Abstract
A monoclonal antibody (MAb) directed against an unknown Chlamydophila pneumoniae epitope has been characterized, and the respective peptide mimotope has been identified. A murine MAb specific for C. pneumoniae was used to select peptides from phage display libraries. The peptides identified from the phage display library clones reacted specifically with the respective target murine MAb and with human sera previously identified as having antibody titers to C. pneumoniae. The selected peptide mimotope sequences tended to be composed of charged residues surrounding a core of hydrophobic residues. The peptide with the best binding could inhibit >95% of binding to the MAb, suggesting that the selected peptide binds the paratope of the respective MAb. The peptide reacted with human sera previously determined by microimmunofluorescence to have anti-C. pneumoniae antibodies. The peptide was competitively competed with the MAb against Renografin-purified, sonicated C. pneumoniae in an enzyme-linked immunosorbent assay and with whole-cell C. pneumoniae in an indirect fluorescence assay format, demonstrating its potential utility in the development of diagnostics. The use of this novel peptide may allow investigators to establish standardized assays free from cross-reactive Chlamydia trachomatis and Chlamydophila psittaci epitopes and immunoreactivity.
Chlamydophila pneumoniae, formerly known as Chlamydia pneumoniae (12), was first isolated in 1965 (15) and is established as an etiologic agent of respiratory tract diseases and related sequelae (5, 14, 16, 18, 19). Chlamydophila and Chlamydia species are gram-negative obligate intracellular bacteria having a biphasic life cycle, consisting of a metabolically inert infectious elementary body (EB) and a metabolically active reticulate body (RB). C. pneumoniae is a respiratory pathogen believed to cause 5% to 20% of community-acquired pneumonias and 5% of bronchitis and sinusitis in adults and children (22, 33, 40, 41, 52).
Seroepidemiology has shown that most C. pneumoniae infections are asymptomatic. Regional and international serology-based epidemiologic studies of C. pneumoniae have shown high prevalences and ubiquitous infection. These studies have indicated that most persons have had their first C. pneumoniae infection before age 20 and commonly become reinfected (1).
The biphasic life cycle of Chlamydophila and Chlamydia as well as their intracellular host cell parasitism could allow for maintenance of a chronic infection. For example, Chlamydophila psittaci can persist in mammals and birds lifelong and only occasionally cause disease, most often after induction of stress (23). C. pneumoniae has been demonstrated to multiply in macrophages, endothelia, and smooth muscle cells in vitro (13, 26), and this multiplication has been associated with cytokine production and induction of adhesions (10, 24, 25). However, demonstration of a state of chronic infection has been more elusive.
Many laboratory methods have been developed for the diagnosis of C. pneumoniae infection, including primary isolation of the organism in cell culture, serologic assays, immunohistochemical assays, and PCR (17). Despite great effort to improve primary culture techniques of C. pneumoniae, isolation and culture still require specialized personnel and considerable laboratory resources. To date, only a few laboratories worldwide have been able to culture primary human isolates. Serologic and PCR assays are the tools most often applied for routine diagnosis of acute C. pneumoniae infection. Serologic assays include complement fixation, microimmunofluorescence (MIF), enzyme-linked immunosorbent assay (ELISA), and immunohistochemistry (2). Each of these assays requires significant technical expertise and is subject to investigator interpretation. The MIF test remains the most sensitive, species-specific assay and is the “gold standard” for determining the prevalence of C. pneumoniae in study populations (45, 46). The traditional MIF assay relies on the use of whole EBs as antigens. Though lacking the necessary species specificity for use as a diagnostic serologic test, indirect immunofluorescence assay (IFA) has been used for culture confirmation of isolates or for laboratory culture standardization. IFA relies on both whole RBs and EBs fixed with methanol as antigens in C. pneumonia-infected cell culture. Whole C. pneumoniae antigen has been observed by investigators to have cross-reactivity in certain serologic and immunohistochemical tests (4). Standardized assays that reduce the requirements for highly specialized, well-trained personnel while still providing species specificity are greatly needed for further investigations into the natural history and epidemiology of C. pneumoniae.
In this study, using phage display, we determined the peptide sequence of the epitope binding a murine C. pneumoniae-specific monoclonal antibody (MAb) designated 8A6 (50), evaluated the peptide sequence, and characterized the immunoreactivity of the peptide using ELISA, MIF, and IFA.
MATERIALS AND METHODS
MAb.
The 8A6 MAb was prepared previously at the Centers for Disease Control and Prevention (CDC, Atlanta, Ga.) (50) by using Renografin-purified C. pneumoniae CWL 029 (ATCC VR-1310) as the immunogen. The primary 8A6 MAb-producing cells were cultured in Iscove's modified Dulbecco's medium (Gibco BRL, Rockville, Md.) supplemented with 10% low-immunoglobulin G (IgG) fetal bovine serum (HyClone, Ogden, Utah). Clone supernatants were assayed for reactivity to C. pneumoniae by IFA (see below). Reactive wells were further subcloned by limiting dilution analysis with methods previously described (49). Subcloning was repeated two more times to ensure that the most-reactive single-cell clones were produced. Clones resulting from single-cell selection were expanded and screened as described previously (49). The clone producing the highest concentration of reactive MAb (based on IFA testing of clones) was then weaned onto BD cell medium (BD Pharmingen, San Diego, Calif.), according to the manufacturer's protocol, for generating a large-scale antibody.
The 8A6 MAb-producing cell line's growth and MAb production were scaled up by using BD cell medium. The cells were incubated in roller bottles at 37°C in a 10% CO2 humidified chamber for an additional 12 days. Cells were collected by centrifugation, and the medium was carefully harvested without disrupting the cell pellet. Reactivity and species specificity of the MAbs selected were determined using indirect IFA with three C. pneumoniae (CWL011, CWL029, CWL050), one Chlamydia trachomatis (UW-F), and two C. psittaci (DD34 and CP3) strains according to standard protocols.
Isotype determinations were made using the Mouse Antibody Isotyping kit (Gibco BRL) and Immunotype Mouse monoclonal antibody typing kit (Sigma Chemical Co., St. Louis, Mo.) following the manufacturer's specified protocol. Monoclonal antibodies of known isotype were used as controls.
Sera.
Human sera were obtained from healthy autologous volunteer donors through blood services at the CDC. Testing protocols for using the donated anonymous sera were covered by a CDC Institutional Review Board (31).
