Abstract
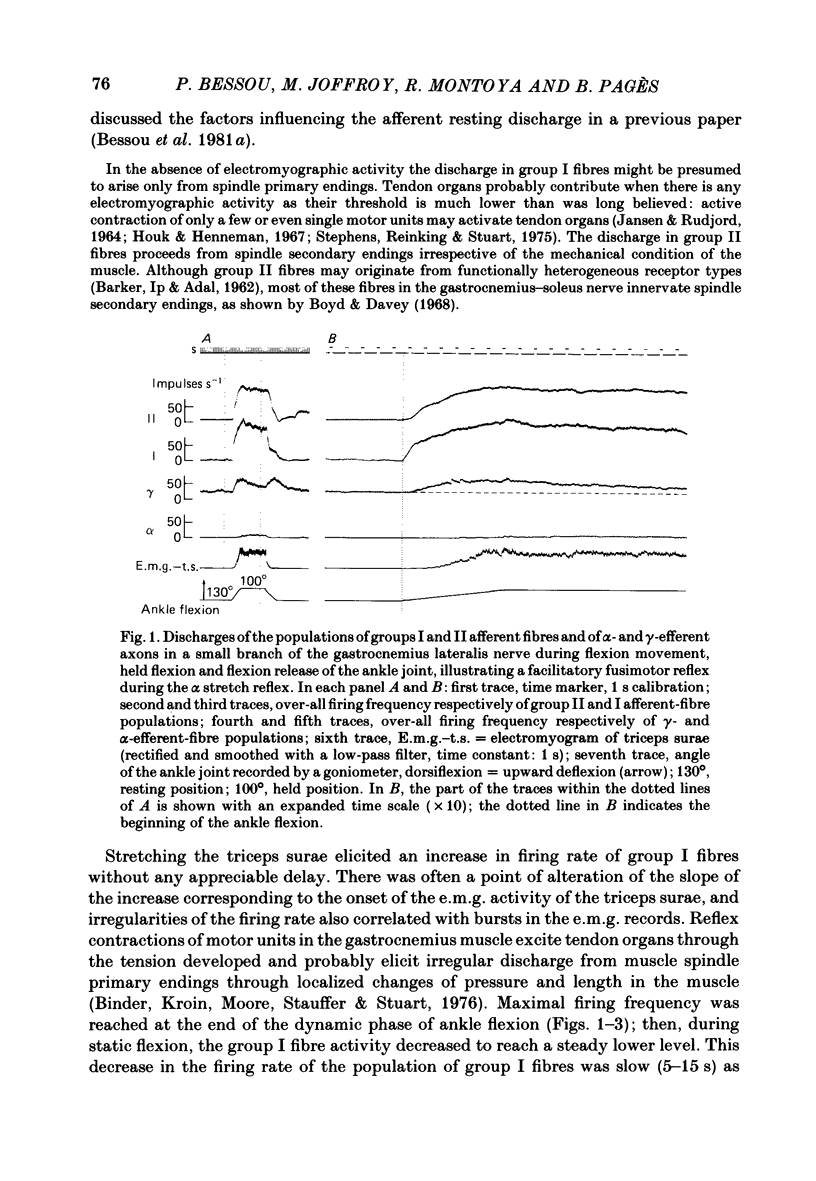
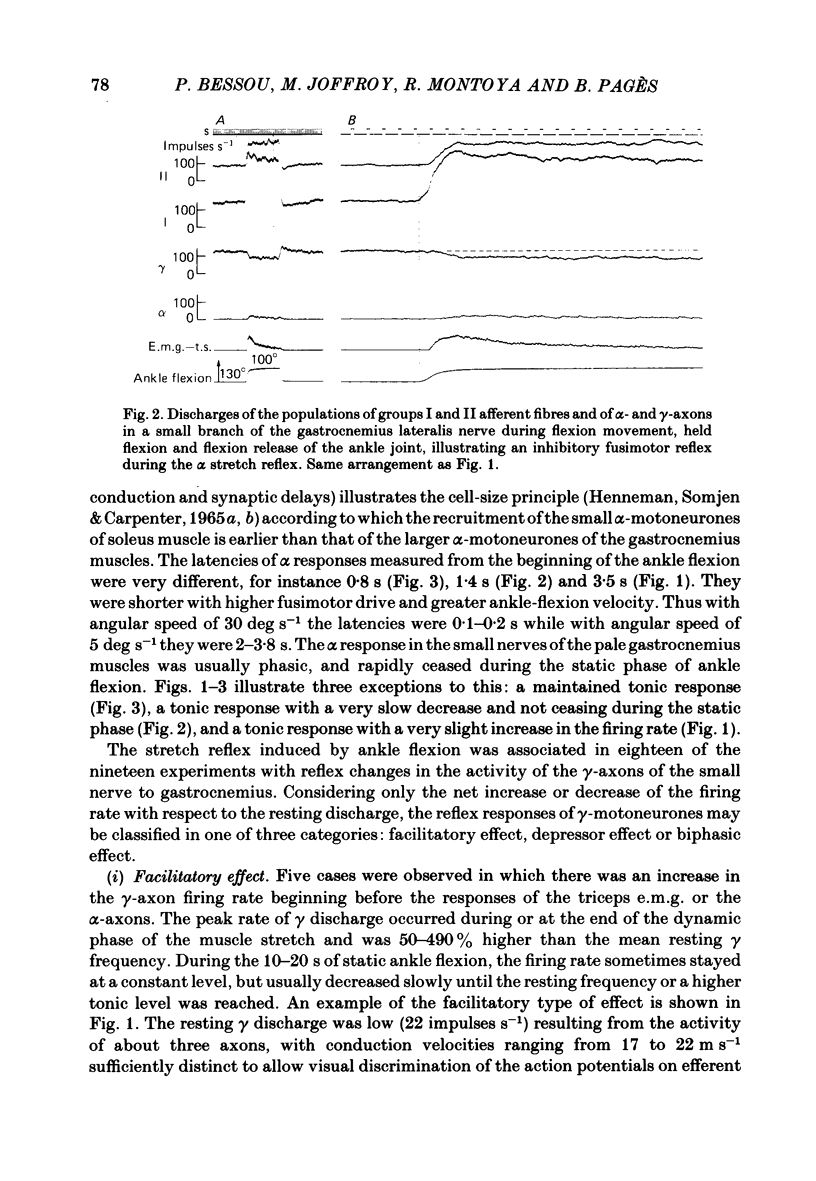
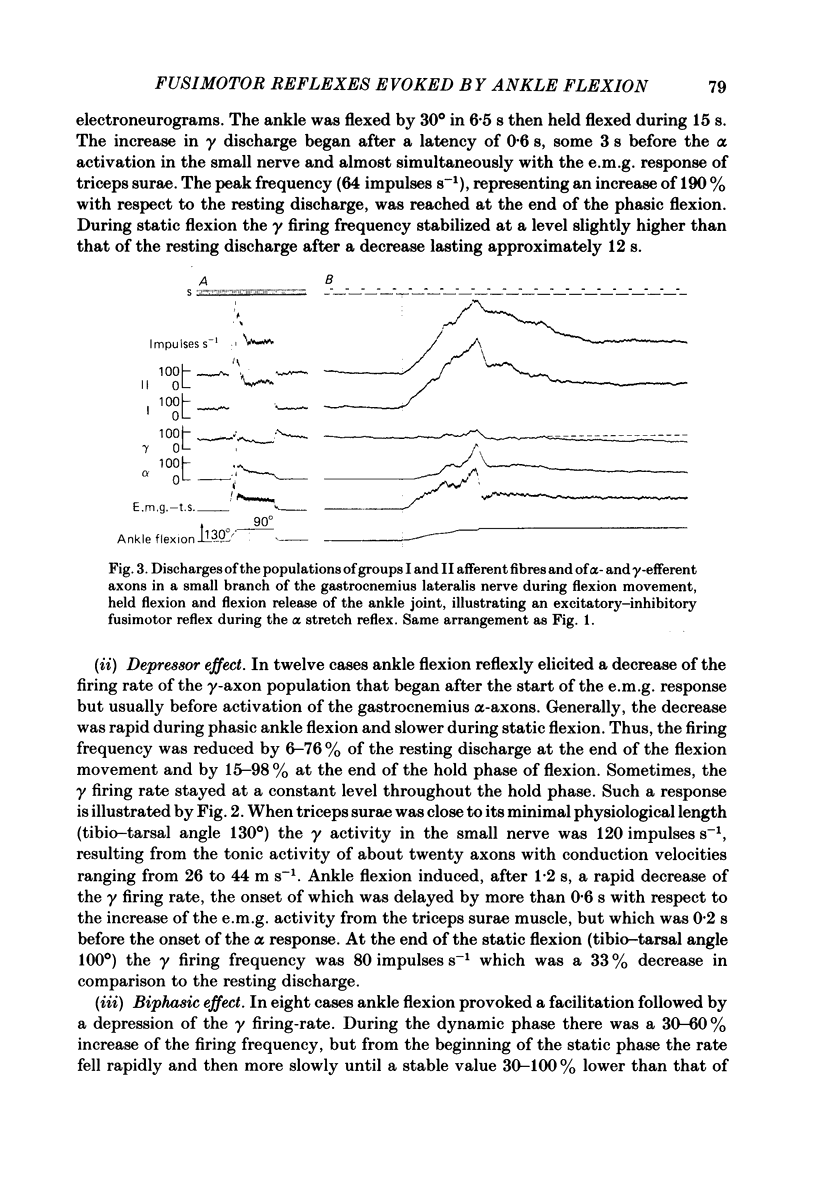
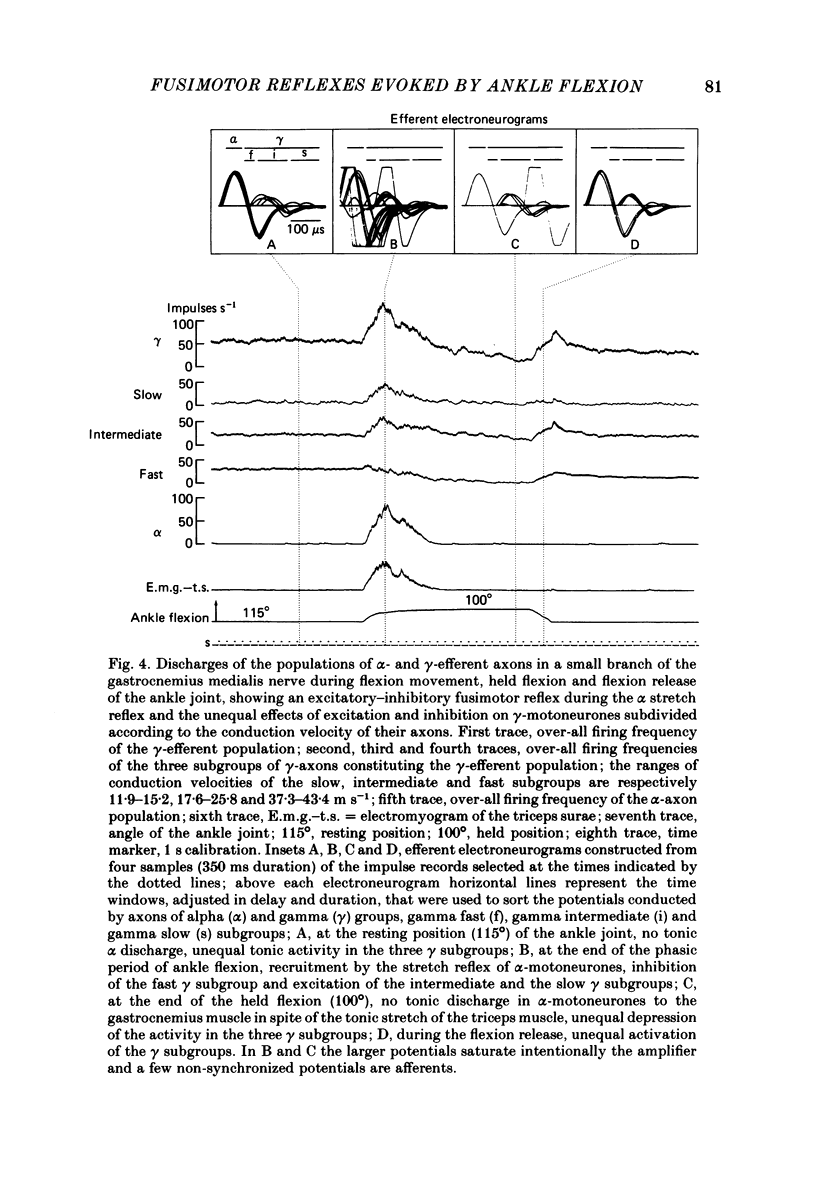
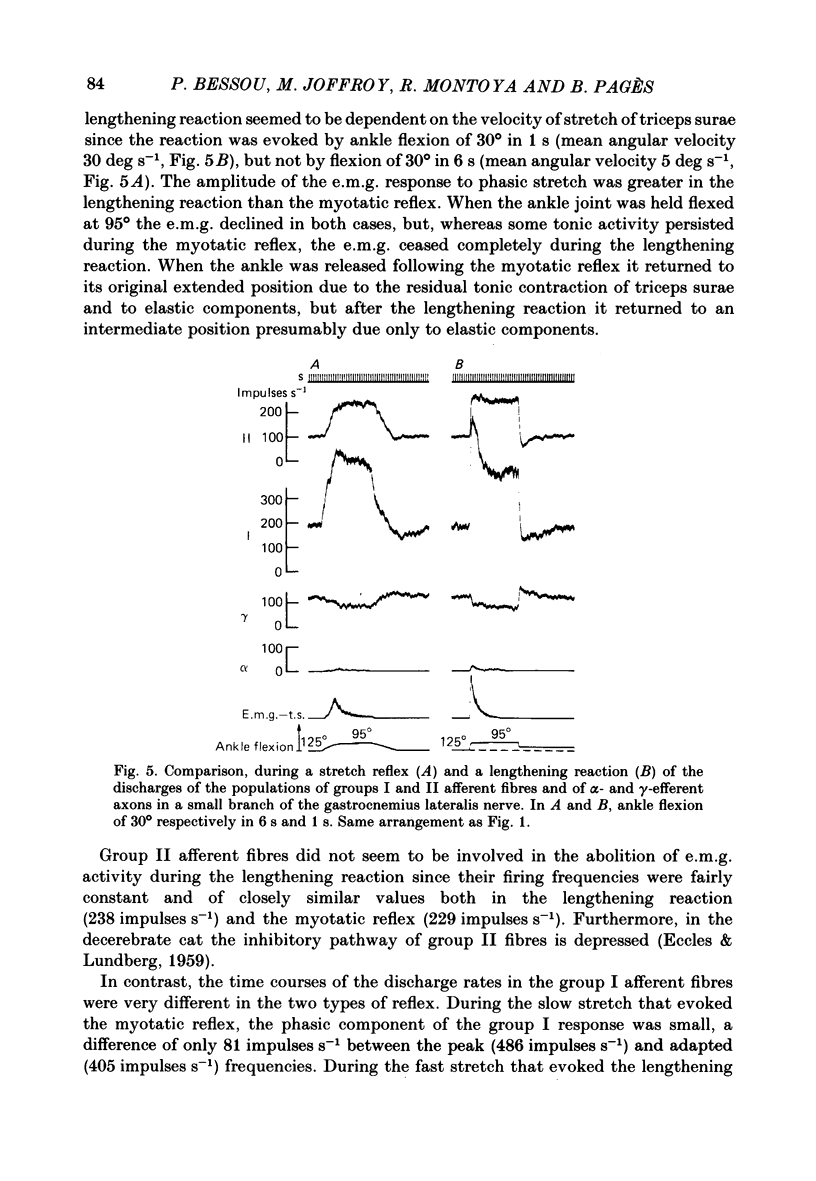
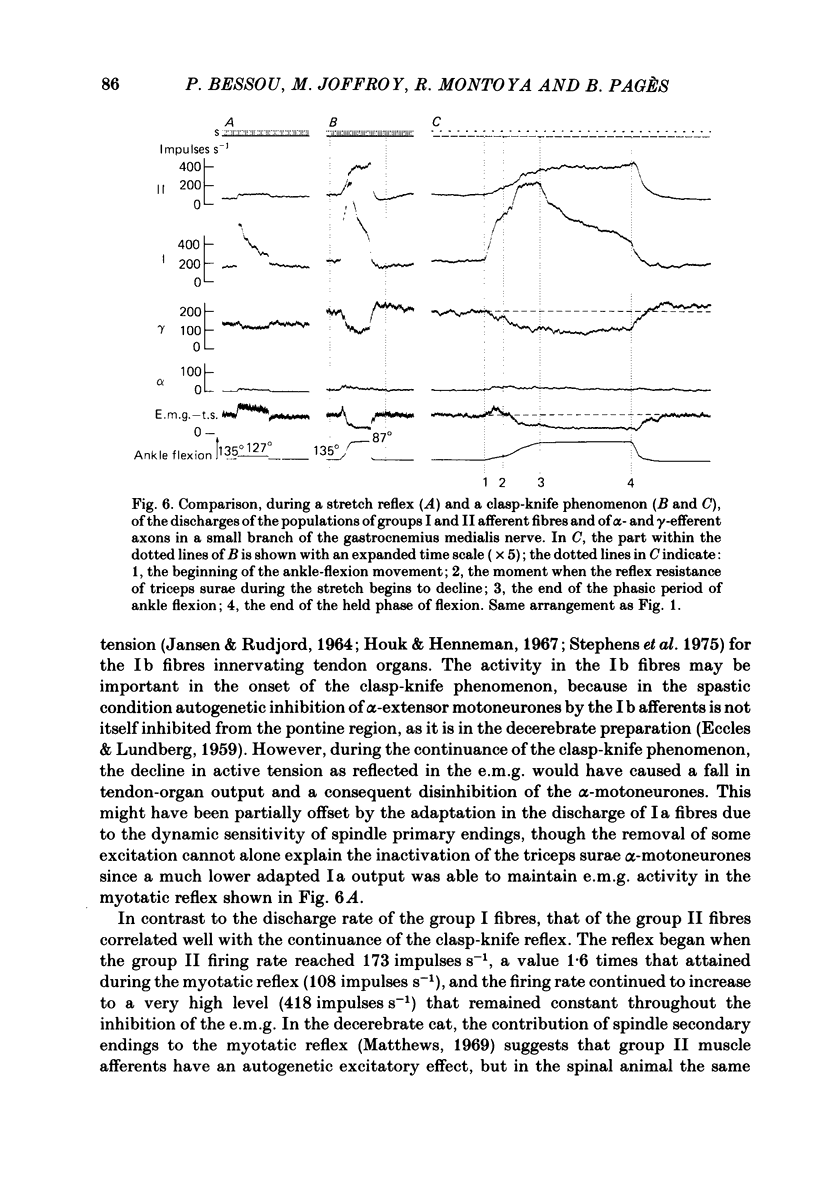
We describe the effects on group I and II afferents as well as alpha- and gamma-efferents of gastrocnemius muscles elicited by stretch and release of the triceps surae muscle performed by a dorsiflexion movement, a maintained dorsiflexion and release of the ankle joint. The experiments were made in decerebrated cats in which the neural loops between the muscles and the spinal cord were intact. Multi-unit discharges of each kind of fibre were obtained, electronically on-line, from two monopolar electrodes 4 mm apart on a small branch of the gastrocnemius lateralis or medialis nerve. Sensory and motor impulses were separated according to the opposite directions of their propagation, then both groups were further subdivided according to conduction velocity. The stretch reflex induced by ankle flexion was associated in eighteen out of nineteen experiments with reflex changes in the activity of the gamma-axon population of the gastrocnemius nerve branch. Facilitatory, depressor and biphasic (facilitatory-depressor) effects were observed, the particular type seeming to depend on the level of gamma tone and gamma-motoneurone size. Flexion release led to cessation of the alpha stretch reflex and to a rebound firing of the gamma-axon population. The lengthening