Abstract
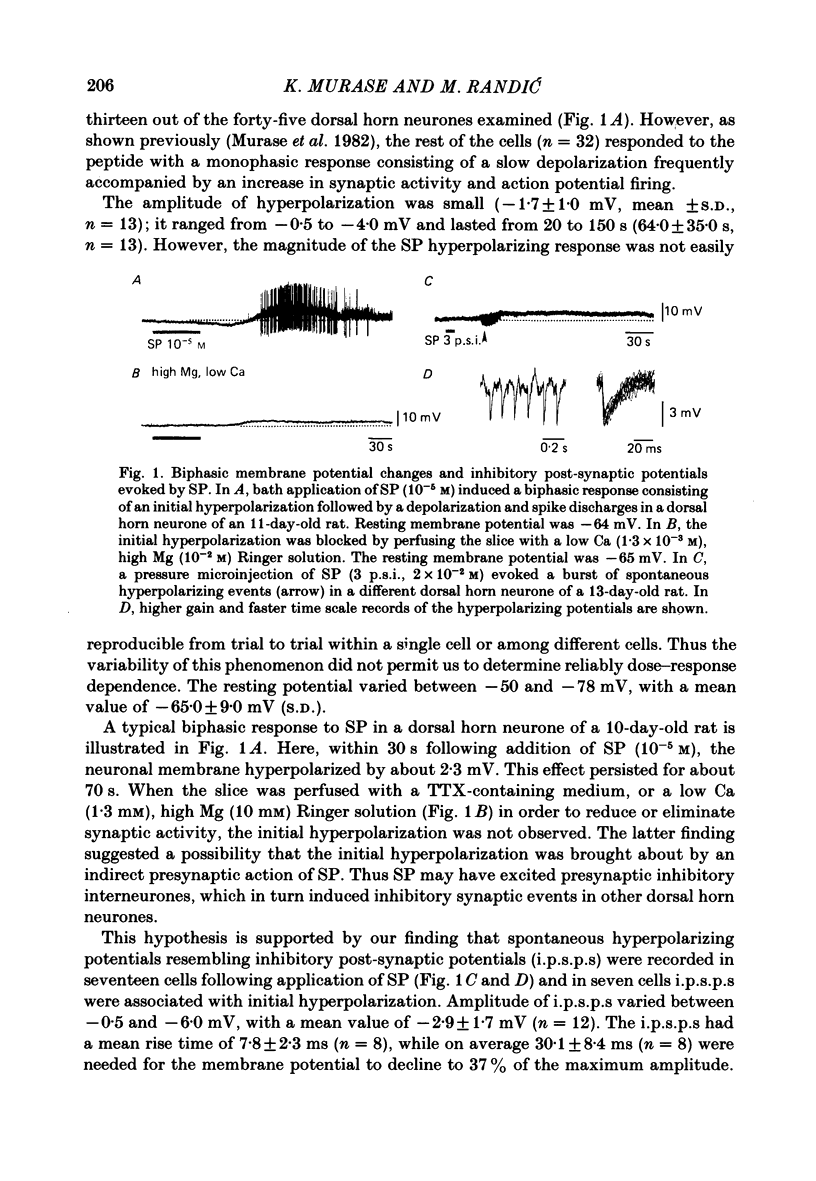
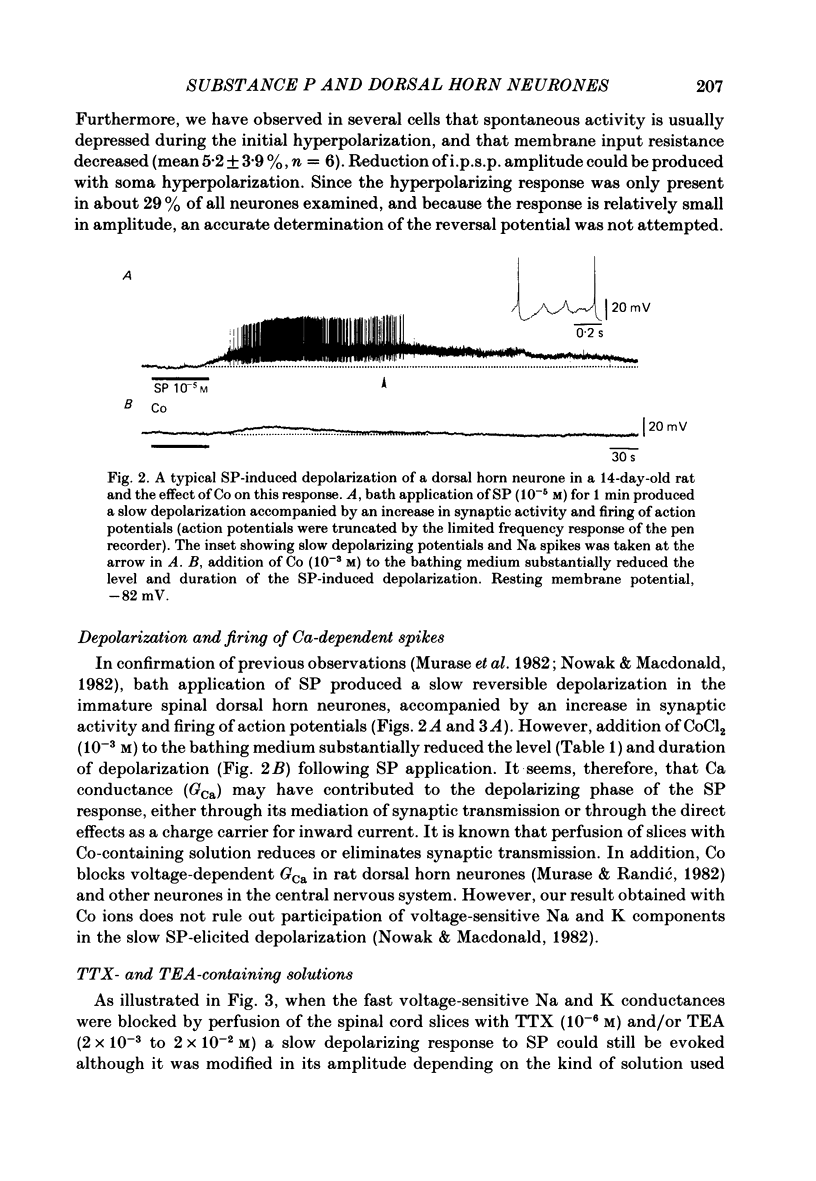
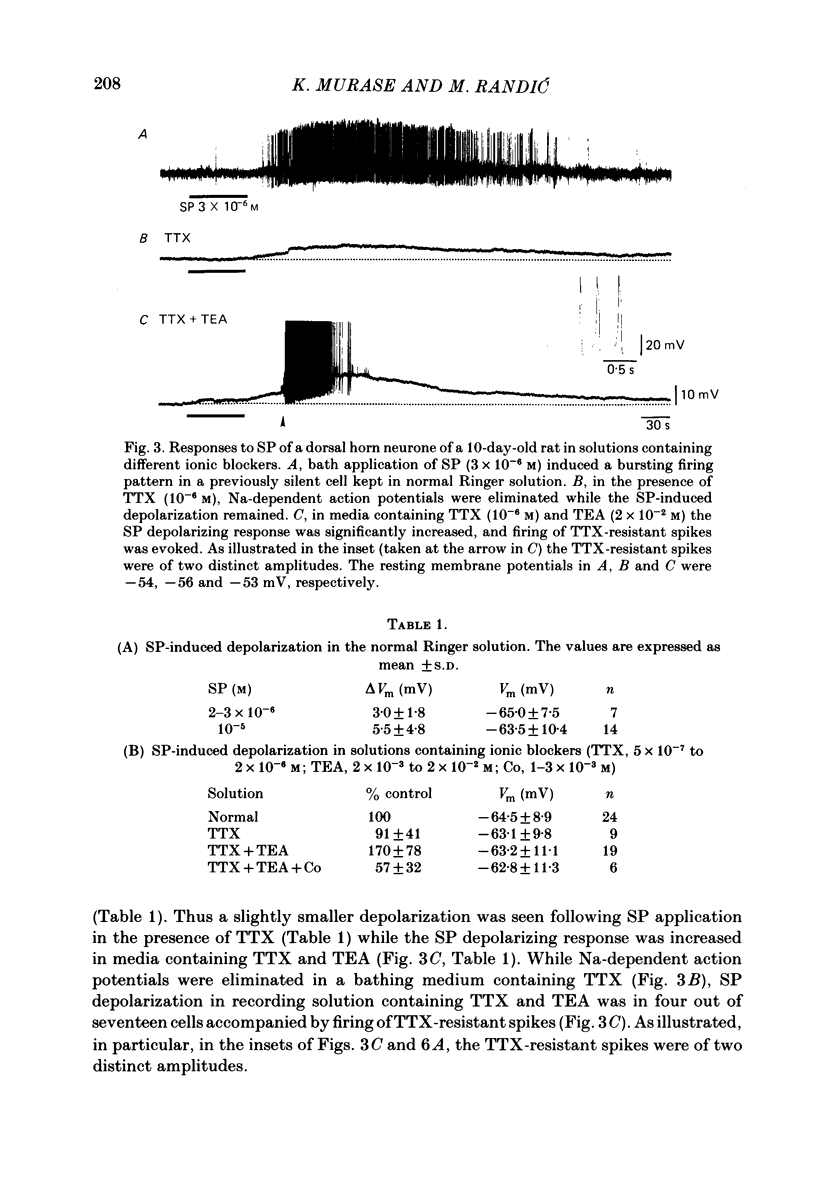
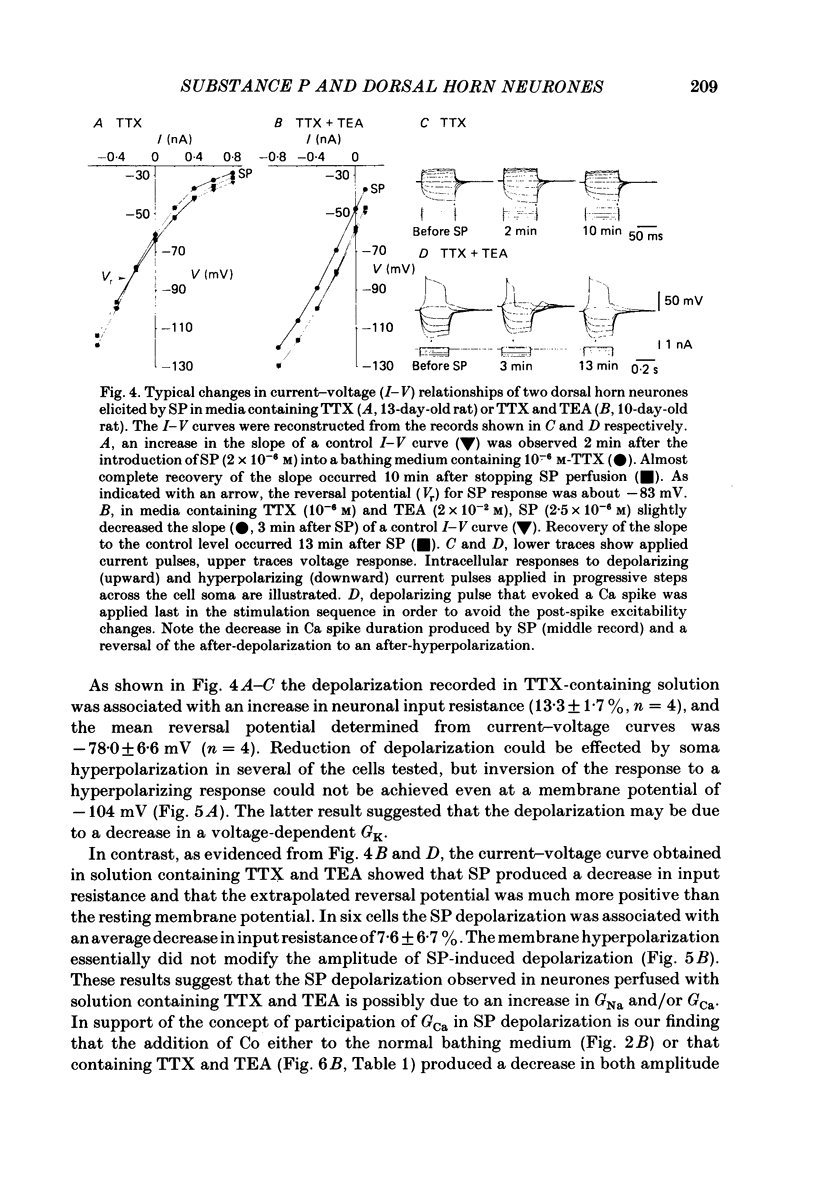
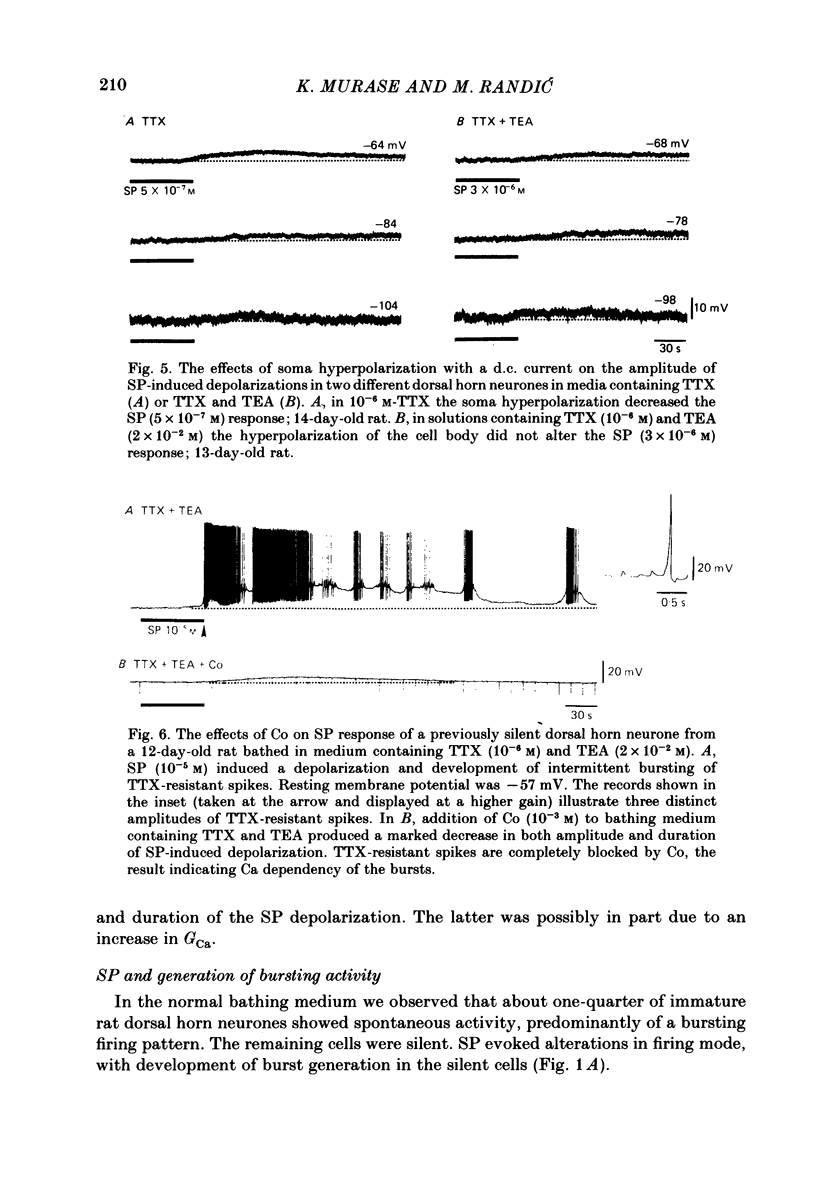
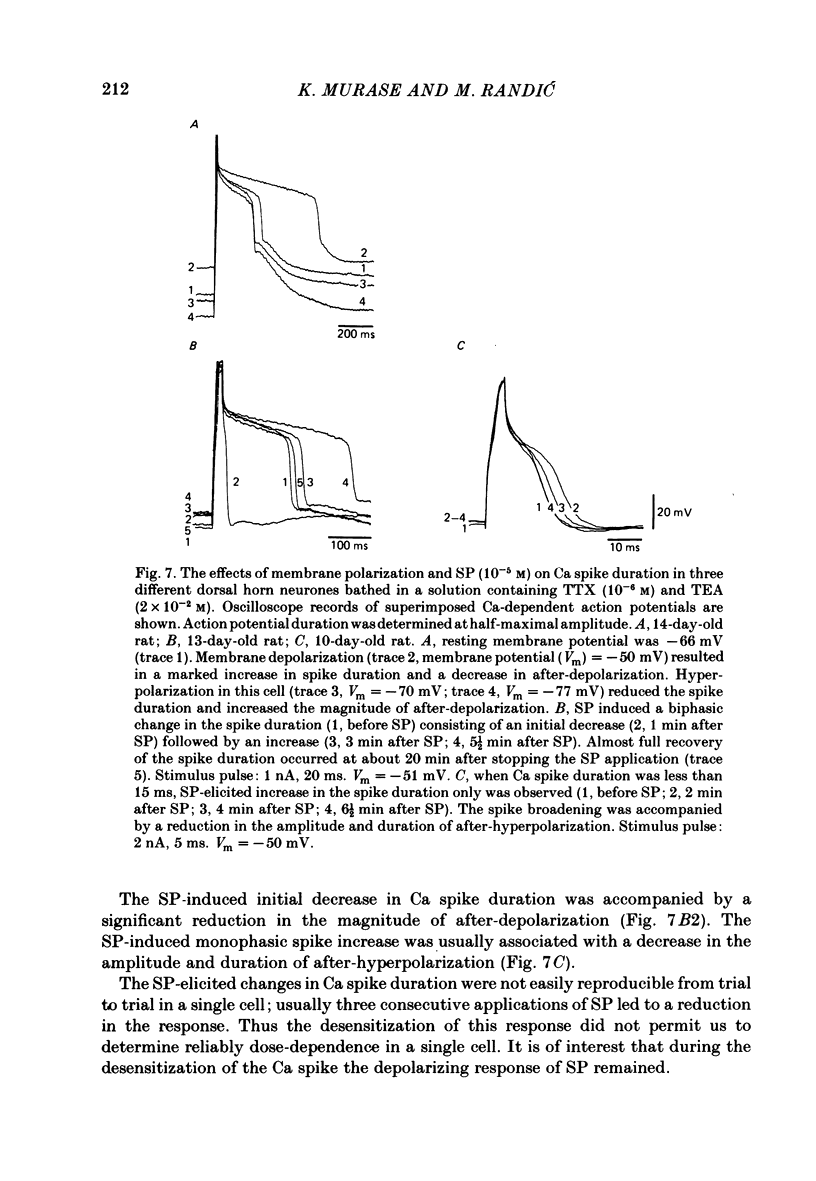
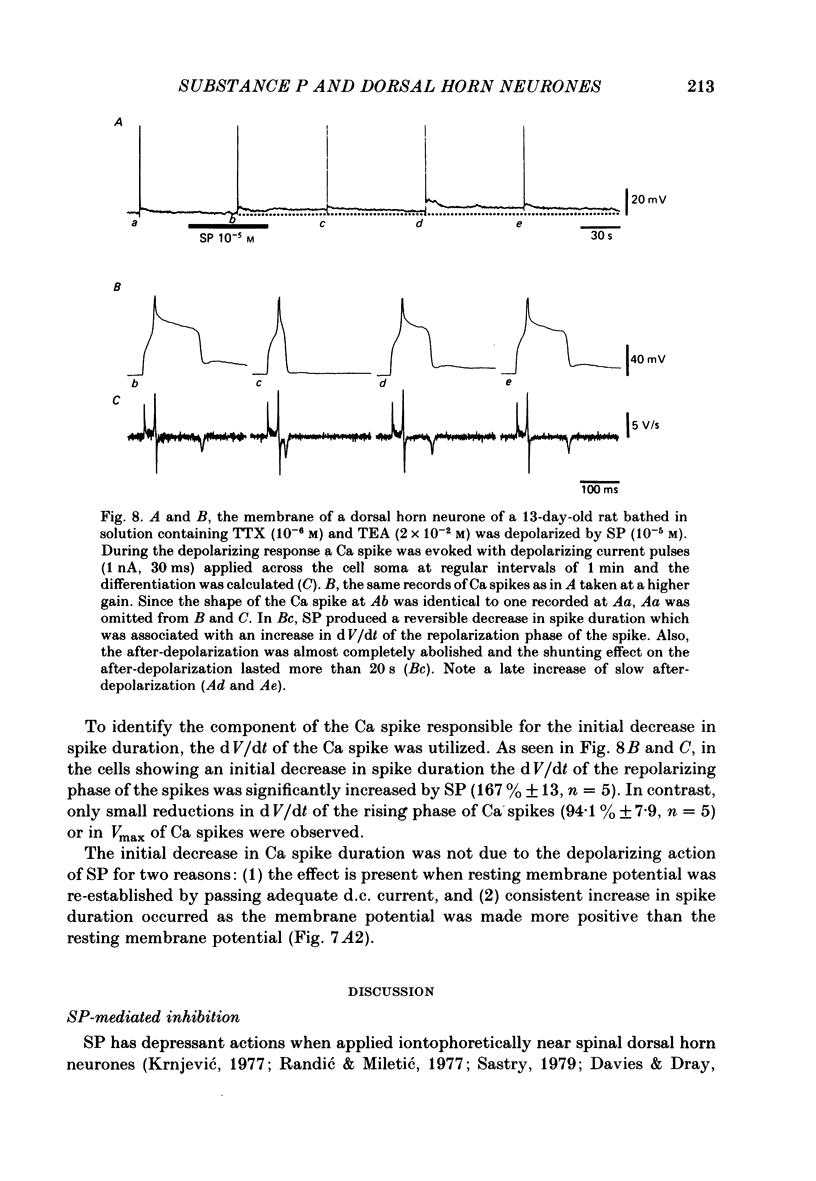
The membrane actions of substance P (SP) and the effects on the Ca-dependent action potential of dorsal horn neurones have been investigated by means of intracellular recording techniques in the immature rat in vitro spinal cord slice preparation. Bath application of SP (2 X 10(-6) to 1 X 10(-5) M) induced a biphasic membrane response consisting of an initial hyperpolarization followed by a depolarization in about one-third of the cells examined. Initial hyperpolarization was not observed when synaptic activity was blocked by perfusing the slice with a tetrodotoxin-containing or low Ca, high Mg Ringer solution. This result is consistent with a presynaptic action of SP mediated through excitation of inhibitory interneurones. This interpretation was supported by recording of repetitive spontaneous inhibitory post-synaptic potential (i.p.s.p.)