocking29. Based on the Williamson–Hall analysis results, NFMMT/NaCaPO4 exhibits the lowest lattice strain (Supplementary Fig. 3b). Therefore, instead of inducing lattice distortion, the introduction of the interface reconstruction layer promoted the formation of an ordered atomic configuration30,31. This behavior is attributed to the introduction of surface reconstruction layers that enhance the structural stability of the interface and reduce TM-ion displacement32. The optimal synthesis conditions for the protective layer were determined based on the long-term cycling stability, as excessively high calcination temperatures and thick coating layers can lead to capacity loss and reduced cyclability (Supplementary Figs. 6 and 7)33. Therefore, the 3 wt.% coated sample, demonstrating better Na storage performance, was chosen as the representative sample for comprehensive structural and electrochemical investigations.
The microstructures of the synthesized cathodes were characterized using scanning electron microscopy (SEM) and dark-field scanning transmission electron microscopy (STEM). As shown in Supplementary Fig. 8 and Fig. 1c, the cathodes synthesized via the traditional solid-state method exhibit a typical sheet-like particle morphology, with a diameter of 1–2 μm and a thickness of approximately 100 nm29. The energy-dispersive X-ray spectroscopy (EDS) mapping revealed a homogeneous distribution of all elements on the surface of NFMMT/NaCaPO4, indicating that NaCaPO4 was uniformly coated on the surface of the cathode particles without agglomeration (Supplementary Fig. 9). The atomic structure and detailed crystallographic information near the particle edges of the cathode cross section prepared by focused ion beam (FIB) milling were analyzed using STEM and selected area electron diffraction (SAED). As shown in Supplementary Fig. 10, the preferred orientation of the layered oxide leads to the NFMMT cathode having nanosheet crystals with well-defined particle edges. The SAED pattern obtained along the [100] zone axis of an individual particle reveals diffraction spots characteristic of an O3-type structure, indicating high crystallinity and single-crystal properties22. High-resolution TEM (HRTEM) revealed ordered lattice fringes with a spacing of 0.532 nm, corresponding to the (003) crystal plane of the O3 structure, consistent with the XRD results14.
The NFMMT/NaCaPO4 exhibits a uniform and continuous surface coating with a thickness of approximately 10 nm, demonstrating the consistency of the wet chemical coating process (Fig. 1f). HRTEM images show that the bulk structure of the modified layered oxide maintains a layered configuration, whereas the surface has lattice fringes with spacings of 0.266 nm, corresponding to the (031) facets of NaCaPO4, confirming the successful formation of a surface protective layer (Fig. 1g). Notably, the epitaxially grown NaCaPO4 is oriented perpendicular to the (003) crystal plane, creating a lattice-anchored interface that can facilitate ion conduction and enhance the air/chemical stability of the layered oxides (Supplementary Fig. 11)11. The relatively bright contrast observed between adjacent TM layers near the interface of the main O3 structure is nearly invisible within the particles (approximately 20 nm) (Fig. 1h). As Na/O atoms are challenging to identify because of their low atomic weights, the pronounced differences in the intensity profile of the Na-layer columns directly indicate the presence of Ca atoms in the Na layer34,35. Similarly, the intensity line profile reveals strong Ca2+ signals near the surface (Fig. 1i). In addition, the interlayer spacing of the O3-type layered structure near the interface (0.532 nm) is slightly smaller than that in the core region (0.535 nm). This slight lattice contraction is attributed to the enhanced interlayer binding energy resulting from Ca2+ intercalation36.
The elemental distributions of the particles were analyzed using TEM combined with EDS mapping. The elemental mapping image shows that the TM elements are uniformly distributed in NFMMT/NaCaPO4, whereas signals corresponding to aggregated Ca and P appear near the particle surface, suggesting a heterogeneous surface structure at the particle level (Fig. 1j). In addition, Ca signals were detected on the surface and within specific regions of the particles, confirming the formation of Ca-doped interphase. To further investigate the position and thickness of the surface coating and Ca-doped interphase, high-resolution EDS line scanning was performed along the arrow in Fig. 1j, with the results presented in Fig. 1k. The signal for Ni/Fe/Mn decreases after passing through the particles, whereas that of Ca/P increases sharply at the particle edges. In addition, the distribution of Ca is approximately 10 nm wider than that of P, indicating that the introduction of the NaCaPO4 protective layer induced gradient Ca doping at the interface28. These results demonstrate the successful synthesis of a reconstruction layer composed of a 10 nm NaCaPO4 coating and a 10 nm gradient Ca-doped interphase, which enhances the stability of the interface structure and prevents surface degradation.
The surface chemical states of the synthesized cathodes were analyzed using X-ray photoelectron spectroscopy (XPS). After etching, the peak positions of Ni and Mn in NFMMT/NaCaPO4 do not shift significantly, indicating that the TM ions are coordinated to O2− rather than PO43− (Supplementary Fig. 12a). In addition, as the etching depth increases, the Ni and Mn peak intensities gradually increase and then stabilize, whereas the Ca peak intensity decreases significantly, confirming the presence of a NaCaPO4 surface protective layer and a gradient Ca-doped interphase (Fig. 2a, b)37. Furthermore, the high-resolution C 1 s XPS spectrum was deconvoluted into two peaks at 284.4 and 288.3 eV, corresponding to CO32− and C–C bonds, respectively (Fig. 2c). Notably, NFMMT/NaCaPO4 exhibited a lower CO32− intensity, indicating that the coating effectively consumes surface residual Na species, consistent with the low surface oxygen content observed in the high-resolution O 1 s XPS spectrum (Supplementary Fig. 12b). The decrease in surface residual alkali species (e.g., NaOH and Na2CO3) significantly enhances the air stability of the layered oxide cathodes18. Based on these results, thermodynamically driven Na/Ca ion exchange leads to the formation of a small amount of the P2 phase and gradient Ca2+ doped interphase. The elemental distribution within the particles was further visualized using time-of-flight secondary ion mass spectrometry (TOF-SIMS)26. As shown in Fig. 2d–f, P is concentrated in the outer layer, while trace amounts of Ca2+ are present in both the outer and inner regions of the particles. These findings confirm the successful formation of a reconstructed layer, featuring a 10 nm NaCaPO4 coating accompanied by gradient Ca2+ doping at the interface.
Fig. 2. Surface characterization of the synthesized cathode.
a Ca 2p and (b) Mn 2p XPS spectra at different depths in NFMMT/NaCaPO4. c C 1 s XPS spectra of NFM, NFMMT, NFMMT-Ca doped, and NFMMT/NaCaPO4. d Spatial distributions of selected secondary ions (Ca, P, Ni, and Mn) in NFMMT/NaCaPO4. Normalized TOF-SIMS depth profiles of Ca, P, Ni, Fe, and Mn in (e) NFMMT and (f) NFMMT/NaCaPO4.
Na storage performance of surface-modified cathode
The electrochemical behavior of the prepared cathodes was first evaluated using a standard coin-type half cell within the voltage range of 2.0–4.2 V (vs. Na+/Na). As revealed by the voltage curves for the initial cycle at 0.1 C (1 C = 150 mA g−1), all the samples undergo similar electrochemical processes (Fig. 3a). The charge/discharge plateaus at approximately 2.7 and 4.1 V correspond to phase transitions from O3 to P3 and P3 to OP2, respectively, whereas the sloping region between 3.0 V and 4.0 V corresponds to the solid-solution reaction of the P3 phase33. Compared with NFM, which has a high discharge capacity of 173.1 mAh g−1, the incorporation of inactive Mg/Ti ions reduces the Ni content of NFMMT, resulting in a lower reversible capacity of 152.7 mAh g−1. The introduction of the NaCaPO4 coating slightly decreased the reversible capacity of NFMMT/NaCaPO4 to 148.2 mAh g−1, which remains within the acceptable range. However, NFMMT/NaCaPO4 achieved the highest initial Coulombic efficiency (ICE) of 97.9% with a capacity loss of only 3.2 mAh g−1, thereby significantly outperforming the original sample (84.6% ICE and 32.4 mAh g−1 capacity loss). The enhanced ICE and reduced voltage hysteresis are attributed to the introduction of an interface reconstruction layer, which effectively reduces residual alkali species on the surface, suppresses parasitic side reactions with the electrolyte, and improves the stability of the interface structure38. During cyclic voltammetry (CV) measurements at a scanning rate of 0.1 mV s−1, the redox peak position of NFMMT/NaCaPO4 remains stable over the first 5 cycles, indicating that the reconstructed interface is beneficial for improving structural reversibility and inhibiting anionic redox reactions (Fig. 3b)16. In contrast, NFMMT exhibits a significantly reduced peak current intensity and pronounced separation of the redox peak potentials (0.223 V), suggesting that irreversible structural damage occurs during the electrochemical process. The rate capabilities of the synthesized cathodes were evaluated at different C rates (0.1–10 C and then returning to 0.2 C) (Fig. 3c). The capacity of NFMMT/NaCaPO4 decays gradually under high current densities, but the initial discharge capacity is almost fully recovered after rate testing, with reversible capacities of 148.3, 142.8, 136.2, 130.5, 124.0, and 119.2 mAh g−1 at 0.1, 0.2, 0.5, 1, 2, and 5 C, respectively. Even at a high current rate of 10 C, NFMMT/NaCaPO4 maintains a capacity of 105.3 mAh g−1, which is approximately 71.0% of the capacity at 0.1 C. In sharp contrast, the discharge capacities of the pristine and doped samples decrease dramatically with increasing current rates. The introduction of NaCaPO4 coating not only increases ionic conductivity but also enhances interface stability by suppressing parasitic side reactions and mitigating surface phase transitions, resulting in significantly improved rate capability (Supplementary Fig. 13).
