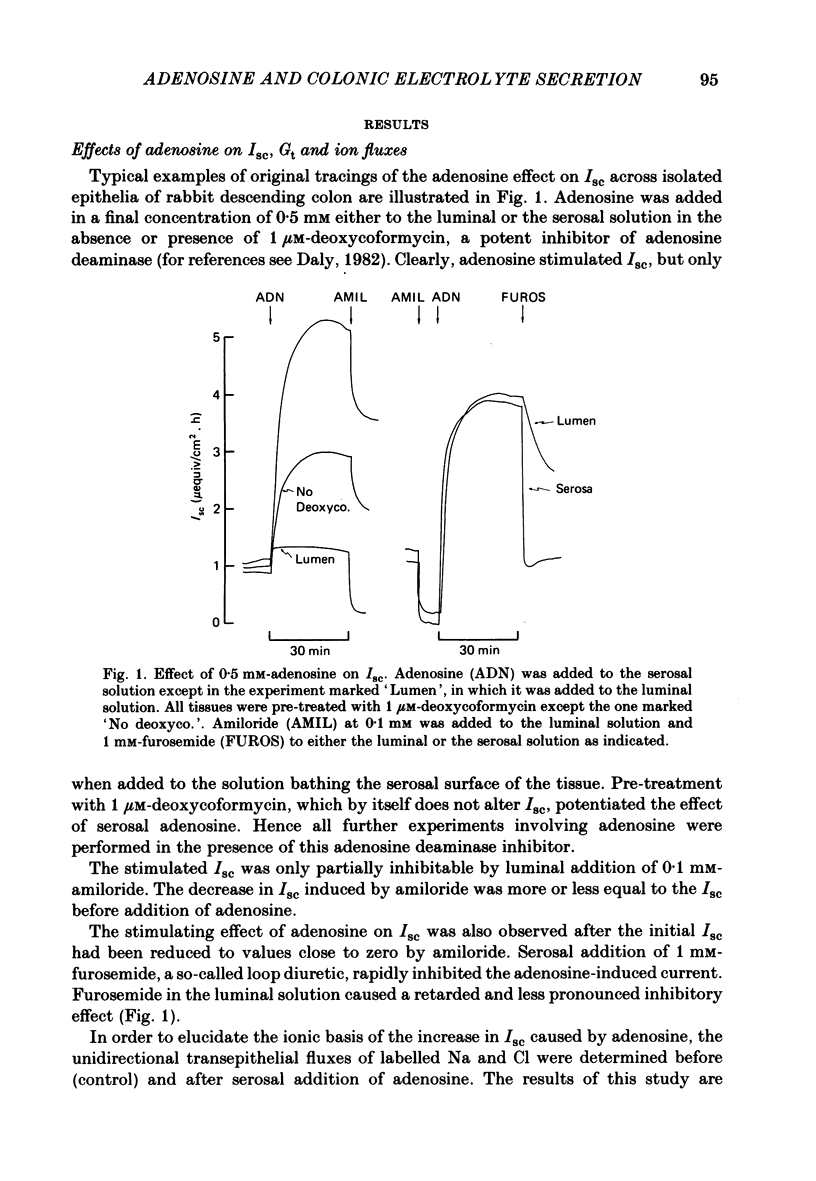
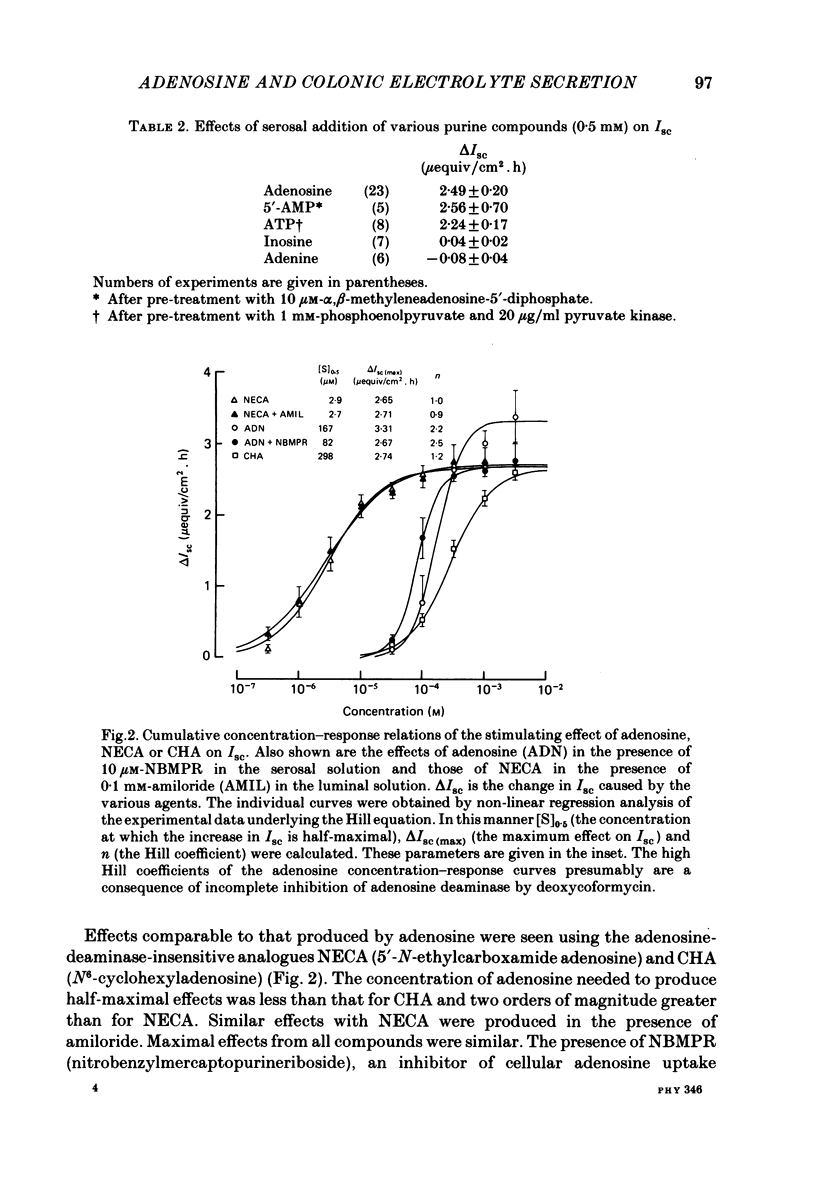
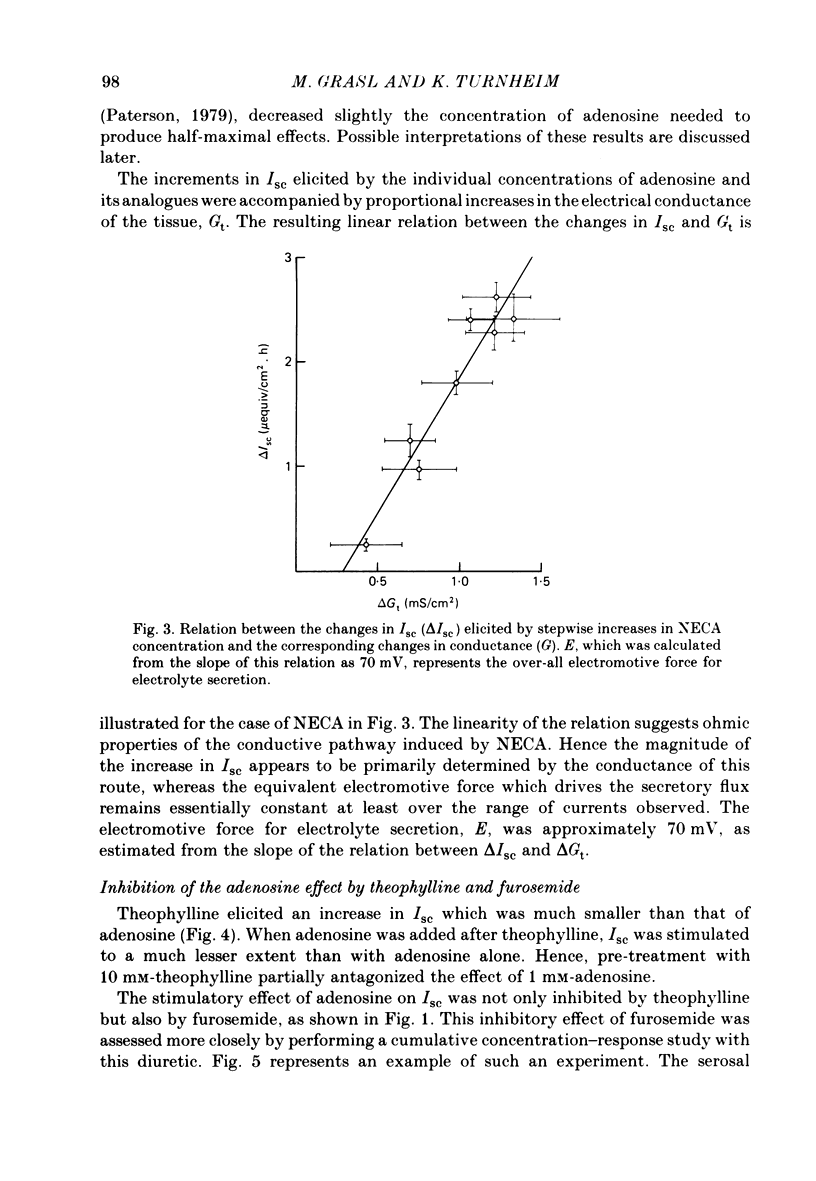
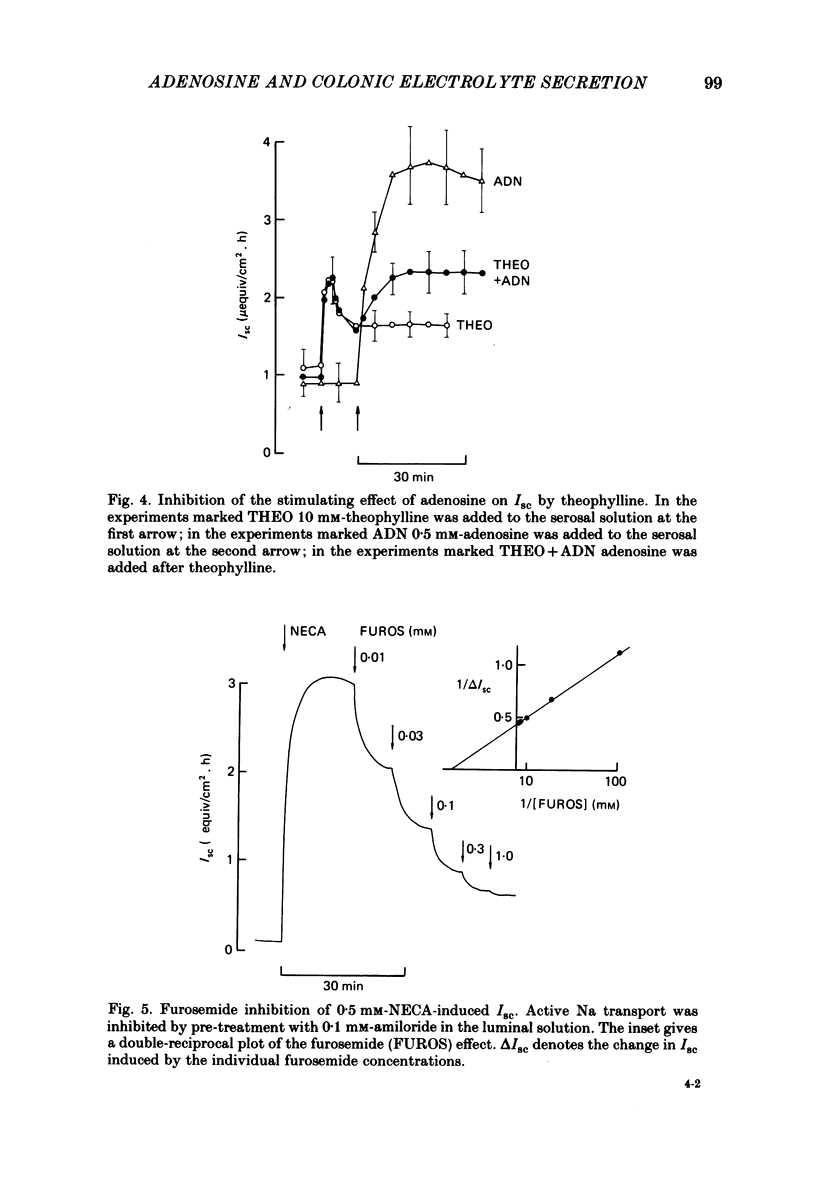
Abstract
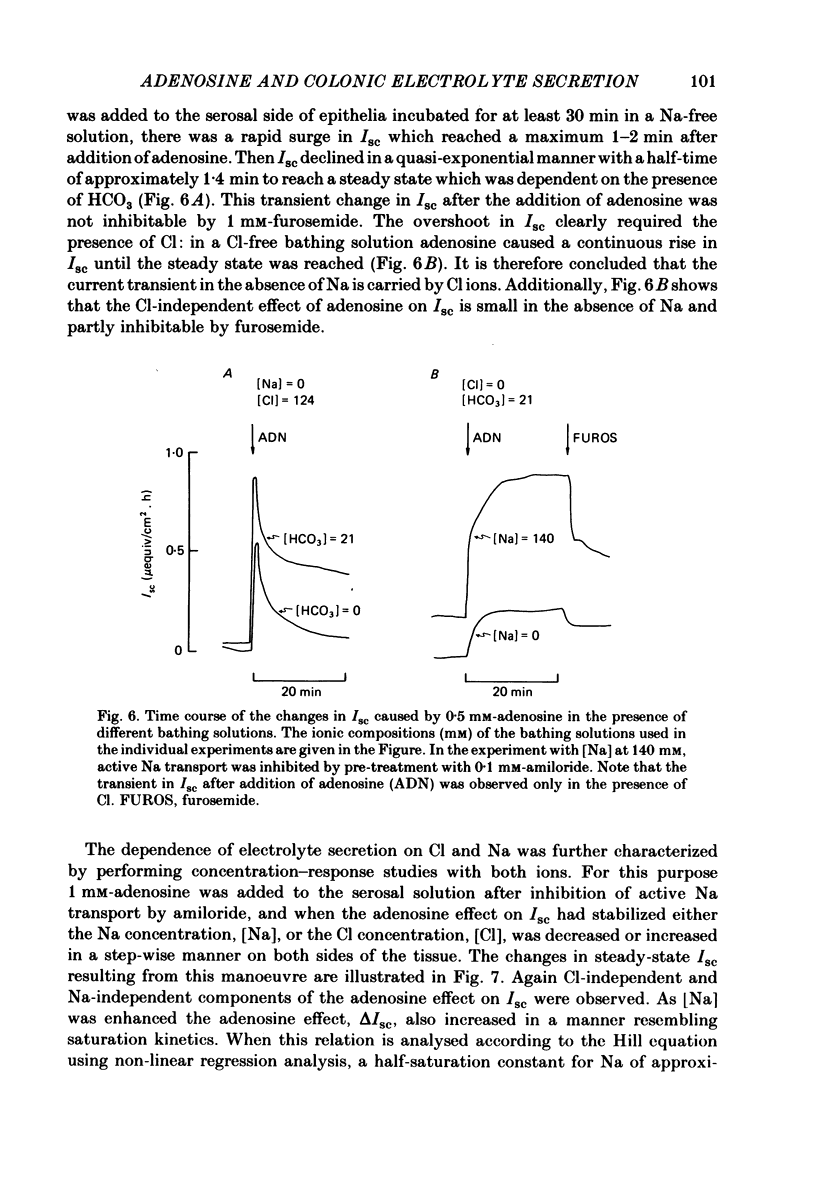
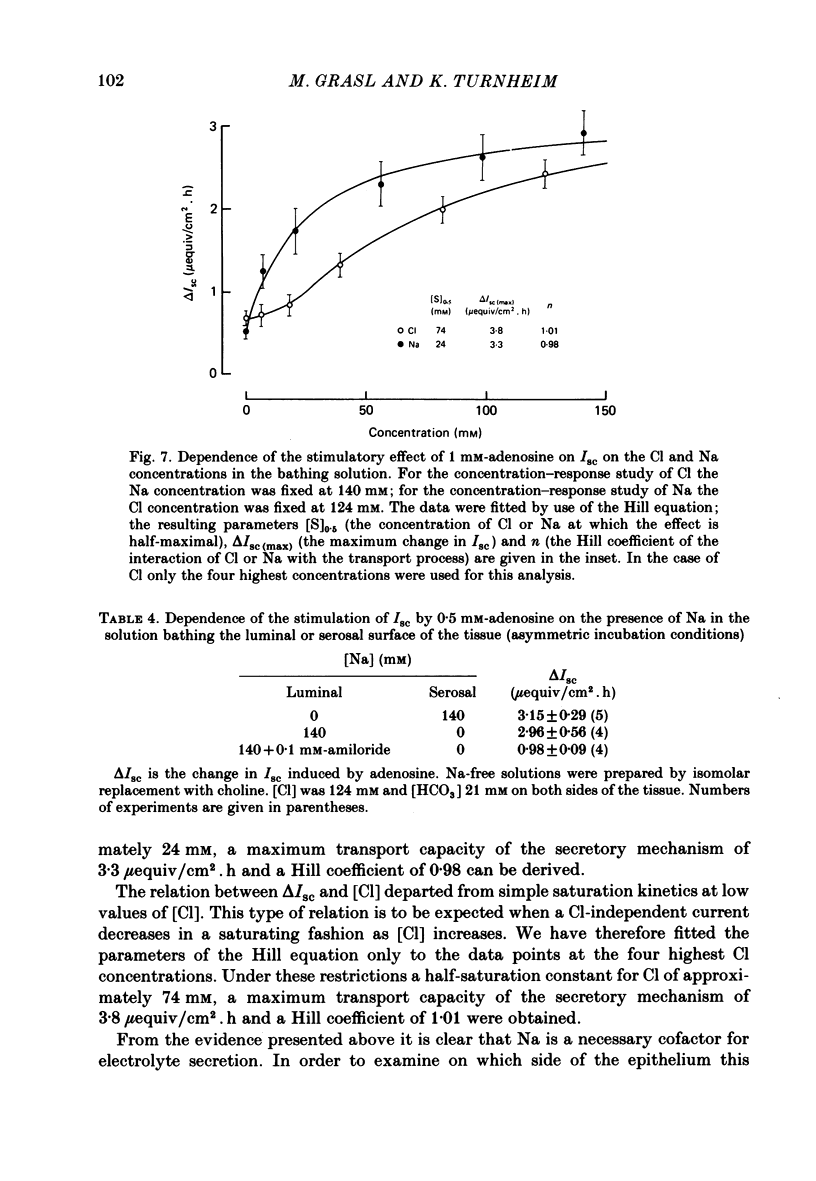
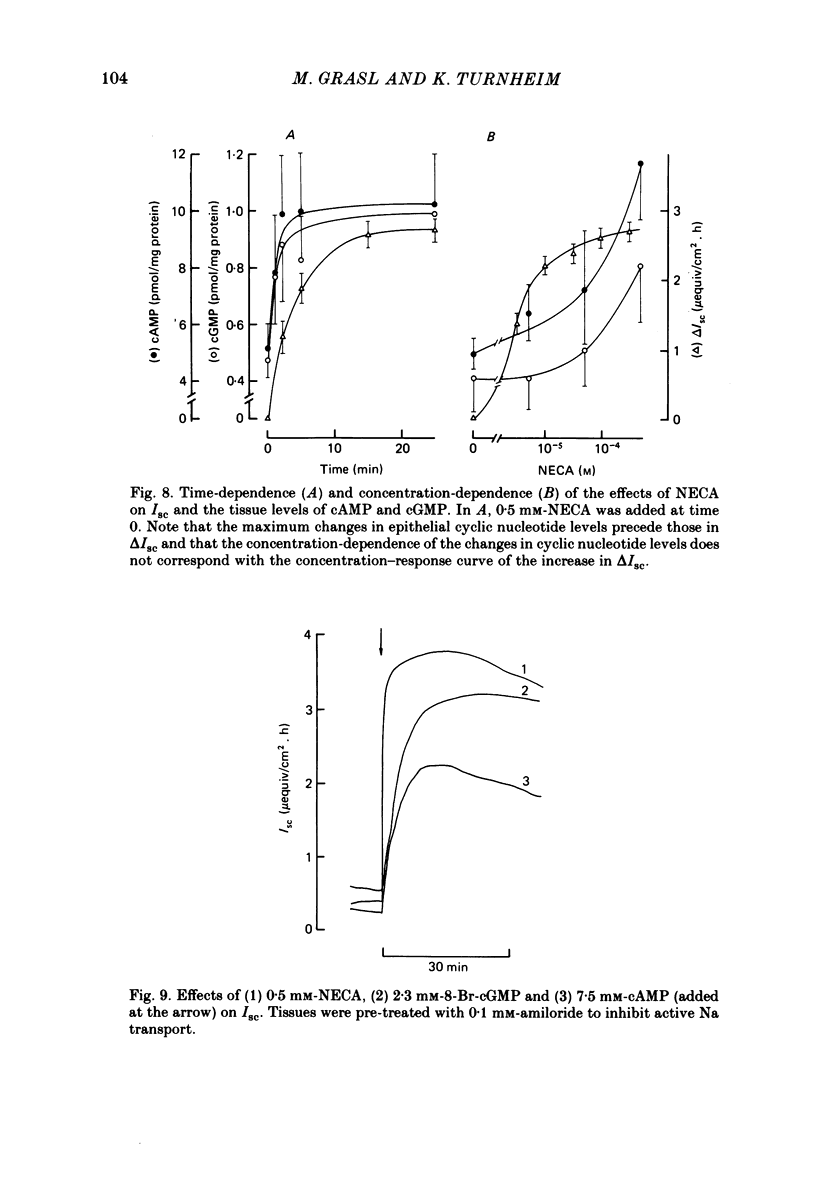
Serosal addition of adenosine after inhibition of adenosine deaminase with deoxycoformycin increases short-circuit current (Isc) and tissue conductance of isolated epithelia of rabbit descending colon. In the presence of Cl this increase in Isc results from a reversal of electrically neutral Cl absorption to rheogenic Cl secretion. When Cl is absent the stimulating effect of adenosine on Isc is reduced to one-third and appears to be brought about by HCO3 secretion. Under all conditions active Na transport remains unaltered. Adenosine-induced electrolyte secretion is markedly decreased by serosal addition of furosemide and depends on the presence of Na on the serosal side of the tissue. The stoichiometry of the interaction of Na and Cl with the