-like hyperpolarizing potentials during the initial hyperpolarization. When Co ions were used to block voltage-dependent Ca conductance and possible indirect presynaptic actions, SP induced only a small depolarization of membrane potential. It seems, therefore, that Ca conductance may have contributed to the depolarizing phase of the SP response, either through its mediation of synaptic transmission or through direct effects as a charge carrier for inward current. When tetrodotoxin was used, the SP-induced increase in neuronal input resistance was not modified, although depolarization was slightly diminished. In contrast, in medium containing tetrodotoxin and tetraethylammonium, the SP-depolarizing response was enhanced and accompanied by a small decrease in input resistance and firing of Ca spikes. These results suggest that SP-induced depolarization might be a consequence of a reduction in a voltage-dependent K conductance allowing Na and/or Ca conductances to dominate. SP modified the duration of Ca-dependent action potentials of dorsal horn neurones, the most consistent change being an initial dose-dependent and reversible decrease in the spike duration. The decrease in Ca spike duration was associated with a small reduction in the rate of rise and peak amplitude, and a significant parallel increase in dV/dt of the falling phase of the Ca spike. Our data indicate that the initial decrease in Ca spike duration was not due to the depolarizing action of SP, although shunting of the membrane resistance, either through presynaptic or post-synaptic mechanisms, has not been ruled out. Alternatively, these data are consistent with the possibility that SP shortens the duration of the Ca spike by decreasing a voltage-sensitive inward Ca current and/or augmenting an outward K current.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A., Constanti A. M-currents and other potassium currents in bullfrog sympathetic neurones. J Physiol. 1982 Sep;330:537–572. doi: 10.1113/jphysiol.1982.sp014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M. Interaction of tetraethylammonium ion derivatives with the potassium channels of giant axons. J Gen Physiol. 1971 Oct;58(4):413–437. doi: 10.1085/jgp.58.