Phage display.
The pIII phage library was a kind gift from George Smith (University of Missouri) and contains a random 15-amino-acid insert located at the N terminus of the pIII coat protein (35, 42). Growth and titration of the phage were performed according to methods previously described (42). A 50-μl aliquot of protein-G 4-Fast Flow Sepharose (Amersham-Pharmacia Biotech, Piscataway, N.J.) slurry was washed 3 times in 0.1 M sodium carbonate buffer (pH 8.6) to remove the alcohol preservative. The protein-G Sepharose was incubated with purified 8A6 MAb for 24 h at 4°C with gentle agitation. The remaining unreacted protein-G binding sites were blocked by incubation with either 10 mg of bovine serum albumin (BSA) (Sigma Chemical Co.) or Superblock (Pierce Scientific, Rockford, Ill.)/ml for at least 3 h at 4°C. The Sepharose was then pelleted and washed 10 times with Tris-buffered saline (TBS) containing BSA (1 mg/ml) and Tween 20 (0.05%) (TBST-B). The beads were stored in TBST-B in a final volume of 0.5 ml. For panning, 1011 phage/ml was added to 8A6-protein-G Sepharose in a final volume of 1 ml in TBST-B. The binding mixture was incubated for 2 h at room temperature with rocking to reach equilibrium. The beads were then washed 10 times with 1 ml of TBS-0.05% Tween 20 (TBST). The bound phage was eluted from the beads by incubation with 1 ml of elution buffer (0.1 M glycine [pH 2.2], BSA [1 mg/ml], and Tween 20 [0.05%]) for 10 min. The elution mixture was neutralized with 125 μl of 1 M Tris-HCl (pH 9.1). The phage titer of the elution mixture was determined as described above.
DNA sequence of selected clones.
Phage clones from the fourth round of biopanning were selected randomly, propagated, and sequenced. Phage DNA was purified by using the QIAprep Spin M13 kit (Qiagen, Inc., Valencia, Calif.) according to the manufacturer's specifications and was then ethanol precipitated. Sequencing reactions were performed by using the dRhodamine dye terminator cycle sequencing kit (Applied Biosystems Incorporated, Foster City, Calif.).
The specificities of the amplified products were confirmed by direct sequencing. The primer fd-tet.27.p (5′-GTAGCATTCCACAGACAGCCCTCATAG-3′) was used to sequence the PCR products. All PCR products were sequenced in both directions with the Prism Dye terminator kit (Applied Biosystems Incorporated) by using an ABI-Prism model 377 autosequencer (Applied Biosystems Incorporated). Sequenced products, DNA and translated amino acid sequences, were compared with C. pneumoniae sequences (TIGR unfinished genome projects) available in GenBank (release 118) by using the FASTA algorithm implemented in the Wisconsin Package (version 10.0.1) from the Genetics Computer Group (Madison, Wis.) as well as locally with all sequences through the Genetics Computer Group package. Additional searches were run externally through the internet using BLAST (www.ncbi.nlm.nih.gov/BLAST/), FASTA-3 (located at the European Bioinformatics Institute, Hinxton, United Kingdom [www2.ebi.ac.uk/fasta3/]), EMBL (at the European Bioinformatics Institute, Cambridge, United Kingdom [www.ebi.ac.uk/embl/]), DDBJ (at the DNA Databank of Japan, National Institute of Genetics, Shizuoka, Japan [http://www.ddbj.nig.ac.jp/fromddbj-e.html]), BLOCKS (located at the Fred Hutchinson Cancer Research Center, Seattle, Wash. [www.blocks.fhcrc.org]), PRINTS (located at the Protein Sequence Analysis Group, Manchester, United Kingdom [www.biochem.ucl.ac.uk/bsm/dbbrowser/PRINTS/]), PENDANT (located at the Munich Information Center for Protein Sequences, National Research Center for Environment and Health at the Max-Planck-Institut für Biochemie, Martinsried, Germany [pedant.mips.biochem.mpg.de/]), and additional search algorithms and methods found at the ExPASy (Expert Protein Analysis System) proteomics server of the Swiss Institute of Bioinformatics (www.expasy.ch/tools/).
Phage ELISA.
A two-step phage ELISA was used to measure 8A6 phage affinity for 8A6 MAb. Immulon II-HB 96-well microtiter plates (Dynex Technologies, Chantilly, Va.) were coated with purified 8A6 MAb (at 5 μg/ml) in 50 mM sodium carbonate (pH 9.6) at 4°C overnight. The plates were then blocked with a solution of 3% nonfat skim milk (Difco, Detroit, Mich.) in TBS-Tween-20 buffer (TBST-MK) for 2 h at 37°C. Serial dilutions of the respective 8A6 phage clone stock were added to the wells and incubated for 2 h at room temperature in TBST-MK at a final volume of 100 μl. The plates were then washed 4 times with TBST, and the bound phage was detected with a mouse anti-M13 polyclonal antibody conjugated to horseradish peroxidase (HRP) (Amersham Pharmacia Biotech). Absorbance at 490 nm was recorded with an enzyme immunoassay reader (Bio-Tek Instruments, Burlington, Vt.). M13 phage without the 12-amino-acid pIII insert was used as a negative control. To determine phage affinity, serial dilutions of 8A6 MAb and a subsaturating concentration of fourth-round-selected phage clone were added to the wells in 100 μl of TBST-MK. After 2 h at 37°C, the wells were washed 4 times with TBST, and bound phage was detected as described above.
Peptide synthesis.
Biotinylated and nonbiotinylated synthetic peptides were obtained from Bethyl Laboratories (Montgomery, Tex.) and the CDC Biotechnology Core Facility. The resulting peptides were purified to ≥90% by high-pressure liquid chromatography. The peptides for the 8A6 mimotope are CP-8A6-A1, CP-8A6-A2, CP-8A6-A3, CP-8A6-B1, CP-8A6-B3, and CP-8A6-B10A (sequences can be found in Table 1). The peptides CP-8A6-A1 and CP-8A6-B3 were reconstituted at 1 mg/ml in 250 μl of sterile 100 mM NaHCO3 and 750 μl of sterile distilled H2O, and peptides CP-8A6-A2, CP-8A6-A3, CP-8A6-B1, and CP-8A6-B10A were reconstituted at 1 mg/ml in 200 μl of sterile 10% acetic acid and 800 μl of sterile distilled H2O.