basolateral Cl entry mechanism appears to be 1:1. Under Na-free conditions adenosine elicits a current transient which is carried by Cl ions and which is not inhibited by furosemide. Hence this current transient seems to be brought about by rheogenic apical Cl efflux. All these findings suggest that the conductive step in transepithelial Cl secretion resides in the apical membrane. Hyperpolarization of the Na-transporting cells by luminal addition of amiloride does not enhance electrolyte secretion. The site of action of adenosine is the extracellular surface of the basolateral membrane, because (a) luminal addition of adenosine is ineffective, (b) nitrobenzylmercaptopurineriboside, a blocker of cellular nucleoside uptake, augments the effect of serosal adenosine, and (c) the intracellular metabolites of adenosine do not mediate the effect. From the rank-order of potency of adenosine and its analogues 5'-N-ethylcarboxamide adenosine and N6-cyclohexyladenosine it is concluded that the adenosine receptors involved in electrolyte secretion are of the Ra subtype. Theophylline partially inhibits the secretory effect. The intracellular mediator of adenosine appears to be cyclic AMP and/or cyclic GMP, since the tissue levels of both compounds are rapidly elevated after addition of adenosine and both cyclic AMP and cyclic 8-bromo-GMP are able to mimic the adenosine action.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruns R. F. Adenosine receptor activation by adenine nucleotides requires conversion of the nucleotides to adenosine. Naunyn Schmiedebergs Arch Pharmacol. 1980;315(1):5–13. doi: 10.1007/BF00504224. [DOI] [PubMed] [Google Scholar]
- Daly J. W. Adenosine receptors: targets for future drugs. J Med Chem. 1982 Mar;25(3):197–207. doi: 10.1021/jm00345a001. [DOI] [PubMed] [Google Scholar]
- Ehrenspeck G. Effect of 3-isobutyl-1-methylxanthine on HCO3- transport in turtle bladder. Evidence for electrogenic HCO3- secretion. Biochim Biophys Acta. 1982 Jan 22;684(2):219–227. doi: 10.1016/0005-2736(82)90009-8. [DOI] [PubMed] [Google Scholar]