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber R. P., Vaughn J. E., Slemmon J. R., Salvaterra P. M., Roberts E., Leeman S. E. The origin, distribution and synaptic relationships of substance P axons in rat spinal cord. J Comp Neurol. 1979 Mar 15;184(2):331–351. doi: 10.1002/cne.901840208. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Adams P. R. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980 Feb 14;283(5748):673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- Davies J., Dray A. Depression and facilitation of synaptic responses in cat dorsal horn by substance P administered into substantia gelatinosa. Life Sci. 1980 Dec 1;27(22):2037–2042. doi: 10.1016/0024-3205(80)90481-6. [DOI] [PubMed] [Google Scholar]
- Dunlap K., Fischbach G. D. Neurotransmitters decrease the calcium conductance activated by depolarization of embryonic chick sensory neurones. J Physiol. 1981 Aug;317:519–535. doi: 10.1113/jphysiol.1981.sp013841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K., Fischbach G. D. Neurotransmitters decrease the calcium ocmponent of sensory neurone action potentials. Nature. 1978 Dec 21;276(5690):837–839. doi: 10.1038/276837a0. [DOI] [PubMed] [Google Scholar]
- Henry J. L. Effects of substance P on functionally identified units in cat spinal cord. Brain Res. 1976 Sep 24;114(3):439–451. doi: 10.1016/0006-8993(76)90965-3. [DOI] [PubMed] [Google Scholar]
- Henry J. L., Krnjevíc K., Morris M. E. Substance P and spinal neurones. Can J Physiol Pharmacol. 1975 Jun;53(3):423–432. doi: 10.1139/y75-061. [DOI] [PubMed] [Google Scholar]
- Heyer E. J., MacDonald R. L., Bergey G. K., Nelson P. G. Calcium-dependent action potentials in mouse spinal cord neurons in cell culture. Brain Res. 1981 Sep 14;220(2):408–415. doi: 10.1016/0006-8993(81)91234-8. [DOI] [PubMed] [Google Scholar]
- Hösli L., Hösli E., Zehntner C., Landolt H. Effects of substance P on neurones and glial cells in cultured rat spinal cord. Neurosci Lett. 1981 Jul 2;24(2):165–168. doi: 10.1016/0304-3940(81)90242-1. [DOI] [PubMed] [Google Scholar]
- Klein M., Kandel E. R. Presynaptic modulation of voltage-dependent Ca2+ current: mechanism for behavioral sensitization in Aplysia californica. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3512–3516. doi: 10.1073/pnas.75.7.