TABLE 1.
Sequences of peptides selected by using phage display with 8A6 MAb
| Peptide | Sequence | pI/Mw |
|---|---|---|
| CP-8A6-A1 | RRLGRQTYDNES | 8.74/1494.59 |
| CP-8A6-A2 | HDEGRQIIQFEE | 4.60/1500.59 |
| CP-8A6-A3 | LRNCEQDFFTLN | 4.37/1499.66 |
| CP-8A6-B1 | PNEPDDLALMRIIRI | 4.56/1766.09 |
| CP-8A6-B3 | AFAQAPTHQLSL | 6.79/1283.45 |
| CP-8A6-B10A | ESNPVDGAHLSL | 4.35/1238.32 |
Peptide ELISA.
Three different plates were used to optimize peptide binding: Reacti-Bind maleic anhydride-activated 96-well polystyrene microtiter plates (Pierce Scientific) (M-plates), Combiplate 8 streptavidin-coated polystyrene microtiter plates (Labsystems, Franklin, Mass.) (S-plates), and Immulon II-HB 96-well microtiter plates (Dynex Technologies) (I-plates). Protocols differed only in antigen coating and blocking procedures. Briefly, M-plates were coated with purified 8A6 MAb (at 5 μg/ml) in 50 mM sodium carbonate (pH 9.6) at 37°C for 1 h. The M-plates were then blocked for 1 h with 1 M glycine to eliminate any remaining unreacted maleic anhydride groups and then blocked with TBST-MK for 2 h at room temperature. S-plates and I-plates were coated with purified 8A6 MAb (at 5 μg/ml) in 50 mM sodium carbonate (pH 9.6) at 4°C overnight. The S-plates were then blocked with a solution of 10% Superblock (Pierce Scientific) in TBS-Tween buffer for 2 h at room temperature. The I-plates were blocked with TBST-MK for 2 h at room temperature. After the antigen was immobilized on the respective plates, the ELISA format followed the same protocol. Briefly, the bound peptide was tested with serially diluted 8A6 MAb (having a starting dilution of 100 μg/ml) and was detected with a goat anti-mouse IgG heavy-plus-light-chain antibody HRP conjugate (Pierce Scientific). All washes were performed 4 times by using TBST. Control peptides of dissimilar sequences were used as negative controls.
Immunoslot blot.
Immunoslot blot analyses were performed as previously described (36). Briefly, 5 μl of antigen was spotted onto nitrocellulose (Invitrogen, Carlsbad, Calif.), air dried, and blocked with casein-thimerosal buffer (27) for 0.5 h. The blots were incubated with 8A6 MAb (1:1,000) diluted in phosphate-buffered saline with 0.005% Tween. The blots were incubated for 2 h at 37°C with shaking and then washed 3 times in phosphate-buffered saline with 0.005% Tween for 5 min (each wash). The blots were probed with goat anti-mouse IgG peroxidase conjugate (Pierce Scientific) for 1 h at 37°C with shaking. They were washed as before and developed with 3′,3′-diaminobenzidine peroxidase substrate (Sigma Chemical Co.). The reaction was stopped by washing the blots with deionized water.
MIF.
The 8A6 MAb was tested with the MRL Diagnostics (Cypress, Calif.) Chlamydia pneumoniae MIF (48) IgG test by following the manufacturer's recommendations.
IFA.
The IFA was performed as described previously (44, 50) with some modifications. Antibiotic-free buffalo African green monkey cells were seeded into 1-dram shell vials or 24-well tissue culture trays containing 12-mm2 coverslips at a density of 3.0 × 105 cells/ml. The cells were incubated for 24 h at 37°C and checked for confluence. Cell cultures were infected by the addition of C. pneumoniae followed by centrifugation at 3,000 × g. Inclusions were counted with the Pathfinder Chlamydia Culture Confirmation System staining kit (Sanofi Diagnostics Pasteur, Redmond, Wash.) according to manufacturer specifications. The 8A6 MAb was used as previously described (50) for the detection of C. pneumoniae inclusions and for the determination of species specificity.
A competitive inhibition IFA was performed by modifying the previously described IFA. The 8A6 peptide was serially diluted in a constant concentration of either 8A6 MAb or Pathfinder detection reagent and incubated for 1 h at room temperature. The material was then used to detect inclusions. Positive controls were 8A6 MAb and Pathfinder reagent without the addition of peptide, and negative controls were 8A6 MAb and Pathfinder reagent with the addition of random, nonspecific peptides generated in the CDC Biotechnology Core Facility.
RESULTS
8A6 MAb.
Although the 8A6 MAb had been previously developed by K. H. Wong at the CDC (in 1992) as a reagent for the detection of C. pneumoniae in cell culture by using indirect IFA, the monoclonal yield was not optimal. In the present study, the monoclonal yield was improved from <0.3 μg of reactive MAb/ml to >1 mg of reactive MAb/ml. Reactivity was defined by assaying the MAb in an IFA with cultured C. pneumoniae. The MAb's specificity for C. pneumoniae was confirmed with three other strains of C. pneumoniae, one strain of C. trachomatis, and two strains of C. psittaci by using the same assay. The MAb was further determined to be an IgG2b(κ) by 2 different commercially available typing kits (see above). The MAb was unable to bind to purified sonicated C. pneumoniae EBs and RBs in a Western immunoblot format (data not shown). However, it was able to bind to whole-purified EBs in an immunoslot blot format (data not shown). No reaction was observed if the EBs were formalin fixed.
Phage display.
A phage display library expressing a linear, random 12-amino-acid sequence (L12) and a phage library expressing a constrained cysteine-looped architecture 7-amino-acid phage display library (C7) were used to select for peptides having the ability to bind the 8A6 MAb. A 12-amino-acid library was selected based on the idea that a 12- to 15-amino-acid length is similar in size to the complementary determining regions (CDR) in antibodies. The CDR has been shown to confer the ability to mimic many anti-idiotypic antibodies (30). A 7-amino-acid phage display library having a cysteine-constrained architecture was used to select for a target that may need additional conformational requirements. Using indirect ELISA, we identified 150 phage clones from the L12 library having binding specificity for the target 8A6 MAb. However, only 10 clones from the C7 library were isolated and screened. The C7 library proved to be difficult to biopan with the 8A6 MAb, as the amplification yields were poor (typically >104 PFU/ml versus yields in excess of 1014 PFU/ml for the L12 library clones) because of low binding as determined by indirect ELISA (data not shown). The C7 library clones were subsequently removed from the peptide candidate pool.