reaction and the clasp-knife phenomenon occurred in certain preparations and were associated with reflex inhibition of the gamma-motoneurones. The over-all responses of group I fibres to triceps stretch showed dynamic and static components, whereas those of the group II fibres were, except in two examples, almost devoid of dynamic sensitivity. During release of stretch and as a result of the simultaneous rebound in gamma activity, the afferent discharges showed a very short pause, no pause, or even a rebound. The origins of the discharges of groups I and II muscle afferents and the factors (muscle length, active and passive muscle tension, reflex changes in fusimotor drive) influencing the activity of the receptors involved are considered as far as possible. Some aspects of the mechanisms of the fusimotor reflex are discussed.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALNAES E., JANSEN J. K., RUDJORD T. FUSIMOTOR ACTIVITY IN THE SPINAL CAT. Acta Physiol Scand. 1965 Mar;63:197–212. doi: 10.1111/j.1748-1716.1965.tb04060.x. [DOI] [PubMed] [Google Scholar]
- Appelberg B., Bessou P., Laporte Y. Action of static and dynamic fusimotor fibres on secondary endings of cat's spindles. J Physiol. 1966 Jul;185(1):160–171. doi: 10.1113/jphysiol.1966.sp007978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg B., Hulliger M., Johansson H., Sojka P. Actions on gamma-motoneurones elicited by electrical stimulation of group I muscle afferent fibres in the hind limb of the cat. J Physiol. 