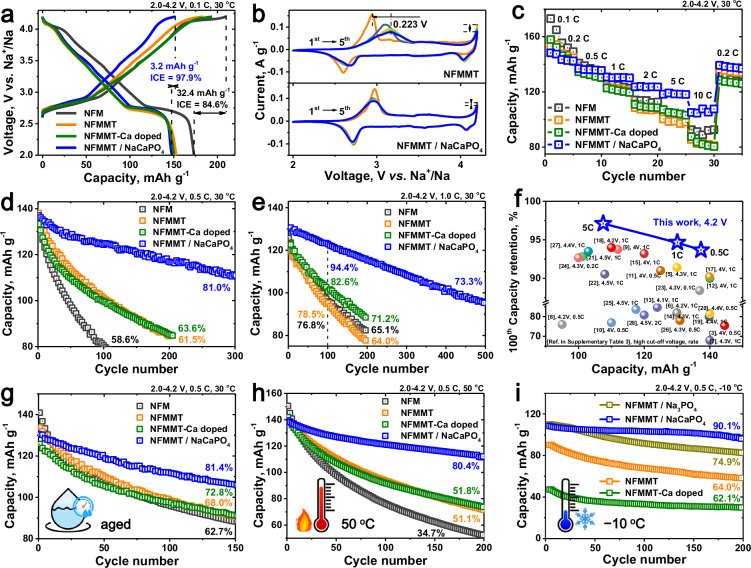
Fig. 3. Electrochemical characteristics of the fabricated cathodes in a Na half-cell system.
a Initial charge–discharge curves, (b) CV curves, (c) rate capability measurements, and cycling performance at (d) 0.5 C and (e) 1 C for NFM, NFMMT, NFMMT-Ca doped, and NFMMT/NaCaPO4 within a 2.0–4.2 V voltage range under normal conditions. f Long-term cycle life of half-cells with NFMMT/NaCaPO4 compared to previously reported layered oxides. Cycling performance of the resulting cathodes after (g) 7 days of air exposure (aged), and under (h) high-temperature (50 °C) and (i) low-temperature (− 10 °C) conditions.
Subsequently, the impact of the interface reconstruction strategy on long-term cycling stability was assessed using galvanostatic charge/discharge tests. As shown in Fig. 3d, NFMMT/NaCaPO4 exhibits the highest structural stability, retaining 81.0% of its initial discharge capacity after 300 cycles at 0.5 C, significantly outperforming NFM and NFMMT. This remarkable improvement in cycling stability is attributed to the enhanced surface chemistry and bulk mechanical stability, as discussed in the following sections. Although the capacity retention of NFMMT-Ca doped is slightly higher than that of NFMMT, it remains inferior to that of NFMMT/NaCaPO4, suggesting that further doping in multicomponent cathodes hinders efficient Na storage24. The prepared cathodes were further tested at high current densities of 1–5 C (Supplementary Figs. 14 and 15). NFMMT/NaCaPO4 delivers terminal capacities of 122.8 and 108.1 mAh g−1 after 100 and 300 cycles at 1 C, corresponding to capacity retention rates of 94.4% and 83.1%, respectively (Fig. 3e). The long-term cycling stability of NFMMT/NaCaPO4 at different current rates is consistent with its enhanced rate performance, which is attributed to the high ionic conductivity and robust interfacial stability of the introduced coating. This Na storage performance exceeds most previously reported results, highlighting the progressive nature of the interface reconstruction strategy (Fig. 3f and Supplementary Table 3)38–49.
The high-voltage performance (4.5 V), all-climate performance (− 10–50 °C), and air stability of the prepared cathode were evaluated to assess its operability in harsh environments. Even at a high cutoff voltage of 4.5 V (Supplementary Fig. 16), the optimized cathode demonstrates a reversible capacity of 109.9 mAh g−1 with a capacity retention of 71.2% after 150 cycles at 0.5 C. In addition, because of their high surface reactivity and strong adsorption capacity, layered cathodes typically exhibit poorer air stability than their Li counterparts, which significantly limits their commercial potential8,50. However, after exposure to humid air (with a relative humidity of 55%) for 7 days, the O3 phase structure of NFMMT/NaCaPO4 is largely retained, with minimal byproduct formation and a small peak shift (0.133°), demonstrating that the uniform reconstruction layer effectively protected the cathode from water insertion (Supplementary Figs. 17 and 18). Consequently, the aged NFMMT/NaCaPO4 maintains a reversible capacity of 130.5 mAh g−1 at 0.5 C, with 81.4% capacity retention after 150 cycles at 0.5 C (Fig. 3g). In contrast, exposure to moisture causes NFMMT to aggregate and form by-products, which increases interfacial resistance and hinders Na-ion diffusion kinetics, leading to poor Na storage performance, with a capacity retention of 68.0% after 150 cycles.
The cycling stabilities of the prepared cathodes were evaluated at an elevated temperature of 50 °C to assess structural integrity29. As shown in Fig. 3h, NFMMT/NaCaPO4 exhibits significantly improved cycling stability in the voltage range of 2–4.2 V, maintaining a reversible capacity of 112.2 mAh g−1 (80.4% capacity retention) after 200 cycles at 0.5 C. The enhanced high-temperature stability is attributed to improved thermal properties, leading to higher exothermic peaks and reduced heat generation in the fully desodiated state (Supplementary Fig. 19), ultimately contributing to increased safety. However, NFMMT retains only 51.1% of its initial capacity under the same conditions owing to dissolution of the active materials and adverse TM-ion crosstalk effects29.
In addition, the feasibility of low-temperature (− 10 °C) operation was evaluated to expand the application range of SIBs (Fig. 3i). Low temperatures limit ion mobility, leading to a relatively low discharge capacity and significantly increased polarization in the synthesized cathode (Supplementary Fig. 20)14. NFMMT delivers reversible capacities of 123.7, 111.4, and 90.3 mAh g−1 at 0.1, 0.2, and 0.5 C, respectively, indicating inferior rate performance at low temperatures. Encouragingly, NFMMT/NaCaPO4 not only exhibits better rate performance at low temperatures (discharge capacities of 129.6, 116.9, and 108.6 mAh g−1 at 0.1, 0.2, and 0.5 C, respectively) but also demonstrates enhanced cycling stability, retaining 90.1% of its initial capacity after 200 cycles at 0.5 C. However, the low-temperature performance of the bulk Ca-doped sample is worse than that of the NFMMT cathode, with a capacity retention of only 62.1% after 200 cycles. This behavior is attributed to the poor reaction kinetics caused by homogeneous Ca2+ doping in the Na layer, which hinders Na-ion diffusion32.
To obtain further insights into the improvement in low-temperature performance provided by the reconstructed coating, a cathode with a Na3PO4 coating (NFMMT/Na3PO4) was synthesized using a similar method. The introduced phosphate coating enhanced Na-ion diffusion kinetics, resulting in a cathode with a rate capability (110.0 mAh g−1 at 0.5 C) comparable to that of NFMMT/NaCaPO4 but slightly inferior cycling stability. These findings indicate that surface-gradient Ca2+ doping significantly enhances interfacial structural stability and extends cycle life, as discussed in subsequent sections. Therefore, the optimized cathode demonstrates good cyclability under harsh conditions, including high cutoff voltages, humid air, and a wide temperature range.
Owing to the superior Na storage performance of NFMMT/NaCaPO4 in Na half cells, this cathode was integrated into Na-ion full cells (with commercial hard carbon (HC) anodes) and anode-free cells (with carbon-coated Al (Al-C) negative-electrode current collectors) to assess its practical applicability51. To compensate for the initial capacity loss (Supplementary Figs. 21 and 22), electrochemical presodiation and sacrificial cathode additives were employed to preactivate HC and Al-C (see the methods section for details). As shown in Supplementary Fig. 23, the constructed full cell delivers a reversible capacity of 149.9 mAh g−1 (based on the cathode mass) at 0.1 C, with an average operating voltage of 3.16 V. The HC | NFMMT/NaCaPO4 full cell exhibits enhanced rate performance, achieving a high reversible capacity of 92.8 mAh g−1 at 10 C, whereas the HC | NFMMT full cell only reaches 66.1 mAh g−1 at the same rate (Fig. 4a). In addition, the cycling stability of the full-cell system is promising. Specifically, the HC | NFMMT/NaCaPO4 full cell delivers an acceptable specific capacity of 138.3 mAh g−1 at 0.5 C, with a capacity loss of only 15.0% after 200 cycles (Fig. 4b). Even after 500 cycles at 1 and 2 C, 76.5% and 75.3% of the reversible capacity are retained, respectively (Fig. 4c). In contrast, the HC | NFMMT full cell retains only 59.6% of its initial capacity after 200 cycles at 0.5 C.