The DNA encoding the displayed peptides from the 8A6-selected phages was sequenced. The DNA and subsequent amino acid sequences obtained from biopanning were sorted into six groups with similar motifs and slightly variable base sequences, which are represented by the peptides in Table 1. The nucleotide and amino acid sequences of the phagotopes were used to search DNA, protein, motif, and structural databases as described above. The sequences could not be matched with any significance or biological relevance to sequences or motifs currently known. Since most of these search algorithms rely on a linear sequence, a discontinuous epitope for which one had sequences would be extremely difficult to identify. The sequences of the epitopes identified here did not match significantly with any known sequences currently in the many databases searched, including the unfinished genomes of Chlamydophila and Chlamydia species. It is therefore likely that the 8A6 epitope is a discontinuous epitope.
Reactivity by ELISA of phage clones with 8A6 MAb.
To demonstrate the specificity of phage binding to the 8A6 MAb, ELISA plates were coated with the 8A6 MAb, the immobilized MAb was incubated with representative phage clones from each selection (1010 PFU/well), and binding was measured (data not shown). The selected phage clones bound to the 8A6 MAb at optical density (OD) levels of >3.0 (on a scale of 0 to 4 using OD at 490 nm) compared with <0.8 observed with the wild-type parental phage. The most reactive 8A6 phage clones (based upon highest OD) were also screened for binding to heterologous MAbs (7D10, 3F12, 3G9.1 [genus specific], and anti-biotin MAbs). The 8A6 phage clones selected did not bind to other anti-C. pneumoniae MAbs (e.g., Pathfinder reagent) for which they were not selected.
Phage and peptides block the binding of antibody to C. pneumoniae in ELISA and IFA.
Being able to demonstrate the specific recognition of the selected peptide sequence by the selecting MAb by using competitive inhibition assays is a good indication that the selected phage binds to the antibody's variable regions. However, to demonstrate that the displayed sequence actually resembles the target epitope for which the 8A6 MAb binds, we needed to demonstrate the ability to block the MAb binding to the C. pneumoniae antigen. To determine this, we used an ELISA in which MAb and inhibitor (phage) were premixed, incubated for 2 h, and then plated onto the microtiter plates with Renografin-purified, sonicated whole-cell C. pneumoniae. Inhibition of the MAb's binding to the respective lysate indicated that the phage was successfully competing with the lysate for MAb binding.
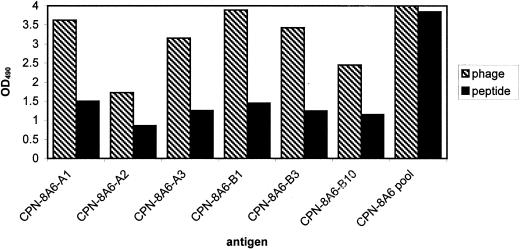
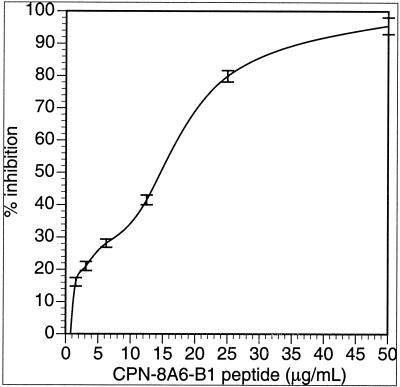
Since the phage clones demonstrated specific binding to 8A6 and an ability to compete against sonicated native C. pneumoniae material, peptides were synthesized to determine if the binding of synthetic peptide had similar or equivalent binding to the respective MAb target (Fig. 1). Several methods were used to determine binding specificity. A direct ELISA demonstrated equivalent binding of the synthetic peptides to their respective MAbs. ELISA plates with three different binding chemistries (see above) were evaluated to determine which plate provided optimal binding of the selected peptides. The maleic anhydride-activated plates and streptavidin-coated plates resulted in high background under the conditions used (data not shown) when compared with the nontreated ELISA plates and were not included in further study of the peptides. Subsequent assays were done with the Immulon II plates. The best synthetic peptides were selected by competing the peptide against the respective native C. pneumoniae lysate for binding to the target MAb. The peptides demonstrating the best binding, as determined by the percent inhibition and 50% inhibitory concentration (IC50), were CP-8A6-B1, CP-8A6-A3, CP-8A6-A2, and CP-8A6-A1, respectively (Table 2). Peptide CP-8A6-B1 was able to inhibit in a dose-dependent manner, with 95.4% at 50 μg of peptide/ml and 50% at 2.9 μg of peptide/ml, indicating that in solution under the conditions used, the peptide could act as an inhibitory mimotope of the 8A6 MAb (Fig. 2). With an IFA, the CP-8A6-B1 peptide demonstrated the ability to completely block any observable binding of 8A6 MAb to fixed, cultured C. pneumoniae cells (RBs and EBs) until a dilution of the peptide to a concentration of 100 ng/ml was reached. Because of the subjective nature of IFAs, it is difficult to standardize a competitive inhibition to obtain fluorescence measures. Instead, slides were scored from 1 to 4 such that a value of 3+ equaled the endpoint titer, and changes due to inhibition were scored empirically from a comparison with control slides. We were unable to compare the IFA with a commercially available MIF assay because the 8A6 MAb did not demonstrate the ability to bind to the proprietary antigen target presented in the commercial MIF kit produced by MRL laboratories.
FIG. 1.
Binding of matched 8A6 MAb-selected phagotopes and respective peptides to 8A6 MAb as demonstrated by ELISA. Phage test wells of the plate used for the negative control and test samples were coated with 8A6 MAb. Peptide test wells of the plate used for the negative control and test samples were coated with peptide. Wild-type phage was used as the negative phage control, and randomly generated 15-mer peptides were used as the negative peptide control. Phage binding was assayed using an anti-M13 MAb HRP conjugate. OD490, OD at 490 nm.
TABLE 2.
Percent inhibition and IC50 from competitive inhibition ELISA
| Peptide | % Inhibition | IC50 (μg/ml) |
|---|---|---|
| CP-8A6-A1 | 84.5 | 12.3 |
| CP-8A6-A2 | 83.5 | 10.6 |
| CP-8A6-A3 | 95.1 | 18.4 |
| CP-8A6-B1 | 95.4 | 14.6 |
| CP-8A6-B3 | 67.8 | 29.6 |
| CP-8A6-B10A | 57.4 | 21.5 |
FIG. 2.