1983 Feb;335:237–253. doi: 10.1113/jphysiol.1983.sp014531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg B., Hulliger M., Johansson H., Sojka P. Actions on gamma-motoneurones elicited by electrical stimulation of group II muscle afferent fibres in the hind limb of the cat. J Physiol. 1983 Feb;335:255–273. doi: 10.1113/jphysiol.1983.sp014532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg B., Hulliger M., Johansson H., Sojka P. Fusimotor reflexes in triceps surae elicited by natural stimulation of muscle afferents from the cat ipsilateral hind limb. J Physiol. 1982 Aug;329:211–229. doi: 10.1113/jphysiol.1982.sp014299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg B., Johansson H., Kalistratov G. The influence of group II muscle afferents and low threshold skin afferents on dynamic fusimotor neurones to the triceps surae of the cat. Brain Res. 1977 Aug 19;132(1):153–158. doi: 10.1016/0006-8993(77)90713-2. [DOI] [PubMed] [Google Scholar]
- Bessou P., Joffroy M., Pagès B. Efferents and afferents in an intact muscle nerve: background activity and effects of sural nerve stimulation in the cat. J Physiol. 1981 Nov;320:81–102. doi: 10.1113/jphysiol.1981.sp013936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder M. D., Kroin J. S., Moore G. P., Stauffer E. K., Stuart D. G. Correlation analysis of muscle spindle responses to single motor unit contractions. J Physiol. 1976 May;257(2):325–336. doi: 10.1113/jphysiol.1976.sp011371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D., Knowles L., Andrews C., Ashby P. Spasticity, decerebrate rigidity and the clasp-knife phenomenon: an experimental study in the cat. Brain. 1972;95(1):31–48. doi: 10.1093/brain/95.1.31. [DOI] [PubMed] [Google Scholar]