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald R. L., Nowak L. M. Substance P and somatostatin actions on spinal cord neurons in primary dissociated cell culture. Adv Biochem Psychopharmacol. 1981;28:159–173. [PubMed] [Google Scholar]
- Mudge A. W., Leeman S. E., Fischbach G. D. Enkephalin inhibits release of substance P from sensory neurons in culture and decreases action potential duration. Proc Natl Acad Sci U S A. 1979 Jan;76(1):526–530. doi: 10.1073/pnas.76.1.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murase K., Nedeljkov V., Randić M. The actions of neuropeptides on dorsal horn neurons in the rat spinal cord slice preparation: an intracellular study. Brain Res. 1982 Feb 18;234(1):170–176. doi: 10.1016/0006-8993(82)90483-8. [DOI] [PubMed] [Google Scholar]
- Murase K., Randić M. Electrophysiological properties of rat spinal dorsal horn neurones in vitro: calcium-dependent action potentials. J Physiol. 1983 Jan;334:141–153. doi: 10.1113/jphysiol.1983.sp014485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NARAHASHI T., MOORE J. W., SCOTT W. R. TETRODOTOXIN BLOCKAGE OF SODIUM CONDUCTANCE INCREASE IN LOBSTER GIANT AXONS. J Gen Physiol. 