Inhibition plot of 8A6 MAb reactivity to C. pneumoniae in the presence of peptide CP-8A6-B1. The plot shows the mean percent inhibition of two independent assays each having three wells per peptide concentration (each error bar represents one standard deviation) compared to peptide-free wells.
Binding of human anti-C. pneumoniae antibodies to peptide.
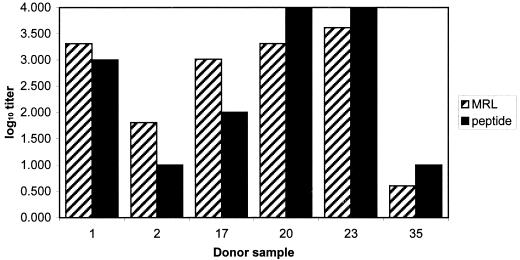
To determine if the epitope identified from the murine MAb was similar to any epitopes found in humans, normal human sera were obtained from a local donor blood bank repository and evaluated. The sera had been screened previously by MIF for C. pneumoniae, C. trachomatis, and C. psittaci and were found to be positive for only C. pneumoniae (31). With direct ELISA and a modified slot immunoblot, the human sera demonstrated the ability to bind to the peptides. The slot blot assay was used to quickly check for binding of the antibody to the various peptides. Though the assay is only qualitative, it demonstrated the ability of the human sera to bind to the peptide (data not shown). The data obtained from the peptide ELISA were compared to a direct ELISA with the sonicated C. pneumoniae as a target. The ELISA data were compared to the respective data sets obtained from MIF by using acute- and convalescent-phase sera from the same donors (the acute- and convalescent-phase designations are based on artificial nomenclature for paired sera collected at the start of the study and after 4 weeks). Though the data set was small, it showed agreement in the results; if sera had a high or low endpoint titer with MIF, these titers were similarly reflected in the endpoints observed with the peptide ELISA (Fig. 3). The geometric mean titers observed using MIF and peptide ELISA were 362.04 and 102.23, respectively.
FIG. 3.
Comparison of relative reactivity of peptide in ELISA with the MRL C. pneumoniae test assay by using the six patient serum samples (randomly coded 1, 2, 17, 20, 23, and 35). Endpoint serum titers were normalized by using log10.
DISCUSSION
The growing significance of C. pneumoniae as an emerging pathogen and its role in chronic disease make accurate diagnosis of infection important; however, the lack of any standardized and fully validated serologic diagnostic methods and problems associated with obtaining appropriately collected, paired sera make it very difficult for investigators to rapidly and efficiently diagnose C. pneumoniae infection. While PCR and other rapid DNA diagnostics have proven to be valuable tools for the detection of pathogens that are difficult to recover in artificial culture, the tests rely on the presence of DNA, which under conditions of antibiotic intervention and/or chronic infection may not be detectable. Additionally, the presence of DNA is not always indicative of current infection and must be viewed cautiously (37). Primary culture of the organism from clinical specimens is technically demanding, time consuming, and resource intensive for most clinical laboratories. Serologic detection provides the best means by which one can assess exposure to and infection with C. pneumoniae. The MIF test has been shown to be species specific with a high specificity and high sensitivity (20, 47, 51). Yet in spite of these published results, several reports indicate that the MIF test, even in expert hands, suffers from a lack of species specificity and sensitivity (3, 28, 34, 51). Additionally, the MIF test is largely subjective in nature, is difficult to standardize, requires experienced personnel, varies in method between laboratories, and may suffer from observer error (51).
An additional difficulty in working with C. pneumoniae antigens is evidence that that the epitopes have labile conformations (3, 6-8, 11, 29). This lability has severely handicapped standardized assay development. Use of immunologically reactive peptides provides the investigator with new tools to develop cost-effective assays, vaccines, and vaccine strategies.
To avoid some of the apparent difficulties encountered with labile C. pneumoniae epitopes, phage display can be used as a tool to identify an interaction between a target molecule and a receptor molecule under native conditions. Phage display is a powerful method of epitope mapping, yielding a source of ligands to antibodies and receptors. These ligands are not necessarily identical or even similar to the natural targets but mimic their binding properties. Phage display systems have been used extensively to select peptides capable of mimicking linear epitopes, nonlinear epitopes, and even nonprotein molecules (9, 21, 38, 39, 43). The use of a 12- to 15-amino-acid phage display library in research is based on the idea that this length is similar to that of the CDR in antibodies. The CDR is believed to confer mimicry capacity to many anti-idiotypic antibodies.
In this study, we used two phage display libraries to identify and characterize the epitope of the 8A6 MAb. The 8A6 MAb was determined to have specific reactivity to C. pneumoniae without any detectible cross-reaction with C. trachomatis or C. psittaci antigens. With an ELISA, a set of peptides was identified as having reactivity with the 8A6 MAb but with no linear sequence identities with any currently known protein or DNA sequences. The peptides with the strongest reactivity to the 8A6 MAb by ELISA did not share any key features with each other. Though dissimilar in sequence, they might have distinct architectural motifs. However, due to their linear nature and effectively different amino acid composition (e.g., peptide CP-8A6-A1 has several helix-breaking amino acids in its sequence and CP-8A6-B1 has a disproportionate amount of hydrophobic residues mixed with charged residues [1:2 ratio]), it is difficult to speculate about the contribution each amino acid has on binding without further study.
Though the 8A6 MAb reacted with the phage mimotopes, peptide mimotopes, cells infected with C. pneumoniae (IFA), and whole-cell lysates of C. pneumoniae (ELISA), it did not react with a proprietary commercial MIF antigen, with C. pneumoniae (CWL029) EBs or RBs on a Western immunoblot, or with formalin-fixed EBs (data not shown). This suggests that the epitope that the 8A6 MAb binds to can be destroyed or rendered inaccessible such that the MAb is no longer able to effectively bind at detectable levels. Additionally, in the present study, phage expressing a constrained 7-amino-acid loop bound by a disulfide bond did not react very well with the 8A6 MAb. The constrained library used is only one of several possible means by which one can introduce a controlled structure to the antigen. Additional methods include binding to carrier proteins or defined protein domains. This is not an unusual finding, given the conformational nature of C. pneumoniae epitopes and the above observations. Further, we found that the phage expressing the peptide sequence was more reactive than the respective synthetic peptide. This too is not an uncommon finding, especially in light of the environment and concomitant architecture in which the sequence exists in the phage tail protein. Though beyond the scope of this study, it is evident that further environmental and structural optimization with rigid, molecular backbones to support optimal presentation of the mimotope is needed.
