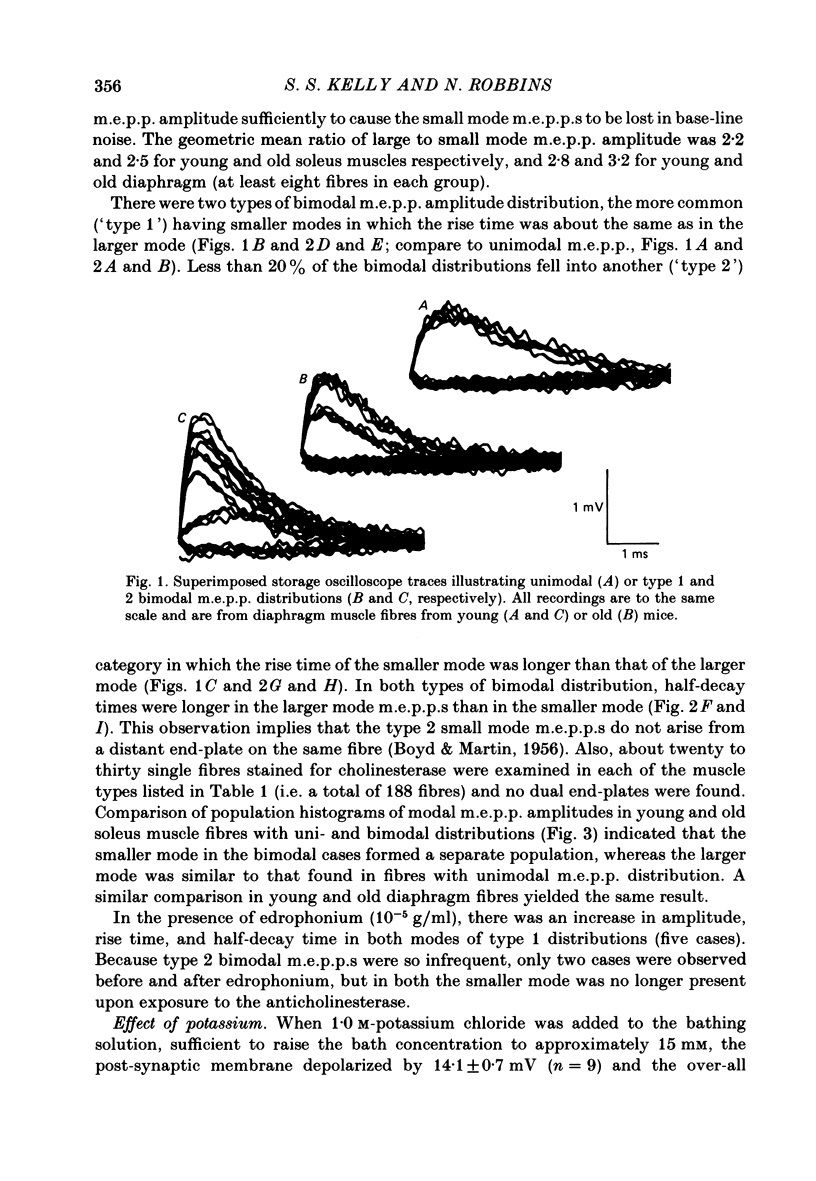
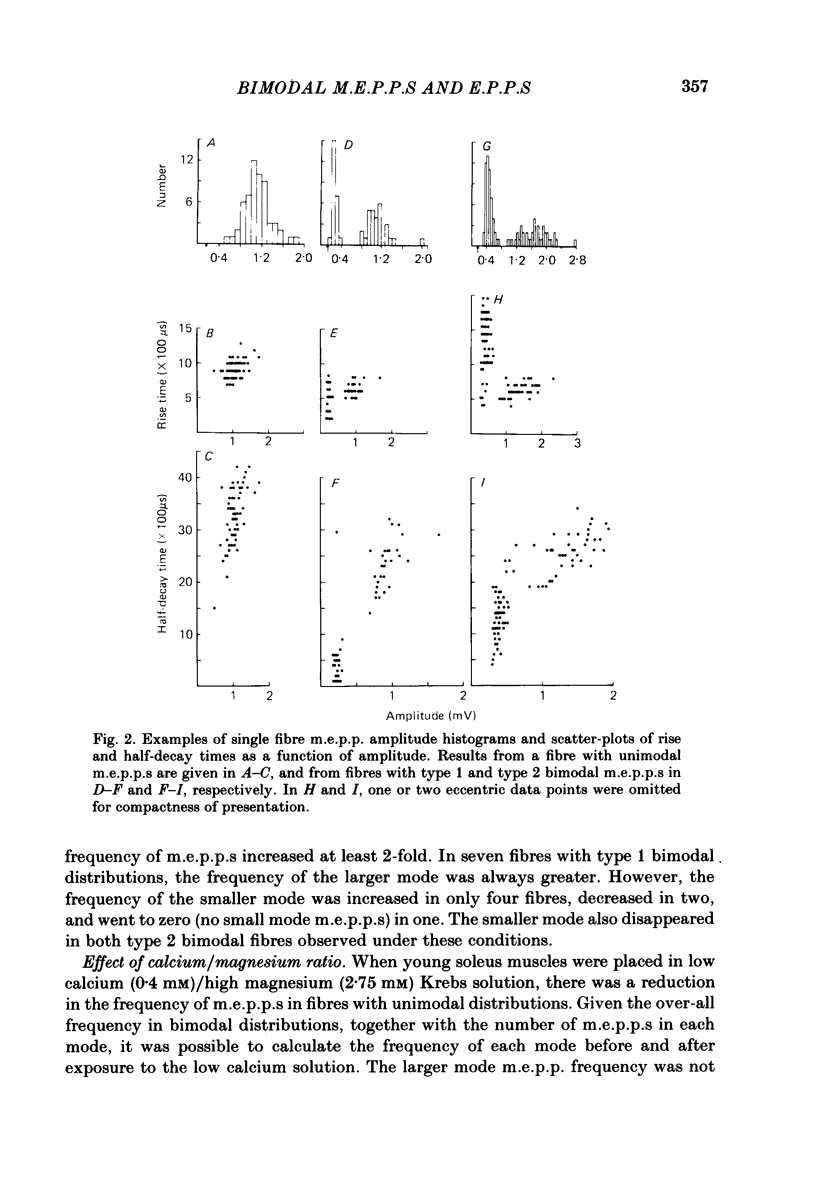
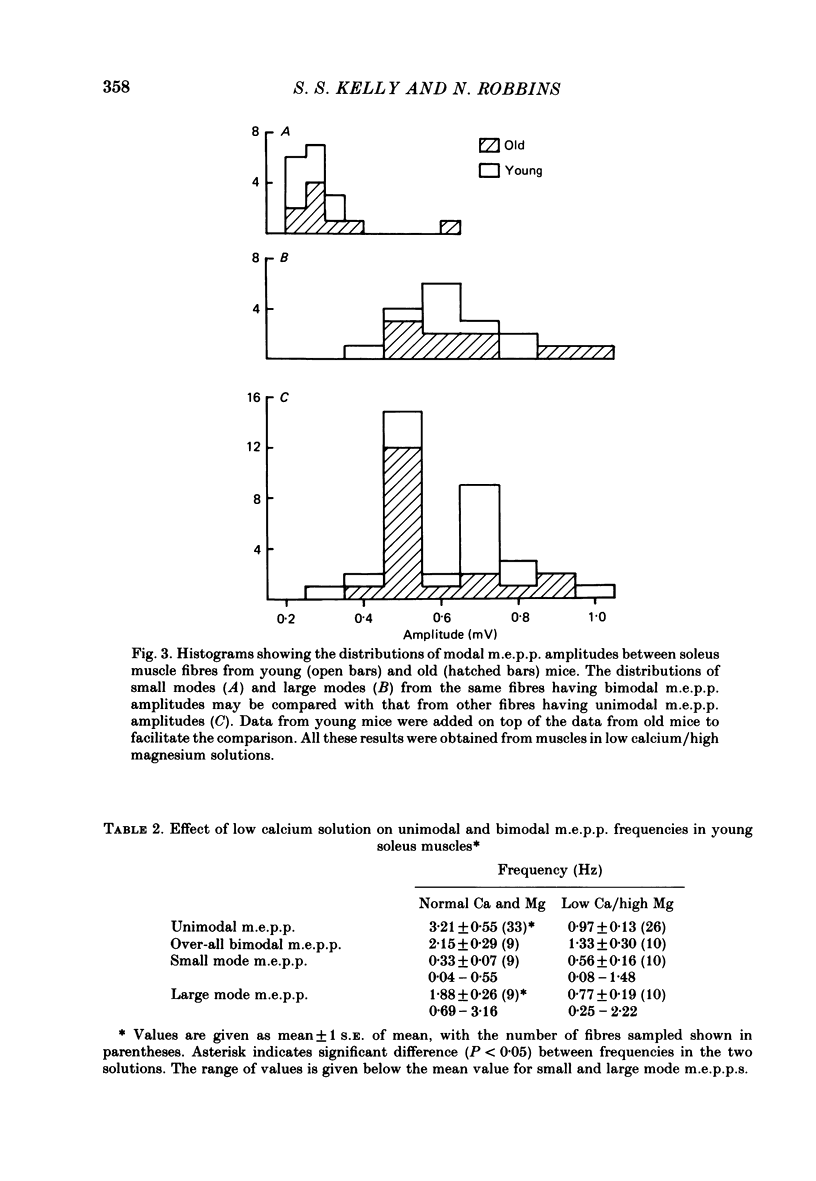
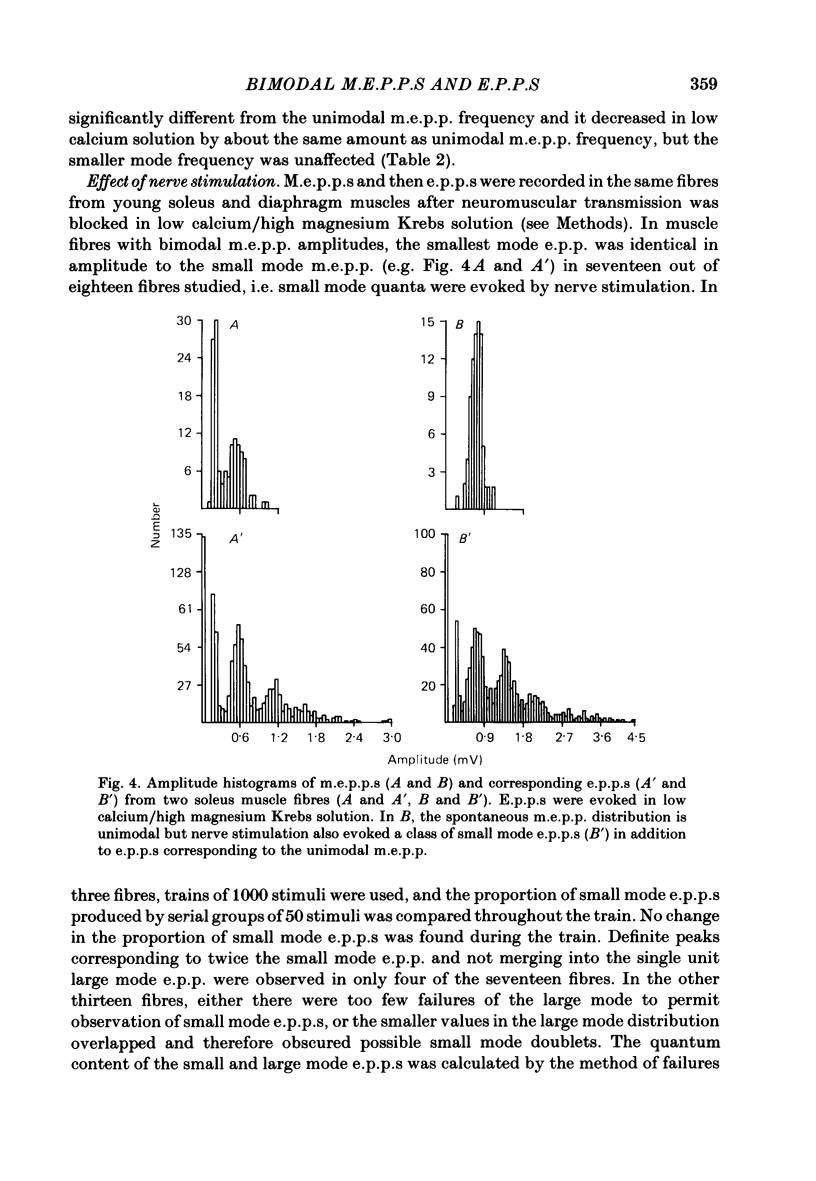
Abstract
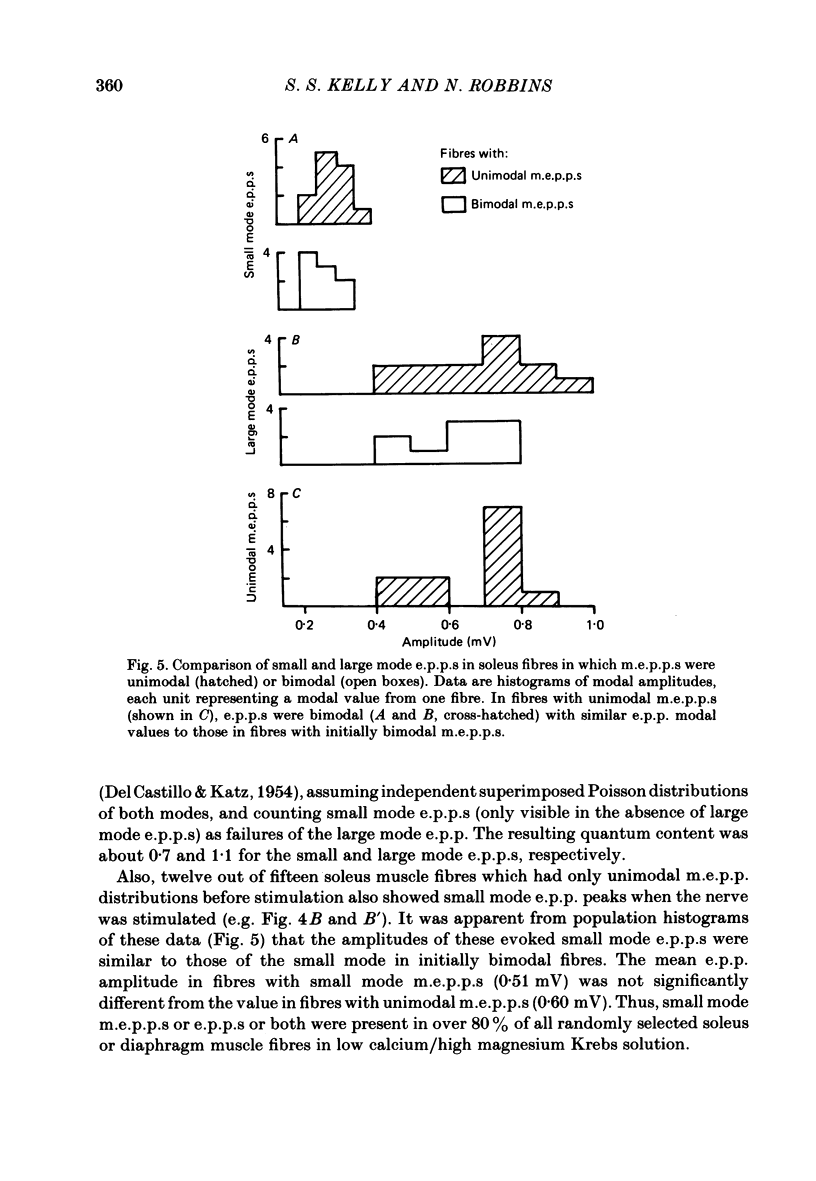
Intracellular recordings of spontaneous miniature end-plate potentials (m.e.p.p.s) in muscles from adult CBF-1 mice revealed a population of muscle fibres in which the amplitude distribution of m.e.p.p.s was bimodal. The large mode m.e.p.p.s were similar to those from fibres having unimodal amplitude distributions and the small mode m.e.p.p.s were about one-half to one-quarter the amplitude of the large mode. In five diverse muscle groups (extensor digitorum communis, gluteus maximus, diaphragm, extensor digitorum longus, and soleus) from mice 10-12 or 31 months of age, bimodal m.e.p.p. amplitude distributions were present in about 20% of fibres sampled. In the common bimodal distribution (type 1), the rise times of small mode m.e.p.p.s were similar to those of large mode m.e.p.p.s. A rare class of small mode m.e.p.p.s (type 2) having long rise times was also observed. Amplitudes and half-decay times of type 1 small mode m.e.p.p.s increased in the presence of an anticholinesterase (edrophonium). Increasing extracellular