Fig. 4. Practicality of the fabricated cathodes in Na full cells and anode-free Na batteries.
a Rate capability measurements and cycling performance at (b) 0.5 C and (c) 1 C for NFM, NFMMT, and NFMMT/NaCaPO4 in Na-ion full cells paired with HC anodes, with an electrochemical pre-sodiation process for the HC. d Rate capability measurements and cycling performance at (e) 0.5 C and (f) 1 C for NFM and NFMMT/NaCaPO4 in anode-free Na batteries, with Na2C2O4 used as the sodium compensator. g Charge/discharge profiles from the 1st to 100th cycle of the HC | NFMMT/NaCaPO4 pouch-type cell at 0.5 C without the pre-sodiation process. h Cycling performance of NFMMT and NFMMT/NaCaPO4 in pouch cells at 0.5 C.
Owing to the absence of active materials on the negative electrode side, anode-free Na batteries, which have ultrahigh energy densities, have recently garnered significant research attention43. After activation52,53, the anode-free Na battery exhibits stable cycling with a reversible capacity of approximately 145 mAh g−1 (Supplementary Fig. 24). Owing to the detrimental crosstalk effect of TM ions derived from the dissolution of the active materials54, Al-C | NFM exhibits significant capacity degradation and inferior rate properties, delivering specific capacities of 166.2 and 65.4 mAh g−1 at 0.1 and 5 C, respectively (Fig. 4d). In contrast, Al-C | NFMMT/NaCaPO4 maintains a high reversible capacity of 96.9 mAh g−1 at 5 C, demonstrating effective suppression of TM dissolution. In addition, long-term cycling durability tests showed that Al-C | NFMMT/NaCaPO4 retains 80.8% of its initial capacity after 300 cycles at 0.5 C, whereas Al-C | NFM lost 83.8% of its reversible capacity after 200 cycles (Fig. 4e). The enhanced cycling stability of Al-C | NFMMT/NaCaPO4 is particularly evident at high current rates, with 74.5% capacity retention after 500 cycles at 1 C (Fig. 4f). In contrast, Al-C | NFM loses nearly all of its capacity within the first 100 cycles.
As a demonstration of the practical feasibility of the proposed cathodes, single-layer pouch cells were assembled under realistic conditions using a high active material loading (12 mg cm−2) and an HC anode without pre-sodiation treatment30,55. Because of the initial irreversible capacity loss of the HC anode, the reversible capacity of the pouch cell (approximately 120 mAh g−1) is slightly lower than that of the half cell (Fig. 4g). However, the HC | NFMMT/NaCaPO4 pouch-type full cell retains 82.9% of its initial specific capacity after 300 cycles at a current density of 0.9 mA cm−2 (0.5 C), whereas the capacity retention of the HC | NFM pouch-type full cell is only 53.9% (Fig. 4h). Thus, the proposed NFMMT/NaCaPO4 cathode exhibits enhanced Na storage performance and has considerable potential for practical applications. In addition, the incorporation of the interface reconstruction layer into the cathode improved surface chemical stability and suppressed parasitic reactions with the electrolyte, resulting in minimal volume expansion (6.1%) in the pouch-type cell (Supplementary Fig. 25).
Structural evolution and reaction mechanism
The improved cycling stability of layered oxides is typically associated with structural evolution during electrochemical processes11,30. Consequently, in situ XRD analysis was used to evaluate the phase transitions of the NFMMT and NFMMT/NaCaPO4 cathodes. Supplementary Fig. 26 illustrates that the prepared cathodes undergo a phase transition from hexagonal O3 to hexagonal P3, subsequently transitioning to the OP2 phase18. As shown in Fig. 5a, for both cathodes, the (003) diffraction peak shifts toward lower diffraction angles during initial charging and then abruptly to higher angles upon charging to ~ 4.0 V. Initially, Na extraction increases the electrostatic repulsion between adjacent oxygen slabs, resulting in an expansion of the c-lattice parameters29. However, at high voltages and low Na contents, peroxo-like (O–O)n− dimers reduce interlayer repulsion, leading to lattice contraction, which is characteristic of the phase transition from P3 to the OP2 phase with TMO2 stacking faults34. Notably, the shift of the (003) peak becomes asymmetric at the end of the charging stage (4.0–4.2 V) and during initial discharging (4.2–3.9 V). This behavior is attributed to ion transport being more rapid in the prismatic (P) layer than in the octahedral (O) layer, which results in the partial loss of O structures5. Obviously, NFMMT/NaCaPO4 displays a more symmetrical shift during sodiation/desodiation (0.067° deviation), indicating enhanced structural reversibility. Although both samples exhibit similar structural evolution, the specific changes in the lattice parameters during charging and discharging differ. As shown in Fig. 5b and Supplementary Fig. 27, smooth variations along the a-axis are accompanied by zigzag changes in the c and V parameters14. The change in the c-axis parameter is significantly smaller for NFMMT/NaCaPO4 (7.2%) than for NFMMT (9.6%). Repeated fluctuations in the lattice parameters and sliding of the TMO2 layer during electrochemical processes lead to the accumulation of internal stress and weakening of the repulsive force between the Na and TM layers, resulting in structural degradation and irreversible capacity loss29,56. Therefore, the reduced lattice parameter changes observed for NFMMT/NaCaPO4 indicate enhanced structural stability and integrity.
Fig. 5. Structural and dynamic analysis of fabricated cathodes.
a 2D contour plot of in situ XRD patterns during charge/discharge of NFMMT (left) and NFMMT/NaCaPO4 (right). b Change in the c parameter during charge/discharge of NFMMT and NFMMT/NaCaPO4. XRD patterns of (c) NFMMT and (d) NFMMT/NaCaPO4 at different charge voltages during the initial cycle. e Diffusion coefficients of NFM, NFMMT, NFMMT-Ca doped, and NFMMT/NaCaPO4 during the electrochemical process. f EIS spectra of NFMMT, NFMMT-Ca doped, and NFMMT/NaCaPO4 at different cycles. SEM images of (g) NFMMT and (h) NFMMT/NaCaPO4 after 200 cycles.
To clarify the underlying mechanism, the evolution of the (003) peak during the O3–P3 phase transition was examined in detail. As illustrated in Fig. 5a, c, d, the (003)O3 peak of NFMMT remained at around 16.7° and partially transitioned from O3 to P3 when charged to 2.8 V, resulting in distinct and well-defined O3 and P3 regions. This behavior was caused by the uneven extraction of Na ions from the particles, resulting in inconsistent desodiation between the bulk and surface, indicating a heterogeneous phase transformation. The differing spatial symmetries of the two phases further contribute to significant interfacial energy, resulting in lattice mismatch, internal stress accumulation, and irreversible structural degradation22. In contrast, the (003)O3 peak of NFMMT/NaCaPO4 shifts uniformly and continuously to lower angles during charging, ultimately forming a mixed-phase structure containing both O3 and P3 phases before fully transitioning into a stable P3 phase. This ordered structural evolution was attributed to enhanced interfacial stability, uniform phase transition, and homogeneous Na-ion distribution enabled by the reconstruction layer. Furthermore, in-situ XRD results of NFMMT after 100 cycles revealed pronounced heterogeneous phase transitions and the disappearance of the high-voltage phase transition process, both of which significantly contribute to cycling performance degradation (Supplementary Fig. 28). In contrast, NFMMT/NaCaPO4 maintained uniform phase transitions even after 100 cycles, underscoring the pivotal role of the reconstruction layer in improving surface structural stability. To further characterize this ordered structural evolution, electrical conductivity maps of the cross-sections of cathodes charged to 2.8 V were investigated using scanning spreading resistance microscopy (SSRM). As shown in Supplementary Fig. 29, NFMMT exhibited significant conductivity fluctuations due to non-uniform phase transitions, resulting in reduced conductivity within the particles. In contrast, NFMMT/NaCaPO4 showed no noticeable inactive regions, further confirming the homogeneous phase transition process facilitated by the interphase reconstruction layer.