The peptide mimotopes identified were able to bind to antibodies found in human sera that appear to be directed to C. pneumoniae. Since pedigreed sera from persons having a confirmed diagnosis of C. pneumoniae infection are difficult to obtain and because current epidemiologic data suggest that a large proportion of the human population has been exposed to C. pneumoniae, our findings with the tested human sera will need further validation. Perhaps the most important utility of the peptides described herein is the identification of a single reactive epitope that was not found to be cross-reactive with other diagnostic MAbs directed against Chlamydia spp. or Chlamydophila spp. epitopes. The peptides do not react with sera obtained from persons having very high antibody titers to C. trachomatis. Cross-reactivity between Chlamydia sp. and Chlamydophila sp. epitopes has resulted in the confounding of epidemiologic studies and patient diagnosis when culture confirmation is not possible or practical.
In a previous study, an epitope discovered by phage display, identified in both the monoclonal and polyclonal screenings, demonstrated reactivity with C. pneumoniae MIF-positive sera and not with sera from patients with other chlamydial infections and nonchlamydial respiratory infections or with normal healthy sera (MIF negative) in an ELISA (32). In our study, we also identified an epitope that reacted with both monoclonal and polyclonal sera in an ELISA.
The C. pneumoniae epitope for which 8A6 binds is currently unknown, and while phage display may potentially allow identification of the actual epitope, this is often not the case and requires additional work with capture systems. Our demonstration of the discovered peptides binding human antibody warrants further investigation of the peptide as a diagnostic assay.
In conclusion, we have screened random peptide display libraries with the MAb 8A6, raised it against C. pneumoniae, and derived peptides that contain discontinuous motifs not yet identified in sequence databases. These peptides strongly inhibit the reactivity of the 8A6 MAb with C. pneumoniae in both ELISA and IFA formats. The peptide mimotopes presumably express important contact residues and intermolecular interactions for the binding of 8A6 to the unknown C. pneumoniae epitope. The unique sequence and specificity of the peptides open up new avenues of development for C. pneumoniae diagnostic assays and investigation of potential vaccine candidates.
Acknowledgments
We thank George Smith for the generous donation of the Fd-tet library. We also thank Arthur Anderson of the U.S. Department of Agriculture, National Animal Disease Center, Ames, Iowa, for the generous contribution of C. psittaci strains.
REFERENCES
- 1.Aldous, M., S.-P. Wang, H. Foy, and T. Grayston. 1990. Chlamydia pneumoniae strain TWAR infection in Seattle children and their families 1965-79, p. 437-440. In W. Bowie, H. Caldwell, R. Jones, P.-A. Mardh, G. Ridgway, J. Schachter, W. Stamm, and M. Ward (ed.), Chlamydial infections. Proceedings of the Seventh International Symposium on Human Chlamydial Infections, Harrison Hot Springs, British Columbia, Canada, 24 to 29 June 1990. Cambridge University Press, New York, N.Y.
- 2.Barnes, R. C. 1989. Laboratory diagnosis of human chlamydial infections. Clin. Microbiol. Rev. 2:119-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bas, S., P. Muzzin, B. Ninet, J. E. Bornand, C. Scieux, and T. L. Vischer. 2001. Chlamydial serology: comparative diagnostic value of immunoblotting, microimmunofluorescence test, and immunoassays using different recombinant proteins as antigens. J. Clin. Microbiol. 39:1368-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brade, L., O. Holst, P. Kosma, Y. X. Zhang, H. Paulsen, R. Krausse, and H. Brade. 1990. Characterization of murine monoclonal and murine, rabbit, and human polyclonal antibodies against chlamydial lipopolysaccharide. Infect. Immun. 58:205-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chirgwin, K., P. M. Roblin, M. Gelling, M. R. Hammerschlag, and J. Schachter. 1991. Infection with Chlamydia pneumoniae in Brooklyn. J. Infect. Dis. 163:757-761. [DOI] [PubMed] [Google Scholar]
- 6.Christiansen, G., T. Boesen, K. Hjerno, L. Daugaard, P. Mygind, A. S. Madsen, K. Knudsen, E. Falk, and S. Birkelund. 1999. Molecular biology of Chlamydia pneumoniae surface proteins and their role in immunopathogenicity. Am. Heart J. 138:S491-S495. [DOI] [PubMed] [Google Scholar]
- 7.Christiansen, G., L. Ostergaard, and S. Birkelund. 1997. Molecular biology of the Chlamydia pneumoniae surface. Scand. J. Infect. Dis. Suppl. 104:5-10. [PubMed] [Google Scholar]
- 8.Christiansen, G., A. S. Pedersen, K. Hjerno, B. Vandahl, and S. Birkelund. 2000. Potential relevance of Chlamydia pneumoniae surface proteins to an effective vaccine. J. Infect. Dis. 181(Suppl. 3):S528-S537. [DOI] [PubMed] [Google Scholar]
- 9.De Bolle, X., T. Laurent, A. Tibor, F. Godfroid, V. Weynants, J. J. Letesson, and P. Mertens. 1999. Antigenic properties of peptidic mimics for epitopes of the lipopolysaccharide from Brucella. J. Mol. Biol. 294:181-191. [DOI] [PubMed] [Google Scholar]
- 10.Dechend, R., M. Maass, J. Gieffers, R. Dietz, C. Scheidereit, A. Leutz, and D. C. Gulba. 1999. Chlamydia pneumoniae infection of vascular smooth muscle and endothelial cells activates NF-kappaB and induces tissue factor and PAI-1 expression: a potential link to accelerated arteriosclerosis. Circulation 100:1369-1373. [DOI] [PubMed] [Google Scholar]
- 11.Essig, A., U. Simnacher, M. Susa, and R. Marre. 1999. Analysis of the humoral immune response to Chlamydia pneumoniae by immunoblotting and immunoprecipitation. Clin. Diagn. Lab. Immunol. 6:819-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Everett, K. D., R. M. Bush, and A. A. Andersen. 1999. Emended description of the order Chlamydiales, proposal of Parachlamydiaceae fam. nov. and Simkaniaceae fam. nov., each containing one monotypic genus, revised taxonomy of the family Chlamydiaceae, including a new genus and five new species, and standards for the identification of organisms. Int. J. Syst. Bacteriol. 49:415-440. [DOI] [PubMed] [Google Scholar]
