Abstract
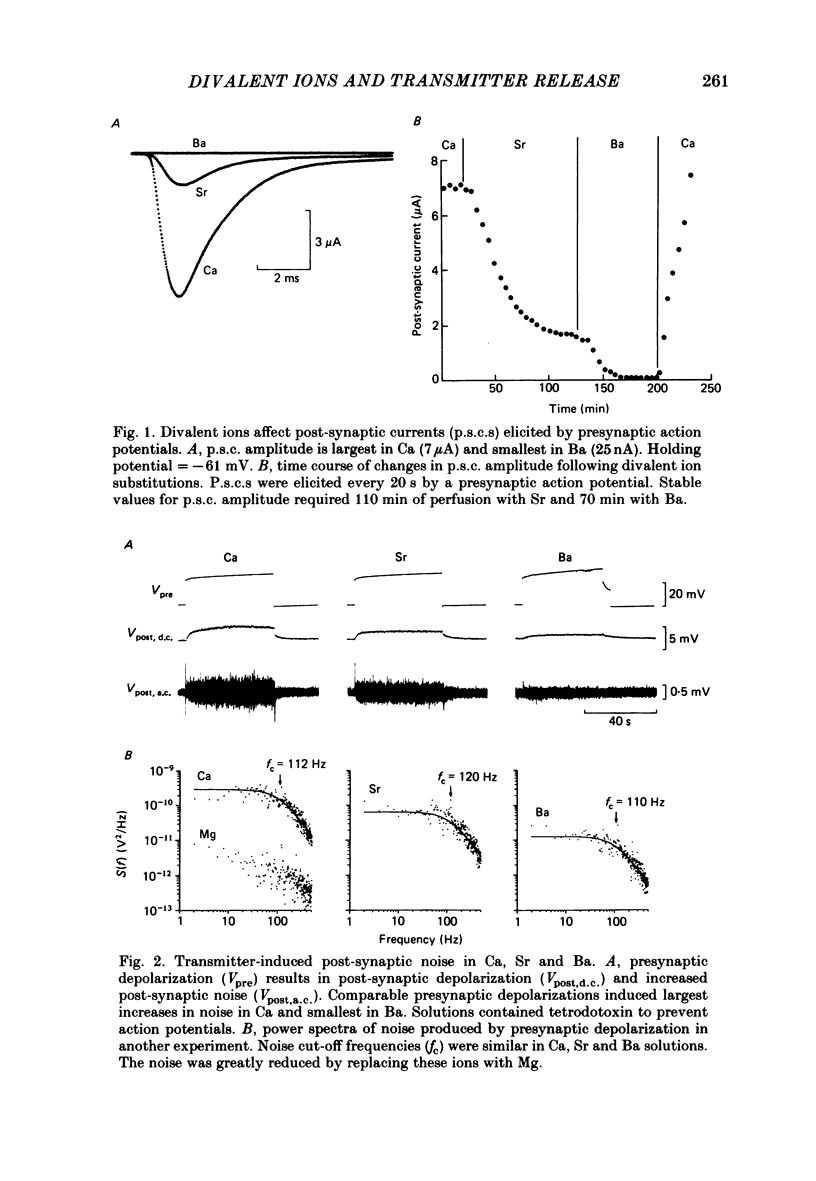
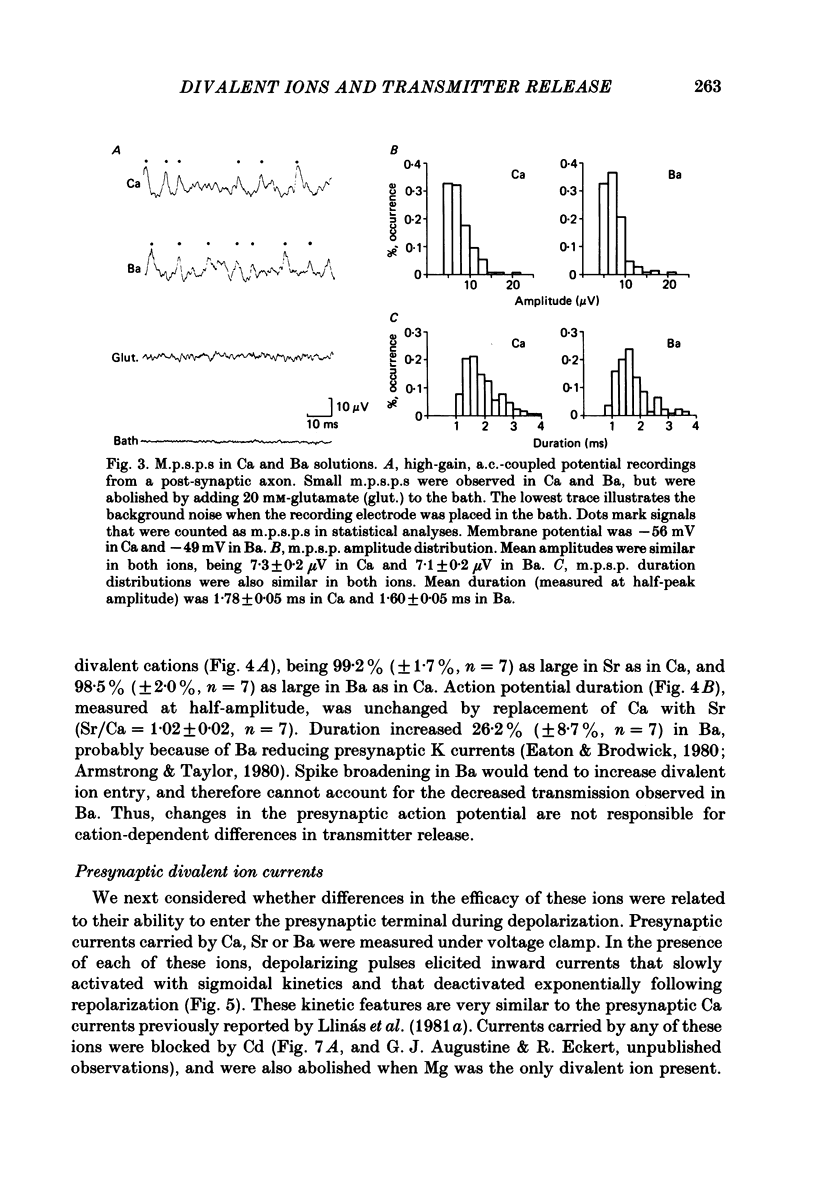
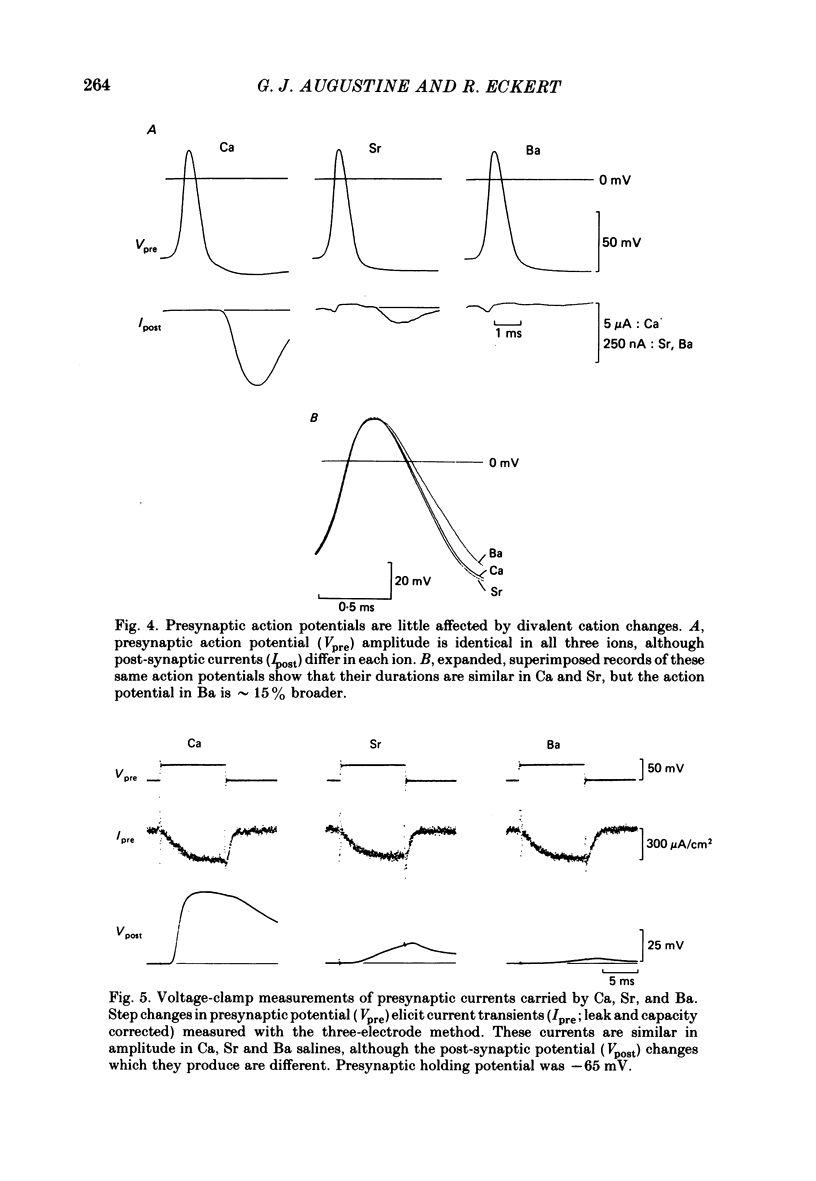
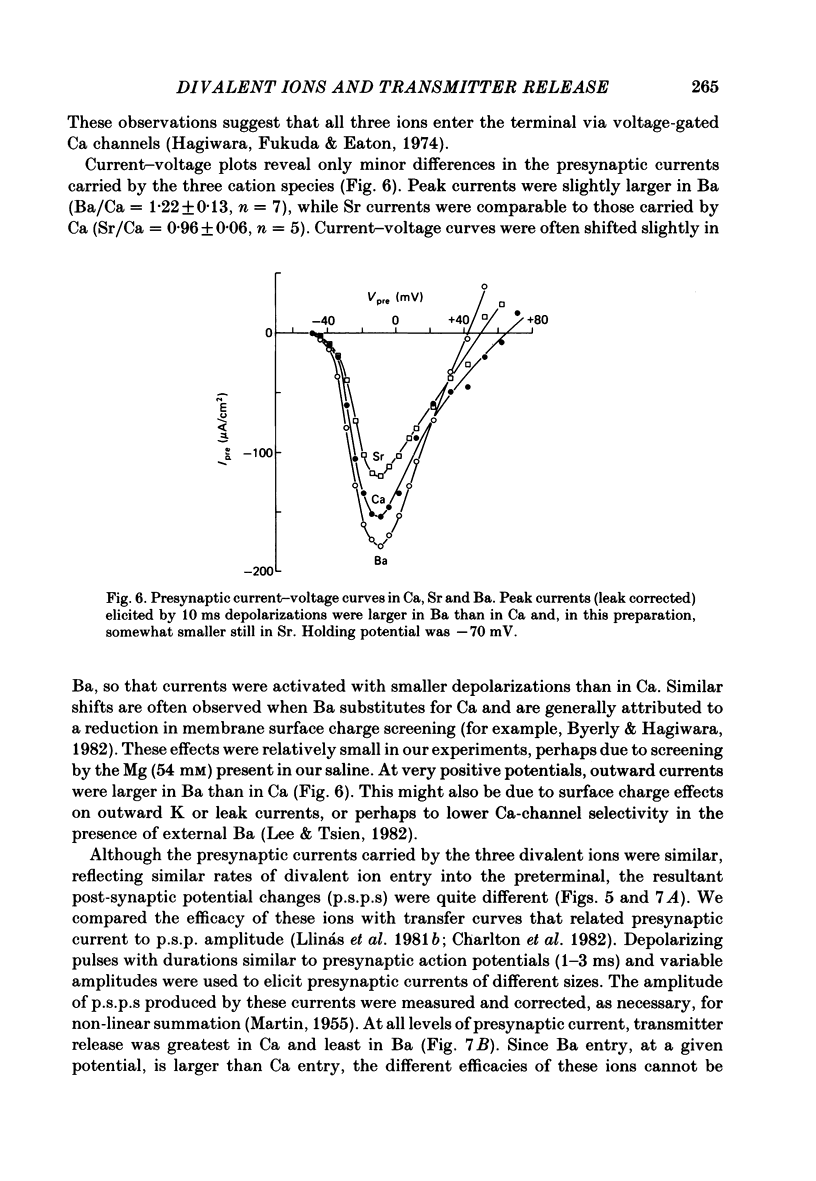
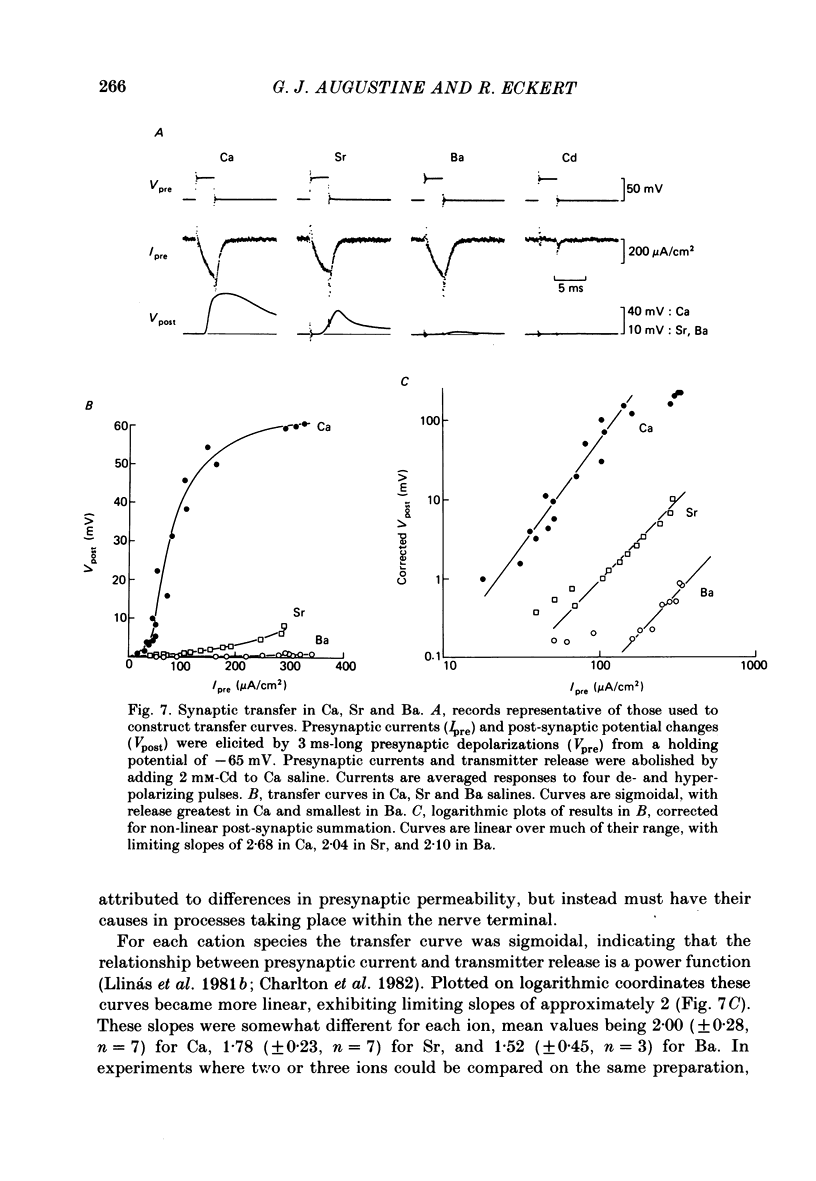
The ability of Ca, Sr and Ba ions to support transmitter release was studied at the squid giant synapse by examining their respective actions on presynaptic current and post-synaptic responses. Transmitter-induced post-synaptic currents were smaller in Sr- than in Ca- containing solutions, and much smaller in Ba-containing solutions. The time course and amplitude of spontaneous miniature post-synaptic potentials were similar in the presence of all three divalent ions. Sr or Ba substitution has little effect on the resting potential of presynaptic terminals. In Sr-containing solutions, action potentials were similar in amplitude and time course to those recorded in Ca. Ba slightly prolonged action potential duration but had no effect on amplitude. Voltage-clamped presynaptic terminals exhibited inward Ca, Sr or Ba currents which were apparently carried through Ca channels. These currents were similar in amplitude and time course in all three ions, being somewhat larger in Ba. Although presynaptic currents were similar in these ions, transmitter release induced by these currents depended upon the divalent species entering the presynaptic terminal. Release was greatest in response to presynaptic current carried by Ca and smallest in response to current carried by Ba. Transfer curves relating presynaptic current to post-synaptic potential were sigmoidal in all three ions, and exhibited limiting slopes of approximately 2. Divalent cations differentially support transmitter release at the squid giant synapse in the sequence Ca greater than Sr much greater than Ba. The differential efficacy of the divalent cations is not due to post-synaptic alterations, presynaptic potential changes or differences in presynaptic divalent cation conductances. This sequence may reflect the cation selectivity of the exocytotic process responsible for transmitter release.