Electrochemical impedance spectroscopy (EIS) and the galvanostatic intermittent titration technique (GITT) were used to evaluate the effect of the reconstruction layer on the reaction kinetics57. In the GITT measurements, current pulses of 0.05 C were applied for 1200 s, followed by a 2 h relaxation period33. All the samples exhibit lower Na+ diffusion coefficients (DNa) at approximately 2.8 and 4.1 V, which is attributed to phase transitions caused by interlayer slipping (Fig. 5e). NFMMT/NaCaPO4 has significantly higher DNa values (3.72 × 10−11– 4.64 × 10−10 cm2 s−1) and a lower overpotential (≈ 15 mV) (Supplementary Fig. 30), indicating that the interface coating effectively enhanced the ion diffusion kinetics. This result is consistent with the DNa value calculated from the CV results using the linear relationship between the peak current and square root of the scan rate (Supplementary Fig. 31). Specifically, owing to the fast ion conductor coating, the DNa value of NFMMT/NaCaPO4 (4.20 × 10−10 cm2 s−1) is nearly twice that of NFMMT (2.21 × 10−10 cm2s−1). Notably, the Ca-doped sample exhibits lower Na+ diffusivity because the Ca2+ ions incorporated into the interlayer partially hinder Na-ion diffusion, consistent with its relatively poor rate performance and low-temperature behavior. However, as the amount of Ca2+ introduced into the Na layer is small, the gradient doping strategy has a minimal negative impact on the reaction kinetics. Consequently, the customized interface reconstruction layer effectively enhances the long-term cycling stability of layered oxides without compromising rate performance, which is essential for developing commercial positive electrodes.
Electrode degradation after long-term cycling was investigated by analyzing the EIS spectra of the cathode at different states of charging (SOC). As shown in Supplementary Figs. 32–34, the Nyquist plots consist of semicircles in the high- and mid-frequency regions and a sloped line in the low-frequency region, corresponding to the surface diffusion resistance (Rsf), charge-transfer resistance (Rct), and Warburg diffusion impedance, respectively22. The Rct value is higher than the Rsf value, indicating that charge-transfer resistance plays a greater role in the electrochemical behavior of layered oxides33. Compared with NFMMT and NFMMT-Ca doped, NFMMT/NaCaPO4 has lower Rct values throughout the electrochemical process owing to the introduction of a surface protective layer (Supplementary Fig. 35)15. However, parasitic side reactions and structural degradation cause the Rct value to increase with cycling (Fig. 5f). Nevertheless, the Rct value of NFMMT/NaCaPO4 increases by only 80.7 Ω after 200 cycles, which is significantly lower than the increases observed for NFMMT (241.9 Ω) and NFMMT-Ca doped (344.5 Ω). These results confirm that the introduced interface reconstruction layer enhances ionic transport and mitigates the intergranular cracking and structural degradation caused by phase transitions, leading to improved cathode durability.
The impact of the interface reconstruction strategy on the structural integrity of layered oxide cathodes was explored using cross-sectional SEM analysis. As shown in Fig. 5g, after 200 cycles, prominent cracks penetrate the NFMMT particles, which are attributed to stress accumulation at the grain boundaries and intergranular crack formation along the c-axis owing to lattice parameter variations and dislocations during the electrochemical process6,58. These microcracks expose fresh surfaces, thereby accelerating electrolyte penetration and leading to the formation of unstable cathode electrolyte interphase (CEI) layers, consistent with the obvious increase in electrochemical impedance29. In contrast, NFMMT/NaCaPO4 exhibits negligible structural damage, even after 200 cycles, demonstrating a strengthened crystal structure and improved mechanical integrity (Fig. 5h). Similarly, XRD analysis (Supplementary Fig. 36) reveals that cycled NFMMT/NaCaPO4 largely retains its initial crystal structure, with minimal changes in the lattice parameters (0.048° shift of the (003) peak). In contrast, NFMMT exhibits a notable shift in the (003) peak toward lower diffraction angles (0.113°) and the formation of a trace amount of a Na-deficient phase, indicating lattice expansion and irreversible loss of active Na after prolonged cycling. This behavior is a key indicator of structural degradation and capacity decay59.
Given the critical role of the interface structure in the cycling stability of layered cathode materials, the surface chemical properties of the electrodes after 200 cycles at a fully charged state (4.2 V) were investigated in detail to highlight the effectiveness of surface engineering (Fig. 6). The SAED pattern of the cycled NFMMT/NaCaPO4 exhibits typical O3 phase characteristics along the [100] zone axis, indicating the preservation of high crystallinity (Fig. 6f). In contrast, the blurred diffraction spots in the cycled NFMMT suggest unfavorable phase transitions from the layered to the rock-salt phase (Fig. 6b). Cross-sectional TEM imaging reveals significant damage on the surface of the fully charged NFMMT cathode after extended cycling, with cracks that are approximately 50 nm deep and 5 nm wide (Fig. 6d). In addition, parasitic reactions and electrolyte corrosion led to the formation of an insulating rock-salt layer with a thickness of approximately 16 nm on the particle surfaces, as confirmed by the corresponding fast Fourier transform (FFT) pattern11. Even within the particles, the accumulation of internal stress induced by non-uniform phase transitions and interlayer slip can result in the formation of intragranular cracks and kinking of delaminated layers (Fig. 6c)30,60. This surface degradation and mechanical damage hinder Na-ion transport, limits the utilization of active materials, and contributes to irreversible capacity loss in NFMMT. Notably, the interface and bulk structures of NFMMT/NaCaPO4 are nearly intact after cycling, indicating enhanced mechanical integrity (Fig. 6e). In addition, the reconstructed layer that integrates the coating and gradient Ca2+ doped interphase remains apparent after extended cycling, effectively preserving the integrity and order of the original O3-type structure and thus enhancing the long-term Na storage performance (Fig. 6g, h and Supplementary Fig. 37)14. In addition, the EDS line scan results indicate a gradual increase in the Na-ion concentration from the surface to the core, stabilizing at ~ 90 nm (Fig. 6i), which directly demonstrates the presence of an ion concentration gradient within the NFMMT particles at the fully charged state. In contrast, the Na-ion concentration in NFMMT/NaCaPO4 is relatively uniform, indicating that the introduced interface reconstruction layer effectively reduces the Na-ion concentration gradient (Fig. 6j)25. To further investigate the structural inhomogeneity within the particles, the average interlayer spacing from the edge to the core was quantitatively assessed using HRTEM and SAED patterns (Supplementary Fig. 38). As shown in Fig. 6k, the interlayer spacing near the edge of NFMMT is relatively small (15.77 Å), corresponding to the OP2 phase in a deep sodiated state, whereas the maximum value (16.28 Å) appears at the center, indicating the presence of two distinct structures with different lattice parameters within the particles61. This structural inhomogeneity generates uneven stress, which is released through dislocations and slips during electrochemical cycling, resulting in microcrack formation19. Interestingly, the interlayer spacing difference within the NFMMT/NaCaPO4 particles is significantly smaller (0.209 Å) than that within the NFMMT particles (0.507 Å), indicating a reduction in structural mismatch. Therefore, the interface reconstruction layer effectively enhances the mechanical stability of layered oxides by reducing the Na-ion concentration gradient and promoting uniform phase transitions.
Fig. 6. Postmortem analysis of crystal structure and surface chemical composition.
STEM and corresponding SAED pattern of (a, b) NFMMT and (e, f) NFMMT/NaCaPO4 after 200 cycles. Dark-field STEM images and related regions’ FFT of (c, d) NFMMT and (g) NFMMT/NaCaPO4. h Fine microstructure of the interphase in the region (4) of NFMMT/NaCaPO4. The STEM images (inset) and corresponding EDS line scan results for Na, Mn, and Fe elements in (i) NFMMT and (j) NFMMT/NaCaPO4. k Comparison of c-axis lattice parameters between center and surface. l 3D render and concentration distribution of species (PO2−, NaF2−, and CHO2 − ) for NFMMT (top) and NFMMT/NaCaPO4 (bottom). Normalized TOF-SIMS depth profiles of (m) NaF2− and (n) CHO2− in NFMMT and NFMMT/NaCaPO4.
The surface chemical states of the cycled electrodes were characterized using XPS. As shown in Supplementary Fig. 39a, CFx bonds related to polyvinylidene fluoride (PVDF) and NaF species formed by electrolyte decomposition were detected on the surface of the cycled electrodes29. Notably, NFMMT exhibits higher content of NaF and (CFx) byproducts, suggesting that electrolyte decomposition on this cathode results in the formation of a thicker CEI layer (Supplementary Fig. 39b). Even after 200 cycles, strong phosphate signals were detected on the surface of NFMMT/NaCaPO4, demonstrating the electrochemical stability of the reconstructed layer (Supplementary Fig. 39c). TOF-SIMS measurements provided 3D distribution and depth profile data, revealing an enriched phosphate (PO2−) coating on NFMMT/NaCaPO4 (Fig. 6l). In addition, NFMMT/NaCaPO4 contained a lower content of organic components (C2HO−) and metal fluorides (NaF2− and MnF3−) resulting from interface degradation and surface side reactions compared to NFMMT (Fig. 6m, n and Supplementary Fig. 39 d, e), demonstrating that the protective layer effectively suppresses adverse parasitic reactions between the positive electrode and electrolyte22.