potassium concentration led to an increase in large mode m.e.p.p. frequency but had more variable effects on small mode frequency. In the few cases available for study, type 2 small mode m.e.p.p.s disappeared after addition of edrophonium or increased potassium. When the extracellular calcium/magnesium ratio was reduced, large mode but not small mode m.e.p.p. frequency decreased. In almost all muscle fibres in which end-plate potentials (e.p.p.s) were evoked by nerve stimulation at 20 Hz in low calcium/high magnesium solution, small mode e.p.p.s similar to small mode m.e.p.p.s appeared during 'failures' of large mode m.e.p.p.s. Also, in twelve out of fifteen fibres which had unimodal m.e.p.p. amplitude distributions, small mode e.p.p.s appeared which were similar in amplitude to small mode m.e.p.p.s in fibres with type 1 bimodal m.e.p.p.s. Thus, if both spontaneous and evoked potentials are included, small mode m.e.p.p.s are present at most CBF-1 mouse adult neuromuscular junctions independent of muscle type or animal age. Small and large mode m.e.p.p.s differ in certain responses but both are evoked by nerve stimulation at physiological frequencies and therefore participate in normal neuromuscular synaptic activity. The possible origin of small mode m.e.p.p.s is discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIRKS R., KATZ B., MILEDI R. Physiological and structural changes at the amphibian myoneural junction, in the course of nerve degeneration. J Physiol. 1960 Jan;150:145–168. doi: 10.1113/jphysiol.1960.sp006379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOYD I. A., MARTIN A. R. The end-plate potential in mammalian muscle. J Physiol. 1956 Apr 27;132(1):74–91. doi: 10.1113/jphysiol.1956.sp005503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banker B. Q., Kelly S. S., Robbins N. Neuromuscular transmission and correlative morphology in young and old mice. J Physiol. 1983 Jun;339:355–377. doi: 10.1113/jphysiol.1983.sp014721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Florin T., Woog R. The formation of synapses in regenerating mammalian striated muscle. J Physiol. 1974 Apr;238(1):79–92. doi: 10.1113/jphysiol.1974.sp010511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Pettigrew A. G., Taylor R. S. The formation of synapses in reinnervated and cross-reinnervated adult avian muscle. J Physiol. 1973 Apr;230(2):331–357. doi: 10.1113/jphysiol.1973.sp010191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. R., Pettigrew A. G. The formation of synapses in amphibian striated muscle during development. J Physiol. 