- Field M., Graf L. H., Jr, Laird W. J., Smith P. L. Heat-stable enterotoxin of Escherichia coli: in vitro effects on guanylate cyclase activity, cyclic GMP concentration, and ion transport in small intestine. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2800–2804. doi: 10.1073/pnas.75.6.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M. Intestinal secretion. Gastroenterology. 1974 May;66(5):1063–1084. [PubMed] [Google Scholar]
- Field M., Plotkin G. R., Silen W. Effects of vasopressin, theophylline and cyclic adenosine monophosphate on short-circuit current across isolated rabbit ileal mucosa. Nature. 1968 Feb 3;217(5127):469–471. doi: 10.1038/217469a0. [DOI] [PubMed] [Google Scholar]
- Flemstrom G., Garner A. Gastroduodenal HCO3(-) transport: characteristics and proposed role in acidity regulation and mucosal protection. Am J Physiol. 1982 Mar;242(3):G183–G193. doi: 10.1152/ajpgi.1982.242.3.G183. [DOI] [PubMed] [Google Scholar]
- Fox I. H., Kelley W. N. The role of adenosine and 2'-deoxyadenosine in mammalian cells. Annu Rev Biochem. 1978;47:655–686. doi: 10.1146/annurev.bi.47.070178.003255. [DOI] [PubMed] [Google Scholar]