To further elucidate the role of the interface reconstruction layer, core–shell-structured positive electrodes with various coating layers (Na3PO4 and NaXPO4, where X = Ba, Sr, Cu, Mg, Sb, or Al) were synthesized (Supplementary Fig. 40). As shown in Supplementary Fig. 41, the introduced polyanionic coatings effectively enhance the ion diffusion kinetics. Consequently, all the coated samples exhibit better Na storage performance than NFMMT18,38. Notably, the Na3PO4 coating significantly enhances the reaction kinetics of the layered oxide, yielding better rate performance than NFMMT/NaCaPO4 (Supplementary Fig. 42). The straightforward surface coating strategy using Na3PO4 enhances the electrochemical performance by facilitating Na-ion diffusion and improving the interfacial stability38. However, the introduction of incompatible cations with relatively large ionic radii or high valence states into the Na layer negatively affects cycling performance (Fig. 7a and Supplementary Fig. 43)30. Therefore, these surface-modified positive electrodes exhibit similar suboptimal cycling stabilities. Interestingly, the introduction of Ca2+, Cu2+, and Mg2+, which have greater compatibility with Na+, markedly improves the cycling stability of NFMMT, with the NaCaPO4-coated sample demonstrating the best cycling performance. Notably, the cycling stabilities of the samples with bulk Ca2+ doping and/or a surface Na3PO4 coating remain inferior to that of NFMMT/NaCaPO4 (Supplementary Fig. 44), suggesting that gradient Ca2+ doping plays a critical role in enhancing structural stability.
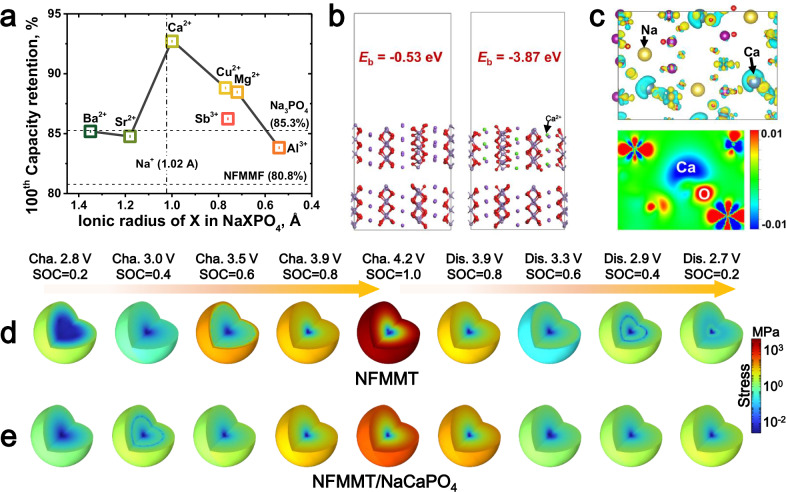
Fig. 7. Theoretical calculations and simulations of the mechanism of the interface reconstruction layer.
a Volcanic curve between dopant ionic radii and the capacity retention of corresponding cathodes. b Interfacial binding energy between F-NFMMT (bottom) and P-NFMMT (top) without (left) and with Ca doping (right). Purple, green, red, and gray represent Na, Ca, O, and TM ions, respectively. c Differential charge density and corresponding 2D projection of P-NFMMT-Ca. Blue and yellow represent the decrease and increase of electron density, respectively. COMSOL simulation of the von Mises stress distribution in (d) NFMMT and (e) NFMMT/NaCaPO4 at different states of charge. The von Mises stress magnitude is represented by the color scale in the legend.
Further insights into the role of the Ca dopant were obtained using density functional theory (DFT) calculations, which disregarded the influence of the coating layer. Figure 7b illustrates the lattice-matching relationship between fresh NFMMT (F-NFMMT) and partially desodiated NFMMT (P-NFMMT) at 25% SOC, highlighting the lattice mismatch caused by uneven Na extraction. After the introduction of Ca2+ into partially desodiated NFMMT (P-NFMMT-Ca), the binding energy between the partially charged and pristine structures decreases from −0.53 eV to − 3.87 eV. This reduction indicates that Ca2+ incorporation significantly alleviates the internal stress caused by lattice mismatch, thereby enhancing the structural stability of the layered framework during desodiation. Supplementary Fig. 45 provides additional evidence, showing that under identical strain conditions, the stress of P-NFMMT-Ca is approximately half that of P-NFMMT, further emphasizing the role of surficial Ca2+ incorporation in mitigating lattice mismatch. Differential charge density analysis revealed the localized effects of the Ca2+ dopant in P-NFMMT (Fig. 7c). In the charge density map, cyan and yellow represent areas of decreased and increased electron density, respectively. The electron density around the Ca2+ ions is significantly reduced, whereas the adjacent oxygen atoms exhibit increased electron density. Furthermore, density of state (DOS) calculations (Supplementary Fig. 46) were performed to elucidate this energy difference. Compared with F-NFMMT, P-NFMMT exhibits a shift in electron density from the valence band to the conduction band owing to the removal of Na ions. However, the introduction of Ca2+ mitigates this shift to some extent, resulting in a smaller difference in the electron distribution before and after desodiation. This optimization of the electron distribution is beneficial for reducing the local energy differences caused by uneven Na extraction. These results indicate a strong interaction between Ca and O, which stabilizes the interlayer structure of the TMO2 framework, mitigating structural changes during desodiation process. Additionally, the Ca–O interaction effectively suppresses oxygen release, thereby enhancing structural stability under high-voltage conditions.
Simulations of the Na+ distribution and von Mises stress evolution in NFMMT and NFMMT/NaCaPO4 were conducted using COMSOL. As illustrated in Supplementary Fig. 47, surface Na ions are preferentially released over internal Na ions. Nonetheless, NFMMT/NaCaPO4, with its uniform phase transition, contains a relatively uniform distribution of Na ions throughout the electrochemical process. In contrast, the uneven phase transition and kinetic differences in NFMMT lead to significant Na-ion concentration gradients, particularly pronounced in the phase transition region (charged to ~ 3.0 and 4.2 V). This concentration gradient results in the accumulation of internal stress and microcrack formation. Notably, NFMMT, characterized by a pronounced Na-ion concentration gradient, displays a marked stress distribution (especially in the fully charged state), which is a key contributor to rapid capacity decay (Fig. 7d). Conversely, minimal internal stress is generated in NFMMT/NaCaPO4 during the electrochemical process, resulting in enhanced structural integrity and extended cycle life (Fig. 7e). Therefore, the proposed interface reconstruction layer facilitates the construction of stable layered oxides by reducing the Na-ion concentration gradient, ensuring uniform phase transitions, and minimizing internal stress.
Discussion
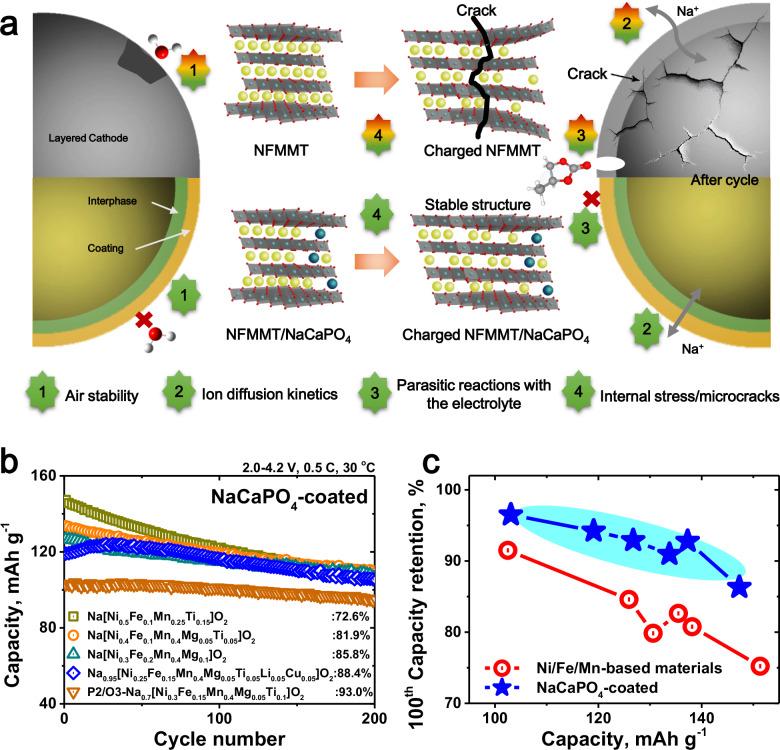
The precisely designed interface reconstruction layer has multiple functions: (1) in situ consumption of surface residual Na species to enhance resistance to humid air; (2) creation of fast ion diffusion channels to improve rate performance; (3) formation of a protective coating to mitigate parasitic reactions with the electrolyte, thereby enhancing the chemical and thermal stabilities; and (4) gradient Ca2+ doped interphase to promote a uniform phase structure and Na-ion concentration within the particles, alleviate internal stress, and minimize microcrack formation, thereby enhancing the mechanical stability (Fig. 8a). As a result, the modified positive electrode with enhanced chemical/mechanical stability demonstrates enhanced Na storage performance and practical feasibility for both Na-ion full cells and anode-free cells.