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADELMAN W. J., TAYLOR R. E. Leakage current rectification in the squid giant axon. Nature. 1961 Jun 3;190:883–885. doi: 10.1038/190883a0. [DOI] [PubMed] [Google Scholar]
- Adrian R. H., Chandler W. K., Hodgkin A. L. Voltage clamp experiments in striated muscle fibres. J Physiol. 1970 Jul;208(3):607–644. doi: 10.1113/jphysiol.1970.sp009139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed Z., Connor J. A. Measurement of calcium influx under voltage clamp in molluscan neurones using the metallochromic dye arsenazo III. J Physiol. 1979 Jan;286:61–82. doi: 10.1113/jphysiol.1979.sp012607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Leefmans F. J., De Santis A., Miledi R. Effects of some divalent cations on synaptic transmission in frog spinal neurones. J Physiol. 1979 Sep;294:387–406. doi: 10.1113/jphysiol.1979.sp012936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong C. M., Taylor S. R. Interaction of barium ions with potassium channels in squid giant axons. Biophys J. 1980 Jun;30(3):473–488. doi: 10.1016/S0006-3495(80)85108-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRYANT S. H. The function of the proximal synapses of the squid stellate ganglion. J Gen Physiol. 1959 Jan 20;42(3):609–616. doi: 10.1085/jgp.42.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULLOCK T. H. Properties of a single synapse in the stellate ganglion of squid. J Neurophysiol. 1948 Jul;11(4):343–364. doi: 10.1152/jn.1948.11.4.343. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Singh R. Metabolism and transport of strontium in giant axons of Loligo. J Physiol. 1982 Sep;330:373–392. doi: 10.1113/jphysiol.1982.sp014346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boullin D. J. The action of extracellular cations on the release of the sympathetic transmitter from peripheral nerves. J Physiol. 