- COOPER S. The responses of the primary and secondary endings of muscle spindles with intact motor innervation during applied stretch. Q J Exp Physiol Cogn Med Sci. 1961 Oct;46:389–398. doi: 10.1113/expphysiol.1961.sp001558. [DOI] [PubMed] [Google Scholar]
- ECCLES R. M., LUNDBERG A. Supraspinal control of interneurones mediating spinal reflexes. J Physiol. 1959 Oct;147:565–584. doi: 10.1113/jphysiol.1959.sp006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELDRED E., GRANIT R., MERTON P. A. Supraspinal control of the muscle spindles and its significance. J Physiol. 1953 Dec 29;122(3):498–523. doi: 10.1113/jphysiol.1953.sp005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway P. H., Murphy P. R., Tripathi A. Closely coupled excitation of gamma-motoneurones by group III Muscle afferents with low mechanical threshold in the cat. J Physiol. 1982 Oct;331:481–498. doi: 10.1113/jphysiol.1982.sp014385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway P. H., Murphy P. R., Trott J. R. Inhibition of gamma motoneurone discharge by contraction of the homonymous muscle in the decerebrated cat. J Physiol. 1979 Jun;291:425–441. doi: 10.1113/jphysiol.1979.sp012823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway P. H. Recurrent inhibition of fusimotor neurones exhibiting background discharges in the decerebrate and the spinal cat. J Physiol. 