Fig. 8. Principles and scalability of the interfacial reconstruction strategy.
a Schematic diagram of an integrated strategy for enhancing structural stability through NaCaPO4 coating and interphase Ca2+ concentration gradient doping. b Cycle performance of other layered oxide materials with a surface reconstruction layer. c Effect of interface reconstruction strategy on Na storage performance of layered oxides.
Furthermore, to validate its versatility, the interface reconstruction strategy was applied to other layered oxides with different Ni contents. As shown in Supplementary Fig. 48, the specific capacity of the layered oxide is closely related to the active Ni content. However, high discharge capacities often lead to large lattice parameter changes and structural degradation, resulting in reduced cycling stability. Fortunately, the introduction of an interface reconstruction layer enhanced the chemical and mechanical stabilities of the layered oxides, significantly extending the cycle life of each coated sample (Fig. 8b). Although the negative correlation between cycling stability and achievable capacity persists, this relationship is significantly weaker in the modified samples (Fig. 8c) and can be further reduced through optimized composition and microstructure1.
In summary, this study successfully integrated a customized reconstruction layer onto the surface of O3-type cathodes using a wet chemistry method to address the challenges of interface instability and mechanical degradation. The protective coating enhanced the rate performance and air/thermal stability of the layered cathode by providing fast ion diffusion channels, consuming surface residual Na species, and suppressing parasitic side reactions. Furthermore, gradient Ca2+ doping reduced internal stress accumulation and enhanced structural integrity by promoting uniform phase transitions and minimizing Na-ion concentration gradients. Consequently, the optimized cathode achieved superior rate performance (105.3 mAh g−1 at 10 C) and long-term cycling stability (81.0% capacity retention after 300 cycles at 0.5 C), as well as robust cyclability under extreme conditions, including high voltage (4.5 V), high temperature (50 °C), and low temperature (− 10 °C). This interface reconstruction strategy, which was also successfully applied to other layered oxide materials, broadens the scope of interface optimization and provides valuable insights into the development of next-generation advanced cathodes for SIBs.
Methods
Synthesis of Na layered oxide materials
NFM, NFMMT, and NFMMT-Ca doped were synthesized by a simple solid-state method. Stoichiometric amounts of CH3COONa (3 mol.% excess, 99%, Sigma–Aldrich), Ni(CH3COO)2 (99%, Sigma–Aldrich), Fe(NO3)3·9H2O (98%, Sigma–Aldrich), Mn(CH3COO)2 (98%, Sigma–Aldrich), Mg(NO3)3 (99%, Sigma–Aldrich), TiO2 (99%, Sigma–Aldrich), Ca(NO3)2 (99%, Sigma–Aldrich), and C6H8O7 (chelating agent, 3 g, Sigma–Aldrich) were dispersed in ethanol and mixed thoroughly in a ball mill for 24 h. The mixture was dried at 80 °C, ground, and then calcined under an oxygen atmosphere (450 °C for 5 h and 900 °C for 12 h) to obtain the final product. For comparison, a series of layered oxide materials with varying compositions and contents was synthesized using a similar method.
Synthesis of coated samples
As-prepared NFMMT was mixed with the desired amount (1, 3, or 5 mol% relative to the total mass of the cathode material) of Ca(NO3)2 (Sigma–Aldrich) in ethanol (99.8%). The resulting solution was magnetically stirred for 20 min. After the dropwise addition of a phosphoric acid solution (0.05 M H3PO4 (Sigma–Aldrich) in ethanol), the mixture was stirred for 1 h, followed by evaporation at 120 °C for 10 h. The obtained powder was ground and calcined under an oxygen atmosphere at 650 °C for 5 h to produce the coated sample. For comparison, the composition of the coating layer was adjusted by varying the type of cation used in the above procedure. All synthesized layered oxides were stored in an Ar-filled glove box with H2O and O2 levels below 0.1 ppm.
Structural characterization
The compositions of the prepared samples were determined using ICP-OES (Optima 8300, PerkinElmer). The crystallographic structure was examined using XRD (Empyrean, Panalytical) with a Cu Kα radiation source. In situ XRD experiments were performed in transmission mode on pouch-type half cells cycled between 2.0 and 4.2 V at 0.05 C (7.5 mA g−1). The XRD data were processed using Rietveld refinement software (FullProf). XPS (PHI5600, Physical Electronics) was used to analyze the oxidation states of the components. The cycled electrodes were retrieved, cleaned with dimethyl carbonate, and vacuum-dried prior to analysis. To prevent exposure to moisture, the layered oxides were protected with ketone films. Microscopic analysis was performed using SEM (Nova Nano SEM 450, FEI) and Cs-corrected TEM (JEM-ARM200F, JEOL). The SEM and TEM samples were prepared by electrode polishing with a cross-sectional polisher (SM-09010, JEOL) and milling with a FIB (FIB, JEM-2100F, JEOL), respectively. The thermal stabilities of the desodiated positive electrodes in the electrolyte were evaluated using DSC (Thermo Plus, Rigaku). The spatial distribution of constituent elements (Ni, Fe, Mn, Ca, P, and F) across the positive electrode cross-sections was examined using TOF-SIMS (TofWerk). A TOF.SIMS5 mass spectrometer (IONTOF) equipped with a 30 keV Bi+ primary ion beam and a 500 eV Cs+ sputter source was used to analyze the depth profiles of the cycled electrodes, with a sputtering area of 400 × 400 µm2.
Electrochemical tests
Slurries containing 85 wt.% active material, 10 wt.% conducting agent (carbon black, Alfa Aesar), and 5 wt.% PVDF (Solef 5130, Solvay) were dispersed in N-methyl-2-pyrrolidone (ARE-310, THINKY Corp.) using a mortar and pestle. The resulting mixture was cast on Al foils and dried at 110 °C for 10 h to produce positive electrodes (working electrodes). Electrode preparation was conducted in a controlled environment (25 °C, dew point lower than − 60 °C) to prevent exposure to moisture. The electrode was rolled using a roller press at a pressure of 5 × 104 N. The working electrodes were punched into 14 mm diameter discs for 2032-type coin-cell assembly. The average active material loading on the positive electrode was 3.6 mg cm−2. Coin-type half cells were assembled in a glove box (O2/H2O content of < 0.1 ppm) using a high-purity Na sheet (99.7%, ~ 1 mm in thickness, 15 mm in diameter) as the counter electrode, 150 μL of electrolyte, a glass fiber separator (Whatman GF/D, ~ 700 μm thick, 19 mm in diameter), and the working electrode. The electrolyte consisted of 1 M NaPF6 in propylene carbonate with 2 vol.% fluoroethylene carbonate as an additive (Panax Etec). To assess the electrochemical performance of the positive electrodes, galvanostatic charge-discharge tests (TOSCAT-3100U, Toyo System) and CV (VMP3, Bio-Logic) were conducted within a voltage range of 2.0–4.2 V (vs. Na+/Na; 1 C = 150 mA g−1). Similar assemblies with commercial HC anodes and commercially available Al-C anode collectors were used for Na-ion full cells and anode-free cells, respectively. In these cells, the initial irreversible capacity loss was mitigated by pre-sodiation of the HC anode or the addition of a sacrificial agent (Na2C2O4) to the cathode. Specifically, the HC anode for the coin-type full cell was pretreated to a fully sodiated state. For the cathode in the anode-free cell, 17 wt.% Na2C2O4 was added to the slurry before casting. The anode-free cells were assembled with the Na2C2O4-containing cathode, an Al-C anode collector, and glass fiber and activated between 2.0 and 4.7 V for one cycle before regular cycling. For the pouch-type Na-ion full cells, a prepared cathode (3 × 5 cm2, mass loading: 12 mg cm−2) was paired with an HC anode, with the negative electrode capacity set to approximately 1.1 times that of the positive electrode (N/P = 1.1). The usage of electrolytes in the pouch-type cell was 1.2 mL. The operating voltage ranges were 1.9–4.19 V for the Na-ion full cells and 2.0–4.2 V for the anode-free cells. GITT and EIS (IM6, Zahner Elektrik) were performed to determine the Na-ion diffusion coefficients and evaluate the electrochemical kinetics of the prepared layered oxides. The frequency range and amplitude for the EIS test is 105–0.01 Hz and 10 mV, respectively. Before the typical cycling test, a formation process was conducted with 1 and 2 cycles at 0.1 and 0.2 C, respectively. Unless otherwise stated, all electrochemical tests were performed at 30 °C in a constant-temperature chamber. To ensure reproducibility, 2–3 cells were tested for each condition.