1967 Mar;189(1):85–99. doi: 10.1113/jphysiol.1967.sp008156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigant J. L., Mallart A. Presynaptic currents in mouse motor endings. J Physiol. 1982 Dec;333:619–636. doi: 10.1113/jphysiol.1982.sp014472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byerly L., Hagiwara S. Calcium currents in internally perfused nerve cell bodies of Limnea stagnalis. J Physiol. 1982 Jan;322:503–528. doi: 10.1113/jphysiol.1982.sp014052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton M. P., Bittner G. D. Facilitation of transmitter release at squid synapses. J Gen Physiol. 1978 Oct;72(4):471–486. doi: 10.1085/jgp.72.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton M. P., Smith S. J., Zucker R. S. Role of presynaptic calcium ions and channels in synaptic facilitation and depression at the squid giant synapse. J Physiol. 1982 Feb;323:173–193. doi: 10.1113/jphysiol.1982.sp014067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I., Van der Kloot W. Effects of [Ca2+] and [Mg2+] on the decay of miniature endplate currents. Nature. 1978 Jan 5;271(5640):77–79. doi: 10.1038/271077a0. [DOI] [PubMed] [Google Scholar]
- Cohen I., Van der Kloot W. The interaction of extracellular H+, Na+, Ca2+ and Sr2+ on the decay of miniature end-plate currents. Brain Res. 1982 Jun 10;241(2):285–290. doi: 10.1016/0006-8993(82)91065-4. [DOI] [PubMed] [Google Scholar]
- Crawford A. C., McBurney R. N. On the elementary conductance event produced by L-glutamate and quanta of the natural transmitter at the neuromuscular junctions of Maia squinado. J Physiol. 1976 Jun;258(1):205–225. doi: 10.1113/jphysiol.1976.sp011415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., LYWOOD D. W., STRAUB R. W. The stimulant effect of barium on the release of acetylcholine from the superior cervical ganglion. J Physiol. 