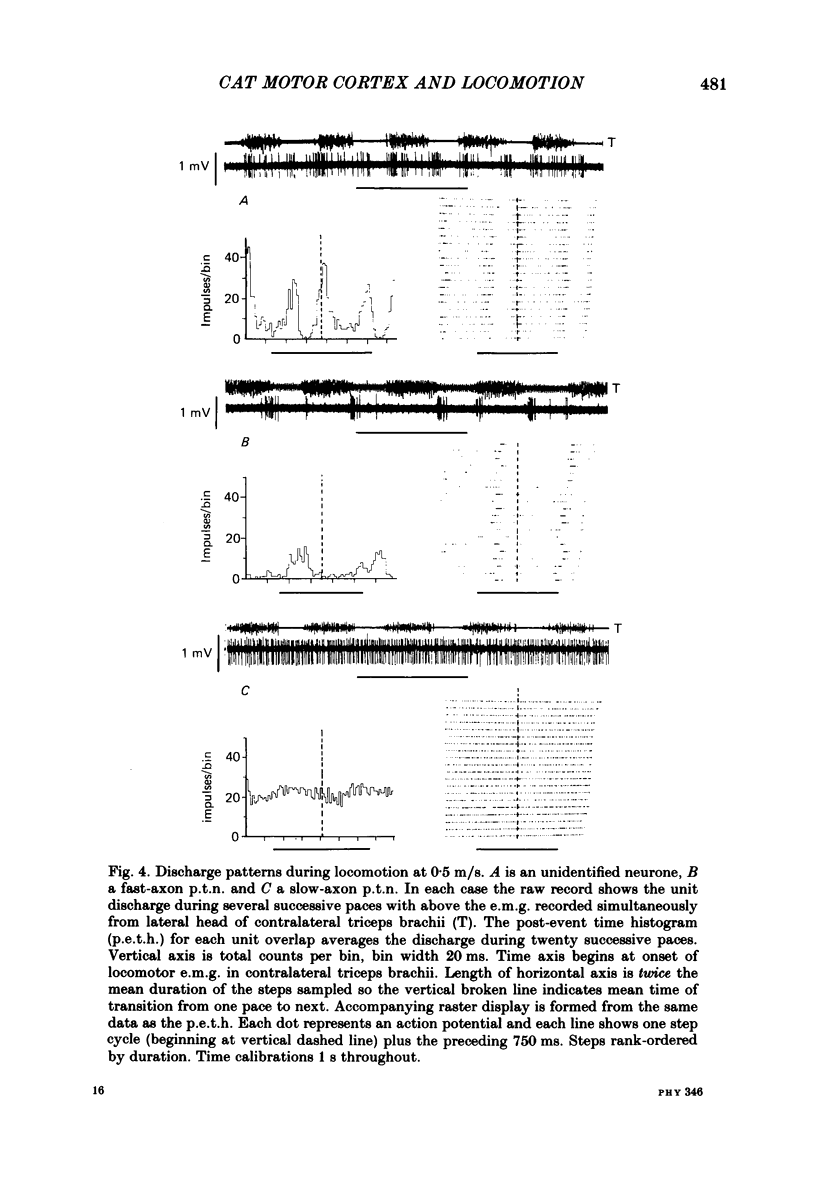
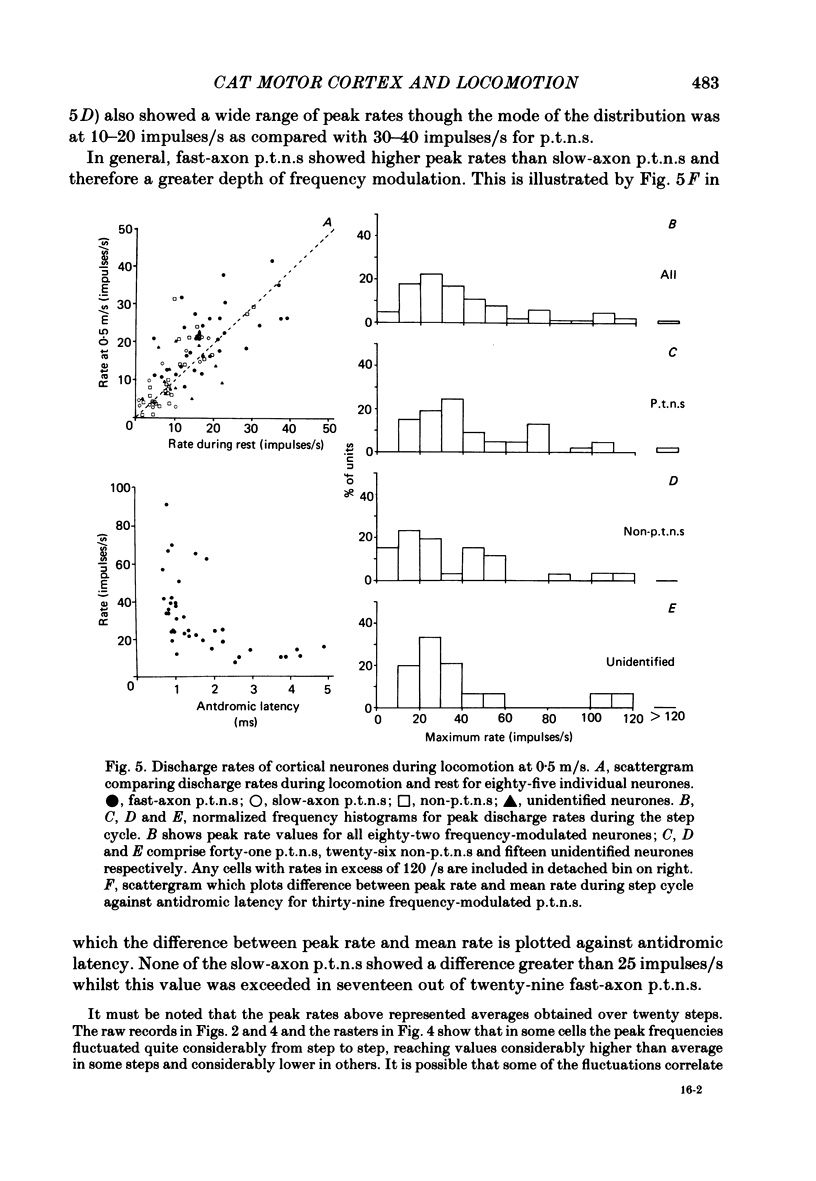
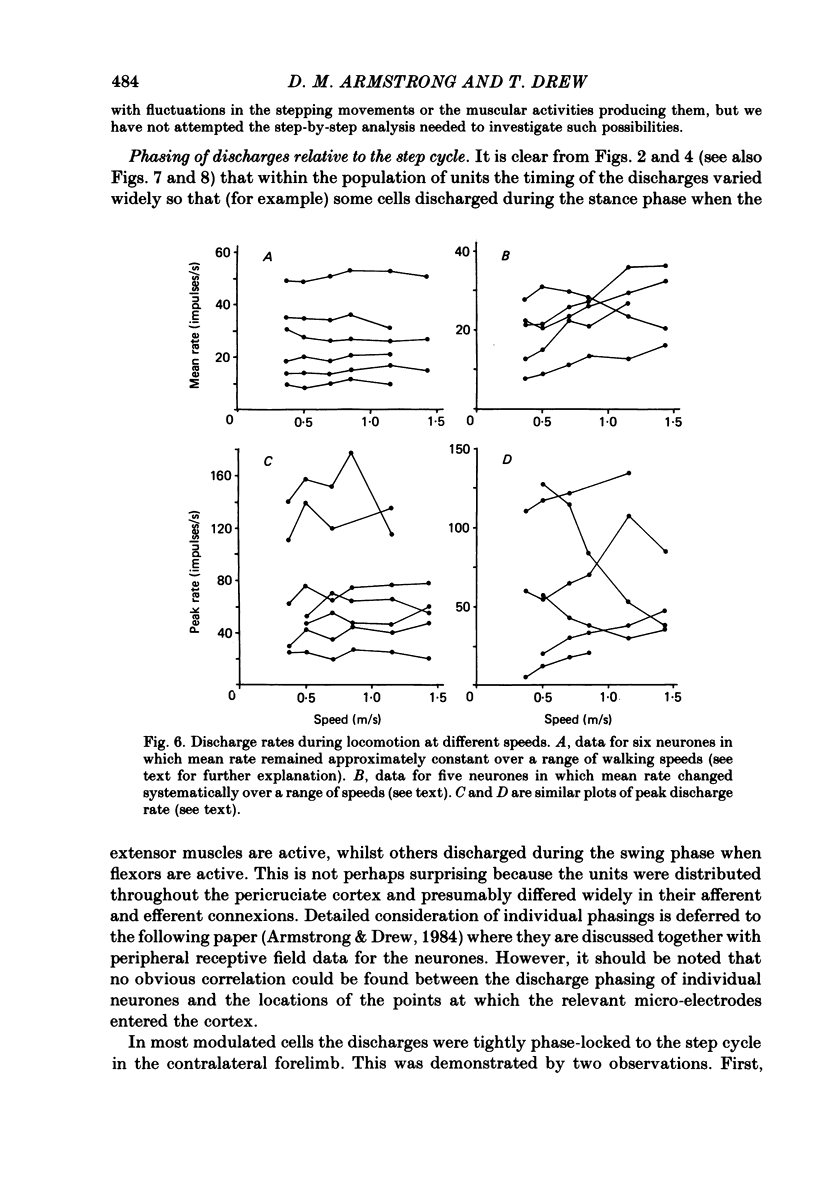
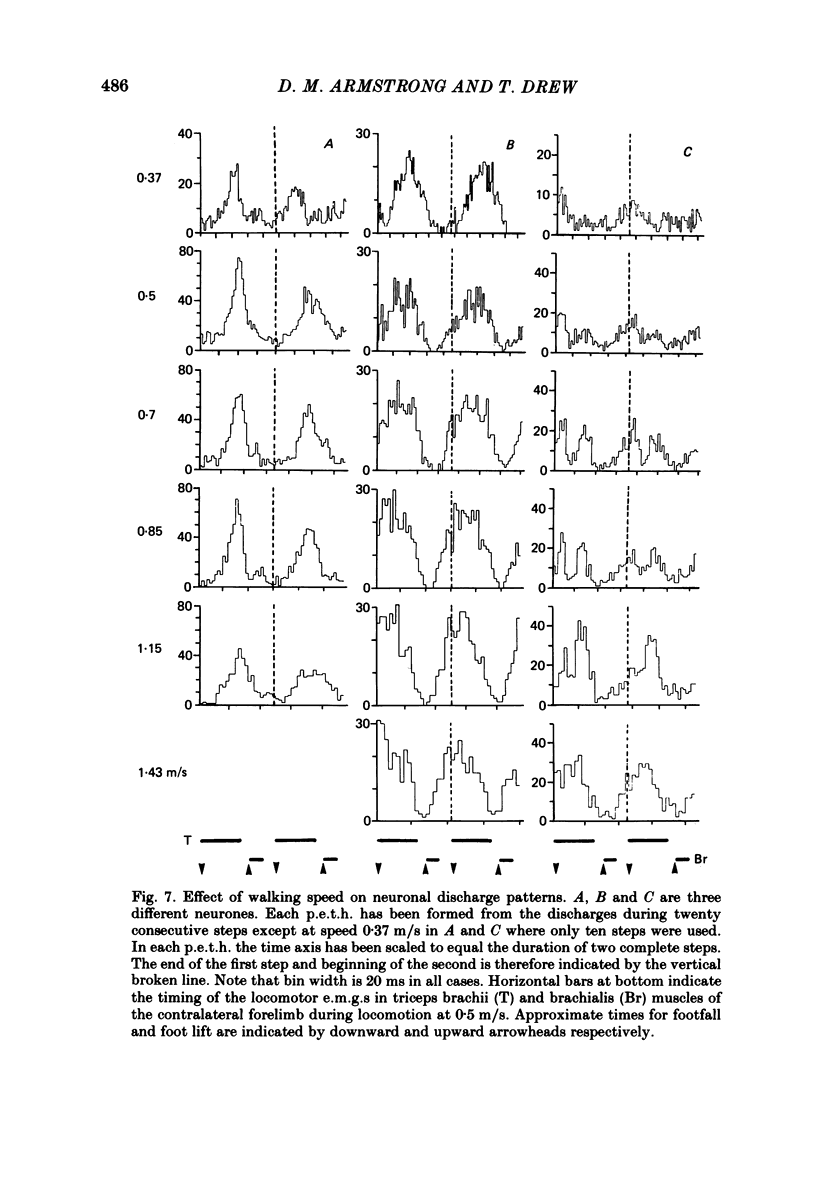
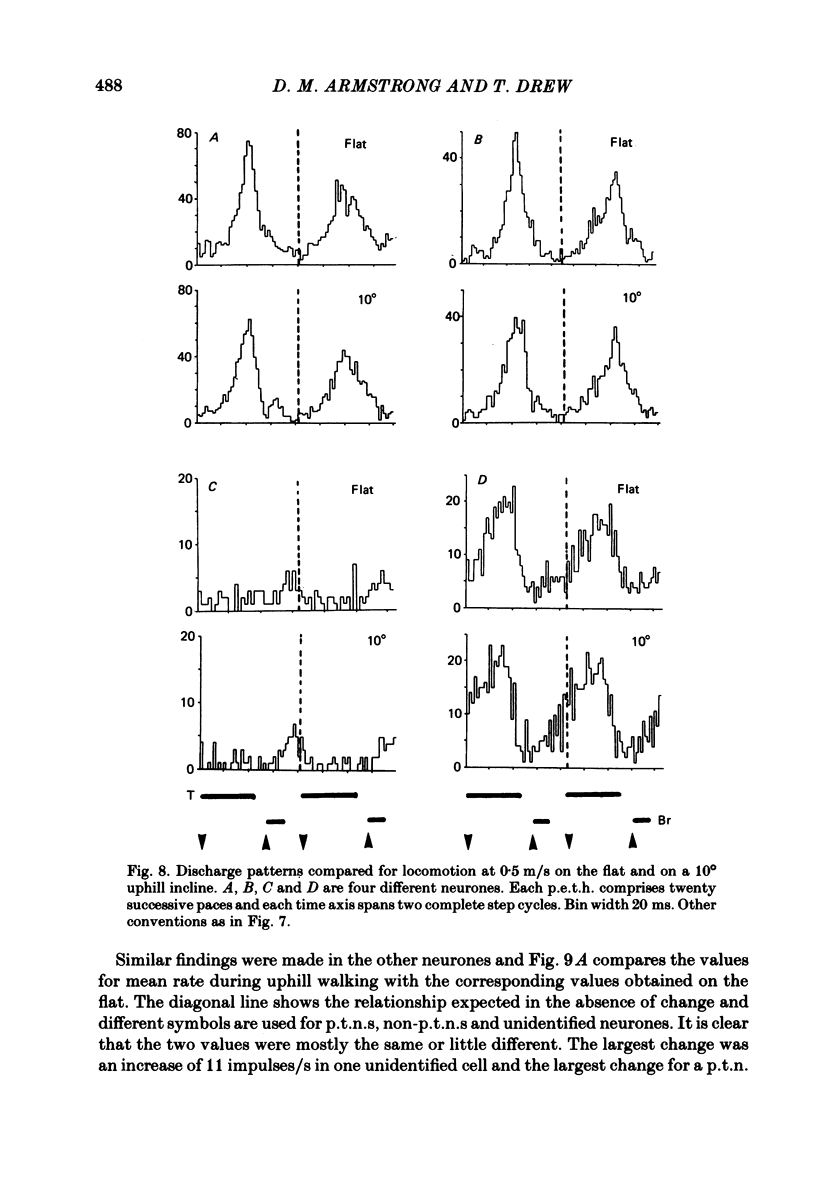
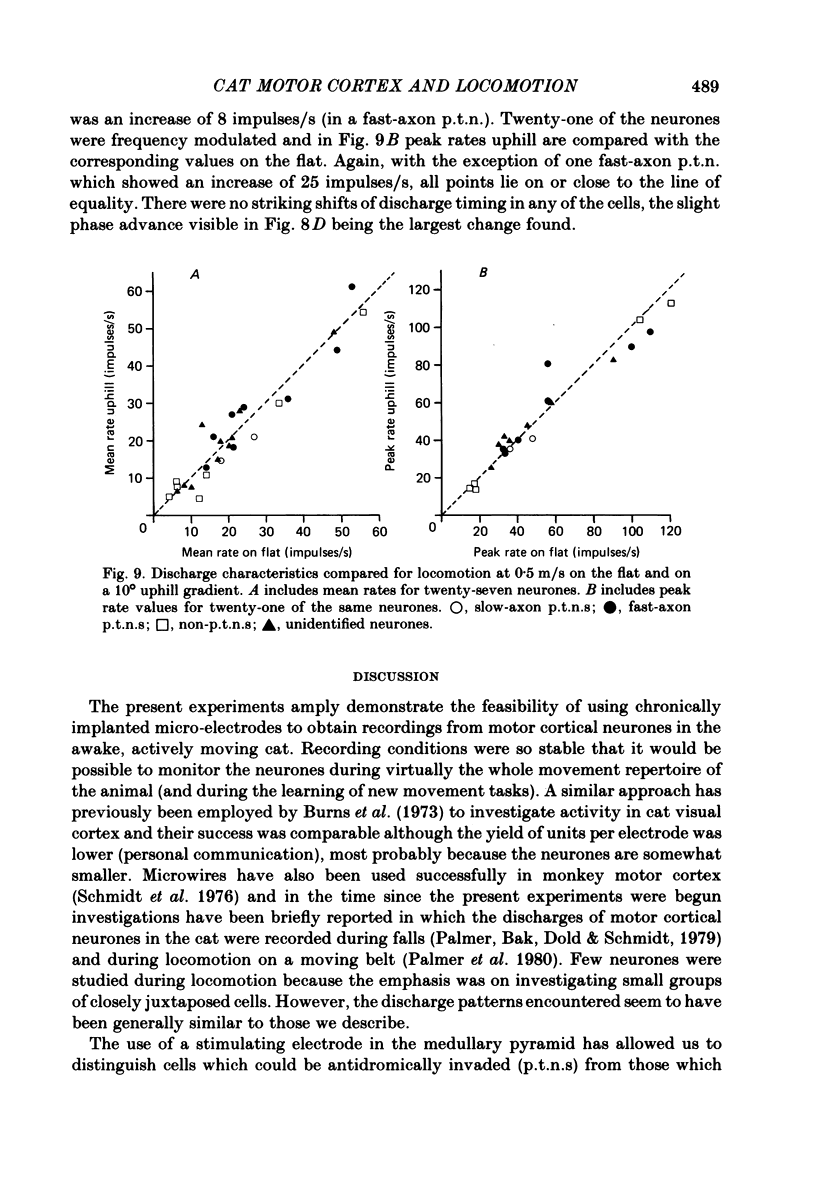
Abstract
A method is described for chronically implanting fine flexible microwires into cat motor cortex, which permitted extracellular recordings to be made from 165 single neurones. Most units were recordable for 12 h and some for up to 2 days. Of the neurones tested, 57% were shown