Modeling
DFT implemented in CP2K was used to study the impact of Ca on the system62. The Perdew–Burke–Ernzerhof (PBE) functional was used to describe the exchange-correlation effects63, and dispersion correction was applied in all calculations using the Grimme D3 method64. The ΔE values were calculated using the following equation:
| 1 |
where Etotal is the energy of the interfacial model, E1 is the energy of F-NFMMT, and E2 is the energy of P-NFMMT or P-NFMMT-Ca. The Hubbard parameter (Ueff = 3.90 eV) was added to correct for the strong correlation of the TM. Brillouin zone integration was conducted by employing Γ 1 × 1 × 1 for geometric optimization. An auxiliary plane-wave basis set with a cutoff value of 400 Ry was used to compute the electrostatic terms. In constructing the P-NFMMT model, the bottom structure represents a fully sodiated state, simulating the kinetically constrained bulk region, while the upper structure represents a 25% deintercalation state, reflecting the surface with preferential Na extraction. The structure of the P-NFMMT-Ca system is similar to that of P-NFMMT, except that 9 additional Ca atoms are introduced in the upper structure to represent the Ca-doped interface (each Ca atom replaces two Na atoms).
Finite element analysis was conducted using COMSOL Multiphysics to simulate the evolution of stress-strain and the Na-ion distribution during the cycling of NFMMT and NFMMT/NaCaPO4. The Na+ concentration within the particles was modeled using Fick’s second law: (∂cNa/∂t = − D∇2cNa), where D represents the Na+ diffusion coefficient, obtained from GITT measurements. The corresponding initial conditions and boundary conditions are expressed as follows: cNa(r, 0) = cmax (41300 mol m-3), ∇cNa (0, t) = 0. The exchange current density on the cathode surface is determined using the Butler–Volmer equation:
| 2 |
where i is the current density, i0 is the exchange current density (related to the sodium ion concentration in the cathode), αa is the anodic charge transfer coefficient, αc is the cathodic charge transfer coefficient, η is the overpotential, and F is the Faraday constant. The exchange current density was implemented into the Transport of Diluted Species module for coupling. The elastic strain in the material follows Hooke’s Law for linear elasticity: = Cstiff , where is the stress tensor, Cstiff is the stiffness tensor and is the elastic strain tensor. The relationship between volumetric strain (ϵvol) and Na+ concentration is described by the following equation:
| 3 |
where β is the coefficient of volume expansion referenced from the results of in-situ XRD, cmax is the maximum concentration of Na+. A free boundary condition is applied at the particle surface, represented by = 0. During the simulation process, changes in Young’s modulus (E) and Poisson’s ratio (ν) were ignored, with E set to 150 GPa and ν to 0.3. Other key parameters were defined as follows: thermal conductivity at 1.58 W m−1 K−1, electrical conductivity at 0.17 S m−1, density at 1.5 g cm−3, and specific heat capacity at constant pressure at 840.1 J kg−1 K−1. The particle model size closely matched the actual crystal particles observed by SEM, with an approximate size of 2 µm.
Supplementary information
Source data
Acknowledgements
This work was supported by the Human Resources Development Program (No. 20214000000320) of the Korea Institute of Energy Technology Evaluation and Planning (KETEP), funded by the Ministry of Trade, Industry, and Energy of the Korean government. This work was also supported by the Korea Institute of Energy Technology Evaluation and Planning (KETEP) grant funded by the Korea government (MOTIE) (RS-2024-00398346, ESS Big Data-Based O&M and Asset Management Technical Manpower Training).
Author contributions
X.L. and Y.-K.S. conceived the idea. X.L. conducted the experiments and drafted the original manuscript. X.S. conducted the theoretical calculations, assisted with in situ tests, and analyzed the corresponding data. H.H.S., H.K., and M.-C.K. carried out and contributed to the discussion of the results. Y.-K.S. supervised the project, revised manuscript, and provided financial support. All authors contributed to the discussion and revision of the manuscript.
Peer review
Peer review information
Nature Communications thanks Linsen Li, Yin Zhang, and the other anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Data availability
The authors declare that all relevant data supporting the findings of this work are included in the Article, with additional data provided in the Supplementary Information. The datasets generated during the current study are available from the corresponding author on request. Source data are provided in this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Xinghui Liang, Xiaosheng Song.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-025-58637-1.
References
- 1.Liang, X., Hwang, J.-Y. & Sun, Y.-K. Practical cathodes for sodium-ion batteries: Who will take the crown? Adv. Energy Mater.13, 2301975 (2023). [Google Scholar]
- 2.Hwang, J.-Y., Myung, S.-T. & Sun, Y.-K. Sodium-ion batteries: present and future. Chem. Soc. Rev.46, 3529–3614 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Hwang, J.-Y. et al. Radially aligned hierarchical columnar structure as a cathode material for high energy density sodium-ion batteries. Nat. Commun.6, 6865 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Sada, K., Kmiec, S. & Manthiram, A. Mitigating sodium ordering for enhanced solid solution behavior in layered NaNiO2 cathodes. Angew. Chem. Int. Ed.136, e202403865 (2024). [DOI] [PubMed] [Google Scholar]
- 5.Yu, T.-Y. et al. High-voltage stability of O3-type sodium layered cathode enabled by preferred occupation of Na in the OP2 phase. Energy Storage Mater.61, 102908 (2023). [Google Scholar]
- 6.Yu, T.-Y., Ryu, H.-H., Han, G. & Sun, Y.-K. Understanding the capacity fading nechanisms of O3-type Na[Ni0.5Mn0.5]O2 cathode for sodium-ion batteries. Adv. Energy Mater.10, 2001609 (2020). [Google Scholar]
- 7.Ryu, H.-H., Park, K.-J., Yoon, C. S. & Sun, Y.-K. Capacity fading of Ni-rich Li[NixCoyMn1–x–y]O2 (0.6 ≤ x ≤ 0.95) cathodes for high-energy-density lithium-ion batteries: Bulk or surface degradation? Chem. Mater.30, 1155–1163 (2018). [Google Scholar]
- 8.Yang, Y. et al. Decoupling the air sensitivity of Na-layered oxides. Science385, 744–752 (2024). [DOI] [PubMed] [Google Scholar]
- 9.Lee, S. et al. Direct observation of the in-plane crack formation of O3-Na0.8Mg0.2Fe0.4Mn0.4O2 due to oxygen gas evolution for Na-ion batteries. J. Mater. Chem. A9, 14074–14084 (2021). [Google Scholar]
- 10.Guo, Y.-J. et al. Boron-doped sodium layered oxide for reversible oxygen redox reaction in Na-ion battery cathodes. Nat. Commun.12, 5267 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng, S. et al. Surface engineering through in situ construction of CoxB-spinel dual coating layers for high-voltage stable sodium-ion batteries. Adv. Energy Mater.14, 2303773 (2024). [Google Scholar]
- 12.Wang, H. et al. Manipulating local chemistry and coherent structures for high-rate and long-life sodium-ion battery cathodes. ACS Nano18, 13150–13163 (2024). [DOI] [PubMed] [Google Scholar]
- 13.Liu, R. et al. Revealing the nature of binary-phase on structural stability of sodium layered oxide cathodes. Adv. Mater.36, 2401048 (2024). [DOI] [PubMed] [Google Scholar]
- 14.Li, X. et al. Multi-level modifications enabling chemomechanically stable Ni-rich O3-layered cathode toward wide-temperature-tolerance quasi-solid-state Na-ion batteries. Adv. Energy Mater.13, 2203701 (2023). [Google Scholar]
- 15.Hong, N. et al. An in situ dual-modification strategy for O3-NaNi1/3Fe1/3Mn1/3O2 towards high-performance sodium-ion batteries. J. Mater. Chem. A11, 18872–18880 (2023). [Google Scholar]
- 16.Xia, X. et al. Suppressing the dynamic oxygen evolution of sodium layered cathodes through synergistic surface dielectric polarization and bulk site-selective Co-doping. Adv. Mater.35, 2209556 (2022). [DOI] [PubMed] [Google Scholar]
- 17.Yu, Y. et al. Revealing the anionic redox chemistry in O3-type layered oxide cathode for sodium-ion batteries. Energy Storage Mater.38, 130–140 (2021). [Google Scholar]
- 18.Xu, W. et al. Conversion of surface residual alkali to solid electrolyte to enable Na-ion full cells with robust interfaces. Adv. Mater.35, 2301314 (2023). [DOI] [PubMed] [Google Scholar]