1961 May;156:515–522. doi: 10.1113/jphysiol.1961.sp006690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS W. W., RUBIN R. P. THE EFFECTS OF ALKALINE EARTHS AND OTHER DIVALENT CATIONS ON ADRENAL MEDULLARY SECRETION. J Physiol. 1964 Dec;175:231–241. doi: 10.1113/jphysiol.1964.sp007514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge F. A., Jr, Miledi R., Rahamimoff R. Strontium and quantal release of transmitter at the neuromuscular junction. J Physiol. 1969 Jan;200(1):267–283. doi: 10.1113/jphysiol.1969.sp008692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C. J., Statham H. E. Interacting effects of temperature and extracellular calcium on the spontaneous release of transmitter at the frog neuromuscular junction. J Physiol. 1977 Jun;268(2):319–333. doi: 10.1113/jphysiol.1977.sp011859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton D. C., Brodwick M. S. Effects of barium on the potassium conductance of squid axon. J Gen Physiol. 1980 Jun;75(6):727–750. doi: 10.1085/jgp.75.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebashi S., Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Erulkar S. D., Weight F. F. Extracellular potassium and trasmitter release at the giant synapse of squid. J Physiol. 1977 Apr;266(2):209–218. doi: 10.1113/jphysiol.1977.sp011764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J. C., Mongar J. L. The role of the alkaline earth ions in anaphylactic histamine secretion. J Physiol. 1972 Aug;224(3):753–769. doi: 10.1113/jphysiol.1972.sp009921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner D., Stevens C. F. Rate-limiting step of inhibitory post-synaptic current decay in Aplysia buccal ganglia. J Physiol. 1980 Jul;304:145–164. doi: 10.1113/jphysiol.1980.sp013316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman A. L., Hermann A. Internal effects of divalent cations on potassium permeability in molluscan neurones. J Physiol. 