- 13.Gaydos, C. A. 2000. Growth in vascular cells and cytokine production by Chlamydia pneumoniae. J. Infect. Dis. 181(Suppl. 3):S473-S478. [DOI] [PubMed] [Google Scholar]
- 14.Gnarpe, H., J. Gnarpe, and A. Lundback. 1999. Evidence of 2 waves of Chlamydia pneumoniae infection in Gavle, Sweden, 1990-96. Scand. J. Infect. Dis. 31:83-86. [DOI] [PubMed] [Google Scholar]
- 15.Grayston, J. T. 2000. Background and current knowledge of Chlamydia pneumoniae and atherosclerosis. J. Infect. Dis. 181(Suppl. 3):S402-S410. [DOI] [PubMed] [Google Scholar]
- 16.Grayston, J. T. 1994. Chlamydia pneumoniae (TWAR) infections in children. Pediatr. Infect. Dis. J. 13:675-684. [DOI] [PubMed] [Google Scholar]
- 17.Grayston, J. T. 1992. Infections caused by Chlamydia pneumoniae strain TWAR. Clin. Infect. Dis. 15:757-761. [DOI] [PubMed] [Google Scholar]
- 18.Grayston, J. T., L. A. Campbell, C. C. Kuo, C. H. Mordhorst, P. Saikku, D. H. Thom, and S. P. Wang. 1990. A new respiratory tract pathogen: Chlamydia pneumoniae strain TWAR. J. Infect. Dis. 161:618-625. [DOI] [PubMed] [Google Scholar]
- 19.Grayston, J. T., V. K. Diwan, M. Cooney, and S. P. Wang. 1989. Community- and hospital-acquired pneumonia associated with Chlamydia TWAR infection demonstrated serologically. Arch. Intern. Med. 149:169-173. [PubMed] [Google Scholar]
- 20.Grayston, J. T., S. P. Wang, C. C. Kuo, and L. A. Campbell. 1989. Current knowledge on Chlamydia pneumoniae, strain TWAR, an important cause of pneumonia and other acute respiratory diseases. Eur. J. Clin. Microbiol. Infect. Dis. 8:191-202. [DOI] [PubMed] [Google Scholar]
- 21.Grothaus, M. C., N. Srivastava, S. L. Smithson, T. Kieber-Emmons, D. B. Williams, G. M. Carlone, and M. A. Westerink. 2000. Selection of an immunogenic peptide mimic of the capsular polysaccharide of Neisseria meningitidis serogroup A using a peptide display library. Vaccine 18:1253-1263. [DOI] [PubMed] [Google Scholar]
- 22.Jokinen, C., L. Heiskanen, H. Juvonen, S. Kallinen, M. Kleemola, M. Koskela, M. Leinonen, P. R. Ronnberg, P. Saikku, M. Sten, A. Tarkiainen, H. Tukiainen, K. Pyorala, and P. H. Makela. 2001. Microbial etiology of community-acquired pneumonia in the adult population of 4 municipalities in eastern Finland. Clin. Infect. Dis. 32:1141-1154. [DOI] [PubMed] [Google Scholar]
- 23.Kalayoglu, M. V., D. L. Hahn, and G. I. Byrne. 1999. Chlamydia infection and pneumonia, p. 302. In L. J. Paradise, H. Friedman, and M. Bendinelli (ed.), Opportunistic intracellular bacteria and immunity: infectious agents and pathogenesis. Plenum Press, New York, N.Y.
- 24.Kaukoranta-Tolvanen, S. S., T. Ronni, M. Leinonen, P. Saikku, and K. Laitinen. 1996. Expression of adhesion molecules on endothelial cells stimulated by Chlamydia pneumoniae. Microb. Pathog. 21:407-411. [DOI] [PubMed] [Google Scholar]
- 25.Kaukoranta-Tolvanen, S. S., A. M. Teppo, K. Laitinen, P. Saikku, K. Linnavuori, and M. Leinonen. 1996. Growth of Chlamydia pneumoniae in cultured human peripheral blood mononuclear cells and induction of a cytokine response. Microb. Pathog. 21:215-221. [DOI] [PubMed] [Google Scholar]
- 26.Kaul, R., and W. M. Wenman. 2001. Chlamydia pneumoniae facilitates monocyte adhesion to endothelial and smooth muscle cells. Microb. Pathog. 30:149-155. [DOI] [PubMed] [Google Scholar]
- 27.Kenna, J. G., G. N. Major, and R. S. Williams. 1985. Methods for reducing non-specific antibody binding in enzyme-linked immunosorbent assays. J. Immunol. Methods 85:409-419. [DOI] [PubMed] [Google Scholar]
- 28.Kern, D. G., M. A. Neill, and J. Schachter. 1993. A seroepidemiologic study of Chlamydia pneumoniae in Rhode Island. Evidence of serologic cross-reactivity. Chest 104:208-213. [DOI] [PubMed] [Google Scholar]
- 29.Knudsen, K., A. S. Madsen, P. Mygind, G. Christiansen, and S. Birkelund. 1999. Identification of two novel genes encoding 97- to 99-kilodalton outer membrane proteins of Chlamydia pneumoniae. Infect. Immun. 67:375-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meloen, R. H., W. C. Puijk, and J. W. Slootstra. 2000. Mimotopes: realization of an unlikely concept. J. Mol. Recognit. 13:352-359. [DOI] [PubMed] [Google Scholar]
- 31.Messmer, T. O., J. Martinez, F. Hassouna, E. R. Zell, W. Harris, S. Dowell, and G. M. Carlone. 2001. Comparison of two commercial microimmunofluorescence kits and an enzyme immunoassay kit for detection of serum immunoglobulin G antibodies to Chlamydia pneumoniae. Clin. Diagn. Lab. Immunol. 8:588-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naidu, B. R., Y. F. Ngeow, L. F. Wang, L. Chan, Z. J. Yao, and T. Pang. 1998. An immunogenic epitope of Chlamydia pneumoniae from a random phage display peptide library is reactive with both monoclonal antibody and patients sera. Immunol. Lett. 62:111-115. [DOI] [PubMed] [Google Scholar]
- 33.Ouchi, K., T. Nakazawa, M. Karita, and Y. Kanehara. 1994. Prevalence of Chlamydia pneumoniae in acute lower respiratory infection in the pediatric population in Japan. Acta Paediatr. Jpn. 36:256-260. [DOI] [PubMed] [Google Scholar]
- 34.Ozanne, G., and J. Lefebvre. 1992. Specificity of the microimmunofluorescence assay for the serodiagnosis of Chlamydia pneumoniae infections. Can. J. Microbiol. 38:1185-1189. [DOI] [PubMed] [Google Scholar]
- 35.Parmley, S. F., and G. P. Smith. 1988. Antibody-selectable filamentous fd phage vectors: affinity purification of target genes. Gene 73:305-318. [DOI] [PubMed] [Google Scholar]
- 36.Pau, C.-P., B. B. Plikaytis, G. M. Carlone, and I. M. Warner. 1988. Purification, partial characterization, and seroreactivity of a genuswide 60-kilodalton Legionella protein antigen. J. Clin. Microbiol. 26:67-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Persing, D. H. (ed.). 1996. PCR protocols for emerging infectious diseases. A supplement to Diagnostic molecular microbiology: principles and applications. ASM Press, Washington, D.C.