1971 Jul;216(2):419–439. doi: 10.1113/jphysiol.1971.sp009533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway P. H., Trott J. R. Autogenetic reflex action on to gamma motoneurones by stretch of triceps surae in the decerebrated cat. J Physiol. 1978 Mar;276:49–66. doi: 10.1113/jphysiol.1978.sp012219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English A. W., Letbetter W. D. Anatomy and innervation patterns of cat lateral gastrocnemius and plantaris muscles. Am J Anat. 1982 May;164(1):67–77. doi: 10.1002/aja.1001640107. [DOI] [PubMed] [Google Scholar]
- Fromm C., Haase J., Noth J. Length-dependent autogenetic inhibition of extensor gamma-motoneurones in the decerebrate cat. Pflugers Arch. 1974;346(3):251–262. doi: 10.1007/BF00595711. [DOI] [PubMed] [Google Scholar]
- Fromm C., Noth J. Reflex responses of gamma motoneurones to vibration of the muscle they innervate. J Physiol. 1976 Mar;256(1):117–136. doi: 10.1113/jphysiol.1976.sp011315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANIT R. Reflex rebound by post-tetanic potentiation; temporal summation-spasticity. J Physiol. 1956 Jan 27;131(1):32–51. doi: 10.1113/jphysiol.1956.sp005442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENNEMAN E., SOMJEN G., CARPENTER D. O. FUNCTIONAL SIGNIFICANCE OF CELL SIZE IN SPINAL MOTONEURONS. J Neurophysiol. 1965 May;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- HUNT C. C. The reflex activity of mammalian small-nerve fibres. J Physiol. 