1979 Nov;296:393–410. doi: 10.1113/jphysiol.1979.sp013012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Fukuda J., Eaton D. C. Membrane currents carried by Ca, Sr, and Ba in barnacle muscle fiber during voltage clamp. J Gen Physiol. 1974 May;63(5):564–578. doi: 10.1085/jgp.63.5.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S., Dennis M. J., Jan Y., Jan L., Evans L. Synaptic vesicle exocytosis captured by quick freezing and correlated with quantal transmitter release. J Cell Biol. 1979 May;81(2):275–300. doi: 10.1083/jcb.81.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner R. W., Moore J. W., Ramón F. Axon voltage-clamp simulations. III. Postsynaptic region. Biophys J. 1975 Jan;15(1):37–54. doi: 10.1016/S0006-3495(75)85790-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. On the factors which determine the amplitude of the miniature end-plate potential. J Physiol. 1957 Jul 11;137(2):267–278. doi: 10.1113/jphysiol.1957.sp005811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. A study of synaptic transmission in the absence of nerve impulses. J Physiol. 1967 Sep;192(2):407–436. doi: 10.1113/jphysiol.1967.sp008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Tetrodotoxin-resistant electric activity in presynaptic terminals. J Physiol. 1969 Aug;203(2):459–487. doi: 10.1113/jphysiol.1969.sp008875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. S., Gage P. W. L-glutamate blockade of transmission at the giant synapse of the squid stellate ganglion. J Neurobiol. 1969;1(2):209–219. doi: 10.1002/neu.480010208. [DOI] [PubMed] [Google Scholar]
- Kita H., Van Der Kloot W. Effects of the ionophore X-537A on acetylcholine release at the frog neuromuscular junction. J Physiol. 1976 Jul;259(1):177–198. doi: 10.1113/jphysiol.1976.sp011460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Tsien R. W. Reversal of current through calcium channels in dialysed single heart cells. Nature. 1982 Jun 10;297(5866):498–501. doi: 10.1038/297498a0. [DOI] [PubMed] [Google Scholar]