1975 Oct;252(1):203–239. doi: 10.1113/jphysiol.1975.sp011141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan S. Sub-miniature end-plate potentials at untreated frog neuromuscular junctions. J Physiol. 1976 Jun;258(1):145–155. doi: 10.1113/jphysiol.1976.sp011411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke J. D., Quastel D. M. Transmitter release by mammalian motor nerve terminals in response to focal polarization. J Physiol. 1973 Jan;228(2):377–405. doi: 10.1113/jphysiol.1973.sp010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cull-Candy S. G., Lundh H., Thesleff S. Effects of botulinum toxin on neuromuscular transmission in the rat. J Physiol. 1976 Aug;260(1):177–203. doi: 10.1113/jphysiol.1976.sp011510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Quantal components of the end-plate potential. J Physiol. 1954 Jun 28;124(3):560–573. doi: 10.1113/jphysiol.1954.sp005129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M. J., Miledi R. Characteristics of transmitter release at regenerating frog neuromuscular junctions. J Physiol. 1974 Jun;239(3):571–594. doi: 10.1113/jphysiol.1974.sp010583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellisman M. H., Rash J. E., Staehelin L. A., Porter K. R. Studies of excitable membranes. II. A comparison of specializations at neuromuscular junctions and nonjunctional sarcolemmas of mammalian fast and slow twitch muscle fibers. J Cell Biol. 1976 Mar;68(3):752–774. doi: 10.1083/jcb.68.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. J., Miledi R. The effect of type D botulinum toxin on frog neuromuscular junctions. J Physiol. 1971 Sep;217(2):497–515. doi: 10.1113/jphysiol.1971.sp009582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly S. S. The effect of age on neuromuscular transmission. J Physiol. 1978 Jan;274:51–62. doi: 10.1113/jphysiol.1978.sp012133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriebel M. E., Gross C. E. Multimodal distribution of frog miniature endplate potentials in adult denervated and tadpole leg muscle. J Gen Physiol. 1974 Jul;64(1):85–103. doi: 10.1085/jgp.64.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriebel M. E., Llados F., Matteson D. R. Histograms of the unitary evoked potential of the mouse diaphragm show multiple peaks. J Physiol. 1982 Jan;322:211–222. doi: 10.1113/jphysiol.1982.sp014033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriebel M. E., Llados F., Matteson D. R. Spontaneous subminature end-plate potentials in mouse diaphragm muscle: evidence for synchronous release. J Physiol. 