- Frizzell R. A., Field M., Schultz S. G. Sodium-coupled chloride transport by epithelial tissues. Am J Physiol. 1979 Jan;236(1):F1–F8. doi: 10.1152/ajprenal.1979.236.1.F1. [DOI] [PubMed] [Google Scholar]
- Frizzell R. A., Koch M. J., Schultz S. G. Ion transport by rabbit colon. I. Active and passive components. J Membr Biol. 1976;27(3):297–316. doi: 10.1007/BF01869142. [DOI] [PubMed] [Google Scholar]
- Frizzell R. A., Welsh M. J., Smith P. L. Electrophysiology of chloride-secreting epithelia. Soc Gen Physiol Ser. 1981;36:137–145. [PubMed] [Google Scholar]
- Gaginella T. S., Bass P. Laxatives: an update on mechanism of action. Life Sci. 1978 Sep 11;23(10):1001–1009. doi: 10.1016/0024-3205(78)90659-8. [DOI] [PubMed] [Google Scholar]
- Granger D. N., Valleau J. D., Parker R. E., Lane R. S., Taylor A. E. Effects of adenosine on intestinal hemodynamics, oxygen delivery, and capillary fluid exchange. Am J Physiol. 1978 Dec;235(6):H707–H719. doi: 10.1152/ajpheart.1978.235.6.H707. [DOI] [PubMed] [Google Scholar]