- Llinás R., Joyner R. W., Nicholson C. Equilibrium potential for the postsynaptic response in the squid giant synapse. J Gen Physiol. 1974 Nov;64(5):519–535. doi: 10.1085/jgp.64.5.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Presynaptic calcium currents in squid giant synapse. Biophys J. 1981 Mar;33(3):289–321. doi: 10.1016/S0006-3495(81)84898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Relationship between presynaptic calcium current and postsynaptic potential in squid giant synapse. Biophys J. 1981 Mar;33(3):323–351. doi: 10.1016/S0006-3495(81)84899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Simon S. M. Transmission by presynaptic spike-like depolarization in the squid giant synapse. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2415–2419. doi: 10.1073/pnas.79.7.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN A. R. A further study of the statistical composition on the end-plate potential. J Physiol. 1955 Oct 28;130(1):114–122. doi: 10.1113/jphysiol.1955.sp005397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. The effect of voltage on the time course of end-plate currents. J Physiol. 1972 May;223(1):151–171. doi: 10.1113/jphysiol.1972.sp009839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Weinstock M. M. Nickel and calcium ions modify the characteristics of the acetylcholine receptor-channel complex at the frog neuromuscular junction. J Physiol. 1980 Feb;299:203–218. doi: 10.1113/jphysiol.1980.sp013120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann D. W., Joyner R. W. Miniature synaptic potentials at the squid giant synapse. J Neurobiol. 1978 Jul;9(4):329–335. doi: 10.1002/neu.480090410. [DOI] [PubMed] [Google Scholar]