1976 Nov;262(3):553–581. doi: 10.1113/jphysiol.1976.sp011610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriebel M. E. Small mode miniature end plate potentials are increased and evoked in fatigued preparations and in high Mg2+ saline. Brain Res. 1978 Jun 16;148(2):381–388. doi: 10.1016/0006-8993(78)90726-6. [DOI] [PubMed] [Google Scholar]
- Kuffler S. W., Yoshikami D. The distribution of acetylcholine sensitivity at the post-synaptic membrane of vertebrate skeletal twitch muscles: iontophoretic mapping in the micron range. J Physiol. 1975 Jan;244(3):703–730. doi: 10.1113/jphysiol.1975.sp010821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letinsky M. S. The development of nerve-muscle junctions in Rana catesbeiana tadpoles. Dev Biol. 1974 Sep;40(1):129–153. doi: 10.1016/0012-1606(74)90114-6. [DOI] [PubMed] [Google Scholar]
- Robbins N., Olek A., Kelly S. S., Takach P., Christopher M. Quantitative study of motor endplates in muscle fibres dissociated by a simple procedure. Proc R Soc Lond B Biol Sci. 1980 Oct 15;209(1177):555–562. doi: 10.1098/rspb.1980.0112. [DOI] [PubMed] [Google Scholar]