1951 Dec 28;115(4):456–469. doi: 10.1113/jphysiol.1951.sp004681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneman E., Somjen G., Carpenter D. O. Excitability and inhibitability of motoneurons of different sizes. J Neurophysiol. 1965 May;28(3):599–620. doi: 10.1152/jn.1965.28.3.599. [DOI] [PubMed] [Google Scholar]
- Houk J., Henneman E. Responses of Golgi tendon organs to active contractions of the soleus muscle of the cat. J Neurophysiol. 1967 May;30(3):466–481. doi: 10.1152/jn.1967.30.3.466. [DOI] [PubMed] [Google Scholar]
- JANSEN J. K., RUDJORD T. ON THE SILENT PERIOD AND GOLGI TENDON ORGANS OF THE SOLEUS MUSCLE OF THE CAT. Acta Physiol Scand. 1964 Dec;62:364–379. doi: 10.1111/j.1748-1716.1964.tb10435.x. [DOI] [PubMed] [Google Scholar]
- Jami L., Petit J. Dynamic and static responses of primary and secondary spindle endings of the cat peroneus tertius muscle [proceedings]. J Physiol. 1979 Nov;296:109P–109P. [PubMed] [Google Scholar]
- Joffroy M. Méthode de discrimination des potentiels unitaires constituant l'activité complexe d'un filet nerveux non sectionné. J Physiol (Paris) 1975 Jul;70(2):239–252. [PubMed] [Google Scholar]
- KUFFLER S. W., HUNT C. C. The mammalian small-nerve fibers: a system for efferent nervous regulation of muscle spindle discharge. Res Publ Assoc Res Nerv Ment Dis. 1952;30:24–47. [PubMed] [Google Scholar]
- MATTHEWS P. B. The differentiation of two types of fusimotor fibre by their effects on the dynamic response of muscle spindle primary endings. Q J Exp Physiol Cogn Med Sci. 1962 Oct;47:324–333. doi: 10.1113/expphysiol.1962.sp001616. [DOI] [PubMed] [Google Scholar]