- McLachlan E. M. The effects of strontium and barium ions at synapses in sympathetic ganglia. J Physiol. 1977 May;267(2):497–518. doi: 10.1113/jphysiol.1977.sp011823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W., Thomas R. C. Effect of measured calcium chloride injections on the membrane potential and internal pH of snail neurones. J Physiol. 1980 Jan;298:111–129. doi: 10.1113/jphysiol.1980.sp013070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiri U., Rahamimoff R. Activation of transmitter release by strontium and calcium ions at the neuromuscular junction. J Physiol. 1971 Jul;215(3):709–726. doi: 10.1113/jphysiol.1971.sp009493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellow A. M. Equivalence of Ca2+ and Sr2+ in transmitter release from K+-depolarised nerve terminals. Nature. 1979 Nov 1;282(5734):84–85. doi: 10.1038/282084a0. [DOI] [PubMed] [Google Scholar]
- Miledi R., Parker I. Effects of strontium ions on end-plate channel properties. J Physiol. 1980 Sep;306:567–577. doi: 10.1113/jphysiol.1980.sp013415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Slater C. R. The action of calcium on neuronal synapses in the squid. J Physiol. 1966 May;184(2):473–498. doi: 10.1113/jphysiol.1966.sp007927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R. Spontaneous synaptic potentials and quantal release of transmitter in the stellate ganglion of the squid. J Physiol. 1967 Sep;192(2):379–406. doi: 10.1113/jphysiol.1967.sp008306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R. Transmitter release induced by injection of calcium ions into nerve terminals. Proc R Soc Lond B Biol Sci. 1973 Jul 3;183(1073):421–425. doi: 10.1098/rspb.1973.0026. [DOI] [PubMed] [Google Scholar]
- Nachshen D. A., Blaustein M. P. Influx of calcium, strontium, and barium in presynaptic nerve endings. J Gen Physiol. 1982 Jun;79(6):1065–1087. doi: 10.1085/jgp.79.6.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazato Y., Onoda Y. Barium and strontium can substitute for calcium in noradrenaline output induced by excess potassium in the guinea-pig. J Physiol. 