- 38.Phalipon, A., A. Folgori, J. Arondel, G. Sgaramella, P. Fortugno, R. Cortese, P. J. Sansonetti, and F. Felici. 1997. Induction of anti-carbohydrate antibodies by phage library-selected peptide mimics. Eur. J. Immunol. 27:2620-2625. [DOI] [PubMed] [Google Scholar]
- 39.Pincus, S. H., M. J. Smith, H. J. Jennings, J. B. Burritt, and P. M. Glee. 1998. Peptides that mimic the group B streptococcal type III capsular polysaccharide antigen. J. Immunol. 160:293-298. [PubMed] [Google Scholar]
- 40.Porath, A., F. Schlaeffer, and D. Lieberman. 1997. The epidemiology of community-acquired pneumonia among hospitalized adults. J. Infect. 34:41-48. [DOI] [PubMed] [Google Scholar]
- 41.Schito, G. C., G. Grazi, B. Dainelli, G. Catamo, G. Satta, R. L. Grillo, S. Stefani, and G. Russo. 1994. Incidence of lower respiratory tract infections caused by Mycoplasma, Chlamydia and Legionella: an Italian multicenter survey. J. Chemother. 6:319-321. [DOI] [PubMed] [Google Scholar]
- 42.Smith, G. P., and J. K. Scott. 1993. Libraries of peptides and proteins displayed on filamentous phage. Methods Enzymol. 217:228-257. [DOI] [PubMed] [Google Scholar]
- 43.Srivastava, N., J. L. Zeiler, S. L. Smithson, G. M. Carlone, E. W. Ades, J. S. Sampson, S. E. Johnson, T. Kieber-Emmons, and M. A. Westerink. 2000. Selection of an immunogenic and protective epitope of the PsaA protein of Streptococcus pneumoniae using a phage display library. Hybridoma 19:23-31. [DOI] [PubMed] [Google Scholar]
- 44.Storey, C., M. Lusher, P. Yates, and S. Richmond. 1993. Evidence for Chlamydia pneumoniae of non-human origin. J. Gen. Microbiol. 139:2621-2626. [DOI] [PubMed] [Google Scholar]
- 45.Verkooyen, R. P., N. A. Van Lent, S. A. Mousavi Joulandan, R. J. Snijder, J. M. van den Bosch, H. P. Van Helden, and H. A. Verbrugh. 1997. Diagnosis of Chlamydia pneumoniae infection in patients with chronic obstructive pulmonary disease by micro-immunofluorescence and ELISA. J. Med. Microbiol. 46:959-964. [DOI] [PubMed] [Google Scholar]
- 46.Verkooyen, R. P., D. Willemse, S. C. A. M. Hiep-van Casteren, S. A. Mousavi Joulandan, R. J. Snijder, J. M. M. van den Bosch, H. P. T. van Helden, M. F. Peeters, and H. A. Verbrugh. 1998. Evaluation of PCR, culture, and serology for diagnosis of Chlamydia pneumoniae respiratory infections. J. Clin. Microbiol. 36:2301-2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang, S. P., and J. T. Grayston. 1986. Microimmunofluorescence serological studies with the TWAR organism, p. 329-332. In J. D. Oriel, G. Ridgway, J. Schachter, D. Taylor-Robinson, and M. Ward (ed.), Chlamydial infections. Proceedings of the Sixth International Symposium on Human Chlamydial Infections, Sanderstead, Surrey, 15 to 21 June 1986. Cambridge University Press, New York, N.Y.
- 48.Wang, S.-P., J. T. Grayston, E. R. Alexander, and K. K. Holmes. 1975. Simplified microimmunofluorescence test with trachoma-lymphogranuloma venereum (Chlamydia trachomatis) antigens for use as a screening test for antibody. J. Clin. Microbiol. 1:250-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Westerwoudt, R. J., A. M. Naipal, and C. M. Harrison. 1984. Improved fusion technique. II. Stability and purity of hybrid clones. J. Immunol. Methods. 68:89-101. [DOI] [PubMed] [Google Scholar]
- 50.Wong, K. H., S. K. Skelton, and Y. K. Chan. 1992. Efficient culture of Chlamydia pneumoniae with cell lines derived from the human respiratory tract. J. Clin. Microbiol. 30:1625-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wong, Y. K., J. M. Sueur, C. H. Fall, J. Orfila, and M. E. Ward. 1999. The species specificity of the microimmunofluorescence antibody test and comparisons with a time resolved fluoroscopic immunoassay for measuring IgG antibodies against Chlamydia pneumoniae. J. Clin. Pathol. 52:99-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wubbel, L., L. Muniz, A. Ahmed, M. Trujillo, C. Carubelli, C. McCoig, T. Abramo, M. Leinonen, and G. H. McCracken, Jr. 1999. Etiology and treatment of community-acquired pneumonia in ambulatory children. Pediatr. Infect. Dis. J. 18:98-104. [DOI] [PubMed] [Google Scholar]