- Matthews P. B. Evidence that the secondary as well as the primary endings of the muscle spindles may be responsible for the tonic stretch reflex of the decerebrate cat. J Physiol. 1969 Oct;204(2):365–393. doi: 10.1113/jphysiol.1969.sp008918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mense S. Nervous outflow from skeletal muscle following chemical noxious stimulation. J Physiol. 1977 May;267(1):75–88. doi: 10.1113/jphysiol.1977.sp011802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAINTAL A. S. Functional analysis of group III afferent fibres of mammalian muscles. J Physiol. 1960 Jul;152:250–270. doi: 10.1113/jphysiol.1960.sp006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rymer W. Z., Houk J. C., Crago P. E. Mechanisms of the clasp-knife reflex studied in an animal model. Exp Brain Res. 1979 Sep;37(1):93–113. doi: 10.1007/BF01474257. [DOI] [PubMed] [Google Scholar]
- Stephens J. A., Reinking R. M., Stuart D. G. Tendon organs of cat medial gastrocnemius: responses to active and passive forces as a function of muscle length. J Neurophysiol. 1975 Sep;38(5):1217–1231. doi: 10.1152/jn.1975.38.5.1217. [DOI] [PubMed] [Google Scholar]
- Trott J. R. The effect of low amplitude muscle vibration on the discharge of fusimotor neurones in the decerebrate cat. J Physiol. 1976 Mar;255(3):635–649. doi: 10.1113/jphysiol.1976.sp011300. [DOI] [PMC free article] [PubMed] [Google Scholar]