1980 Aug;305:59–71. doi: 10.1113/jphysiol.1980.sp013349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M. Can barium support the release of acetylcholine by nerve impulses? Br J Pharmacol. 1977 Jan;59(1):215–217. doi: 10.1111/j.1476-5381.1977.tb06997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinsky E. M. On the role of barium in supporting the asynchronous release of acetylcholine quanta by motor nerve impulses. J Physiol. 1978 Jan;274:157–171. doi: 10.1113/jphysiol.1978.sp012141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K., Gage P. W., Barry P. H. Effects of divalent cations on toad end-plate channels. J Membr Biol. 1982;64(1-2):55–66. doi: 10.1007/BF01870768. [DOI] [PubMed] [Google Scholar]
- Teo T. S., Wang J. H. Mechanism of activation of a cyclic adenosine 3':5'-monophosphate phosphodiesterase from bovine heart by calcium ions. Identification of the protein activator as a Ca2+ binding protein. J Biol Chem. 1973 Sep 10;248(17):5950–5955. [PubMed] [Google Scholar]
- Tsien R. W. Calcium channels in excitable cell membranes. Annu Rev Physiol. 1983;45:341–358. doi: 10.1146/annurev.ph.45.030183.002013. [DOI] [PubMed] [Google Scholar]
- Zengel J. E., Magleby K. L. Changes in miniature endplate potential frequency during repetitive nerve stimulation in the presence of Ca2+, Ba2+, and Sr2+ at the frog neuromuscular junction. J Gen Physiol. 1981 May;77(5):503–529. doi: 10.1085/jgp.77.5.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker R. S., Stockbridge N. Presynaptic calcium diffusion and the time courses of transmitter release and synaptic facilitation at the squid giant synapse. J Neurosci. 1983 Jun;3(6):1263–1269. doi: 10.1523/JNEUROSCI.03-06-01263.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker R. S. Tetraethylammonium contains an impurity which alkalizes cytoplasm and reduce calcium buffering in neurons. Brain Res. 1981 Mar 16;208(2):473–478. doi: 10.1016/0006-8993(81)90580-1. [DOI] [PubMed] [Google Scholar]