1964 May;47:965–974. doi: 10.1085/jgp.47.5.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll R. A., Schenker C., Leeman S. E. Substance P as a transmitter candidate. Annu Rev Neurosci. 1980;3:227–268. doi: 10.1146/annurev.ne.03.030180.001303. [DOI] [PubMed] [Google Scholar]
- Nowak L. M., Macdonald R. L. Substance P: ionic basis for depolarizing responses of mouse spinal cord neurons in cell culture. J Neurosci. 1982 Aug;2(8):1119–1128. doi: 10.1523/JNEUROSCI.02-08-01119.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillis J. W. The role of cyclic nucleotides in the CNS. Can J Neurol Sci. 1977 Aug;4(3):151–195. doi: 10.1017/s031716710002521x. [DOI] [PubMed] [Google Scholar]
- Priestley J. V., Somogyi P., Cuello A. C. Immunocytochemical localization of substance P in the spinal trigeminal nucleus of the rat: a light and electron microscopic study. J Comp Neurol. 1982 Oct 10;211(1):31–49. doi: 10.1002/cne.902110105. [DOI] [PubMed] [Google Scholar]
- Randić M., Carstens E., Zimmermann M., Klumpp D. Dual effects of substance P on the excitability of single cutaneous primary afferent C- and A-fibers in the cat spinal cord. Brain Res. 1982 Feb 11;233(2):389–393. doi: 10.1016/0006-8993(82)91211-2. [DOI] [PubMed] [Google Scholar]
- Sastry B. R. Substance P effects on spinal nociceptive neurones. Life Sci. 1979 Jun 4;24(23):2169–2177. doi: 10.1016/0024-3205(79)90115-2. [DOI] [PubMed] [Google Scholar]
- Werz M. A., Macdonald R. L. Opioid peptides decrease calcium-dependent action potential duration of mouse dorsal root ganglion neurons in cell culture. Brain Res. 1982 May 6;239(1):315–321. doi: 10.1016/0006-8993(82)90859-9. [DOI] [PubMed] [Google Scholar]
- Yeh J. Z., Oxford G. S., Wu C. H., Narahashi T. Interactions of aminopyridines with potassium channels of squid axon membranes. Biophys J. 1976 Jan;16(1):77–81. doi: 10.1016/S0006-3495(76)85663-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieglgänsberger W., Tulloch I. F. Effects of substance P on neurones in the dorsal horn of the spinal cord of the cat. Brain Res. 1979 Apr 27;166(2):273–282. doi: 10.1016/0006-8993(79)90213-0. [DOI] [PubMed] [Google Scholar]