- Grasl M., Krivanek P., Turnheim K. Does tissue ATP content limit active sodium transport across intestinal epithelia in vitro? Pflugers Arch. 1982 Nov 11;395(3):257–259. doi: 10.1007/BF00584820. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Londos C., Cooper D. M., Wolff J. Subclasses of external adenosine receptors. Proc Natl Acad Sci U S A. 1980 May;77(5):2551–2554. doi: 10.1073/pnas.77.5.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz S. G., Frizzell R. A., Nellans H. N. Active sodium transport and the electrophysiology of rabbit colon. J Membr Biol. 1977 May 12;33(3-4):351–384. doi: 10.1007/BF01869524. [DOI] [PubMed] [Google Scholar]
- Sheerin H. E., Field M. Ileal mucosal cyclic AMP and Cl secretion: serosal vs. mucosal addition of cholera toxin. Am J Physiol. 1977 Feb;232(2):E210–E215. doi: 10.1152/ajpendo.1977.232.2.E210. [DOI] [PubMed] [Google Scholar]
- Spinowitz B. S., Zadunaisky J. A. Action of adenosine on chloride active transport of isolated frog cornea. Am J Physiol. 1979 Aug;237(2):F121–F127. doi: 10.1152/ajprenal.1979.237.2.F121. [DOI] [PubMed] [Google Scholar]
- Turnheim K., Plank B., Kolassa N. Inhibition of adenosine uptake in human erythrocytes by adenosine-5'-carboxamides, xylosyladenine, dipyridamole, hexobendine, and p-nitrobenzylthioguanosine. Biochem Pharmacol. 1978;27(18):2191–2197. doi: 10.1016/0006-2952(78)90076-x. [DOI] [PubMed] [Google Scholar]
- Walling M. W., Mircheff A. K., Van Os C. H., Wright E. M. Subcellular distribution of nucleotide cyclases in rat intestinal epithelium. Am J Physiol. 1978 Nov;235(5):E539–E545. doi: 10.1152/ajpendo.1978.235.5.E539. [DOI] [PubMed] [Google Scholar]
- Walus K. M., Fondacaro J. D., Jacobson E. D. Effects of adenosine and its derivatives on the canine intestinal vasculature. Gastroenterology. 1981 Aug;81(2):327–334. [PubMed] [Google Scholar]
- de Jonge H. R. Cyclic nucleotide-dependent phosphorylation of intestinal epithelium proteins. Nature. 1976 Aug 12;262(5569):591–593. doi: 10.1038/262590a0. [DOI] [PubMed] [Google Scholar]