- 19.Chae, B.-G., Park, S. Y., Song, J. H., Lee, E. & Jeon, W. S. Evolution and expansion of Li concentration gradient during charge-discharge cycling. Nat. Commun.12, 3814 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang, J., Liang, X., Ryu, H.-H., Yoon, C. S. & Sun, Y.-K. Ni-rich layered cathodes for lithium-ion batteries: From challenges to the future. Energy Storage Mater.63, 102969 (2023). [Google Scholar]
- 21.Gao, Z. et al. Kirkendall effect-induced uniform stress distribution stabilizes nickel-rich layered oxide cathodes. Nat. Commun.15, 1503 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang, W. et al. Surface atomic rearrangement with high cation ordering for ultra-stable single-crystal Ni-rich Co-less cathode materials. Adv. Funct. Mater.34, 2409956 (2024). [Google Scholar]
- 23.Yin, X. et al. Synergetic modulation of interlayer–intralayer spacings for P2-type layered oxide cathode with superior rate performance. ACS Energy Lett.9, 3922–3930 (2024). [Google Scholar]
- 24.Li, R. et al. High-entropy and multiphase cathode materials for sodium-ion batteries. Adv. Energy Mater.14, 2400127 (2024). [Google Scholar]
- 25.Jiang, N. et al. Surface gradient desodiation chemistry in layered oxide cathode materials. Angew. Chem. Int. Ed.63, e202410080 (2024). [DOI] [PubMed] [Google Scholar]
- 26.Jo, C. H. et al. Bioinspired surface layer for the cathode material of high-energy-density sodium-ion batteries. Adv. Energy Mater.8, 1702942 (2018). [Google Scholar]
- 27.Ou, X. et al. Mn doped NaV3(PO4)3/C anode with high-rate and long cycle-life for sodium ion batteries. Energy Storage Mater.12, 153–160 (2018). [Google Scholar]
- 28.Gao, X. et al. Post-substitution modulated robust sodium layered oxides. Small Methods7, 2300635 (2023). [DOI] [PubMed] [Google Scholar]
- 29.Liang, X. & Sun, Y.-K. A novel pentanary metal oxide cathode with P2/O3 biphasic structure for high-performance sodium-ion batteries. Adv. Funct. Mater.32, 2206154 (2022). [Google Scholar]
- 30.Ding, F. et al. Tailoring planar strain for robust structural stability in high-entropy layered sodium oxide cathode materials. Nat. Energy9, 1529–1539 (2024). [Google Scholar]
- 31.Yu, T.-Y. & Sun, Y.-K. A fluorinated O3-type layered cathode for long-life sodium-ion batteries. J. Mater. Chem. A10, 23639–23648 (2022). [Google Scholar]
- 32.Hou, Y. et al. New insights into the critical role of inactive element substitution in improving the rate performance of sodium oxide cathode material. Energy Storage Mater.56, 87–95 (2023). [Google Scholar]
- 33.Liang, X., Yu, T.-Y., Ryu, H.-H. & Sun, Y.-K. Hierarchical O3/P2 heterostructured cathode materials for advanced sodium-ion batteries. Energy Storage Mater.47, 515–525 (2022). [Google Scholar]
- 34.Jin, J. et al. Unveiling the complementary manganese and oxygen redox chemistry for stabilizing the sodium‐ion storage behaviors of layered oxide cathodes. Adv. Funct. Mater.32, 2203424 (2022). [Google Scholar]
- 35.Zhuang, Z. et al. Ultrahigh-voltage LiCoO2 at 4.7 V by interface stabilization and band structure modification. Adv. Mater.35, 2212059 (2023). [DOI] [PubMed] [Google Scholar]
- 36.Yu, T.-Y. et al. High-energy O3-Na1-2xCax[Ni0.5Mn0.5]O2 cathodes for long-life sodium-ion batteries. J. Mater. Chem. A8, 13776–13786 (2020). [Google Scholar]
- 37.Liang, J. et al. A gradient oxy-thiophosphate-coated Ni-rich layered oxide cathode for stable all-solid-state Li-ion batteries. Nat. Commun.14, 146 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang, H. et al. In situ plastic-crystal-coated cathode toward high-performance Na-ion batteries. ACS Energy Lett.8, 1434–1444 (2023). [Google Scholar]
- 39.Xu, G. L. et al. Native lattice strain induced structural earthquake in sodium layered oxide cathodes. Nat. Commun.13, 436 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hong, N. et al. Regulating phase transition and restraining Fe distortion at high potential window via rare earth metal incorporation on O3‐type layered cathodes. Adv. Funct. Mater.34, 2402398 (2024). [Google Scholar]
- 41.Yu, Y. et al. Triggering reversible anion redox chemistry in O3-type cathode through tuning Na/Mn anti-site defects. Energy Environ. Sci.16, 584–597 (2023). [Google Scholar]
- 42.Joshi, A. et al. High-entropy Co-free O3-type layered oxyfluoride: A promising air-stable cathode for sodium-ion batteries. Adv. Mater.35, 2304440 (2023). [DOI] [PubMed] [Google Scholar]
- 43.Li, Y. et al. Interfacial engineering to achieve an energy density of over 200 Wh kg−1 in sodium batteries. Nat. Energy7, 511–519 (2022). [Google Scholar]
- 44.Zhang, K. et al. Regulating phase transition and oxygen redox to achieve stable high-voltage O3-type cathode materials for sodium-ion batteries. Adv. Energy Mater.13, 2302793 (2023). [Google Scholar]
- 45.Cheng, Z. et al. A rational biphasic tailoring strategy enabling high-eerformance layered cathodes for sodium-ion batteries. Angew. Chem. Int. Ed.61, 202117728 (2022). [DOI] [PubMed] [Google Scholar]
- 46.Zhang, Y. et al. Revisiting the Na2/3Ni1/3Mn2/3O2 cathode: oxygen redox chemistry and oxygen release suppression. ACS Cent. Sci.6, 232–240 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang, Y. et al. Vitalization of P2–Na2/3Ni1/3Mn2/3O2 at high-voltage cyclability via combined structural modulation for sodium-ion batteries. Energy Storage Mater.29, 182–189 (2020). [Google Scholar]
- 48.Lee, S. Y. et al. Enhanced fast-discharging performance and cyclability in oxygen-redox-based P3-type Na-layered cathode via vacancies in TM layers. Adv. Energy Mater.14, 2402412 (2024). [Google Scholar]
- 49.Tang, Y. et al. Sustainable layered cathode with suppressed phase transition for long-life sodium-ion batteries. Nat. Sustain.7, 348–359 (2024). [Google Scholar]
- 50.Ryu, H.-H., Lim, H.-W., Lee, S. G. & Sun, Y.-K. Near-surface reconstruction in Ni-rich layered cathodes for high-performance lithium-ion batteries. Nat. Energy9, 47–56 (2024). [Google Scholar]
- 51.Oh, S.-M. et al. High capacity O3-type Na[Li0. 05(Ni0. 25Fe0. 25Mn0. 5)0.95]O2 cathode for sodium ion batteries. Chem. Mater.26, 6165–6171 (2014). [Google Scholar]
- 52.Niu, Y. B. et al. High-efficiency cathode sodium compensation for sodium-ion batteries. Adv. Mater.32, 2001419 (2020). [DOI] [PubMed] [Google Scholar]
- 53.Zhang, Y. Y. et al. Refined electrolyte and interfacial chemistry toward realization of high-energy anode-free rechargeable sodium batteries. J. Am. Chem. Soc.145, 25643–25652 (2023). [DOI] [PubMed] [Google Scholar]
- 54.Ma, M., Chen, B., Tu, Y. & Pan, H. Ternary electrolyte enables high-voltage and high-temperature Na-ion batteries. ACS Energy Lett.9, 4655–4665 (2024). [Google Scholar]
- 55.Yu, T.-Y., Hwang, J.-Y., Bae, I. T., Jung, H.-G. & Sun, Y.-K. High-performance Ti-doped O3-type Na[Tix(Ni0.6Co0.2Mn0.2)1-x]O2 cathodes for practical sodium-ion batteries. J. Power Sources422, 1–8 (2019). [Google Scholar]
- 56.Liang, X., Kim, H., Jung, H. G. & Sun, Y.-K. Lithium-substituted tunnel/spinel heterostructured cathode material for high-performance sodium-ion batteries. Adv. Funct. Mater.31, 2008569 (2021). [Google Scholar]
- 57.Liang, X. et al. Surface modification of Na3V2(PO4)3 by nitrogen and sulfur dual-doped carbon layer with advanced sodium storage property. ACS Appl. Mater. Interfaces9, 13151–13162 (2017). [DOI] [PubMed] [Google Scholar]
- 58.Hwang, J.-Y. et al. Novel cathode materials for Na-ion batteries composed of spoke-like nanorods of Na[Ni0.61Co0.12Mn0.27]O2 assembled in spherical secondary particles. Adv. Funct. Mater.26, 8083–8093 (2016). [Google Scholar]
- 59.Li, M. et al. Tailoring Cu-rich surface spinel phase for high-performance O3-type layered cathode naterials for sodium-ion batteries. Nano Energy123, 109375 (2024). [Google Scholar]
- 60.Wang, K. et al. Precipitate-stabilized surface enabling high-performance Na0.67Ni0.33-xMn0.67ZnxO2 for sodium-ion battery. eScience2, 529–536 (2022). [Google Scholar]
- 61.Ryu, H.-H. et al. Capacity fading mechanisms in Ni-rich single-crystal NCM cathodes. ACS Energy Lett.6, 2726–2734 (2021). [Google Scholar]
- 62.Vandevondele, J. et al. Quickstep: Fast and accurate density functional calculations using a mixed Gaussian and plane waves approach. Comput. Phys. Commun.167, 103–128 (2005). [Google Scholar]
- 63.Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett.77, 3865 (1996). [DOI] [PubMed] [Google Scholar]
- 64.Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys.132, 154104 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all relevant data supporting the findings of this work are included in the Article, with additional data provided in the Supplementary Information. The datasets generated during the current study are available from the corresponding author on request. Source data are provided in this paper.