to project to the medullary pyramid (pyramidal tract neurones, p.t.n.s). Antidromic latencies corresponded to a range of conduction velocities from 63 to 9 m/s. In the animal at rest neurones discharged at rates from 0.5 to 44 impulses/s. During locomotion at 0.5 m/s (a slow walk) 56% of cells discharged faster than at rest and 80% showed frequency modulations time-locked to the step cycle. Most fired one discrete burst of impulses per step or one peak period superimposed on a maintained discharge. In different cells peak activity occurred at widely different times during the step cycle. A few cells peaked twice per step. Peak rates (averaged over twenty steps) ranged from 10 to over 120 impulses/s, the values for most slow-axon p.t.n.s (conduction velocity less than 21 m/s) being lower than for any of the fast-axon p.t.n.s. For locomotion at speeds between 0.37 and 1.43 m/s a roughly linear relationship existed between discharge rate and speed in 14% of cells. However, the changes were modest and in most cells both mean rate and peak rate were unrelated to speed. In some cells discharge phasing was fixed (relative to the step cycle in the contralateral forelimb); in others there were progressive phase shifts (or more complex changes) as speed increased. During locomotion up a 10 degrees incline discharge phasings were the same as on the flat in all of the twenty-seven neurones studied and most showed no substantial change in mean rate or peak rate (although there were substantial increases in limb muscle electromyogram amplitudes).
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong D. M., Drew T. Locomotor-related neuronal discharges in cat motor cortex compared with peripheral receptive fields and evoked movements. J Physiol. 1984 Jan;346:497–517. doi: 10.1113/jphysiol.1984.sp015037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. M., Rawson J. A. Responses of neurones in nucleus interpositus of the cerebellum to cutaneous nerve volleys in the awake cat. J Physiol. 1979 Apr;289:403–423. doi: 10.1113/jphysiol.1979.sp012744. [DOI] [PMC free article] [PubMed] [Google Scholar]
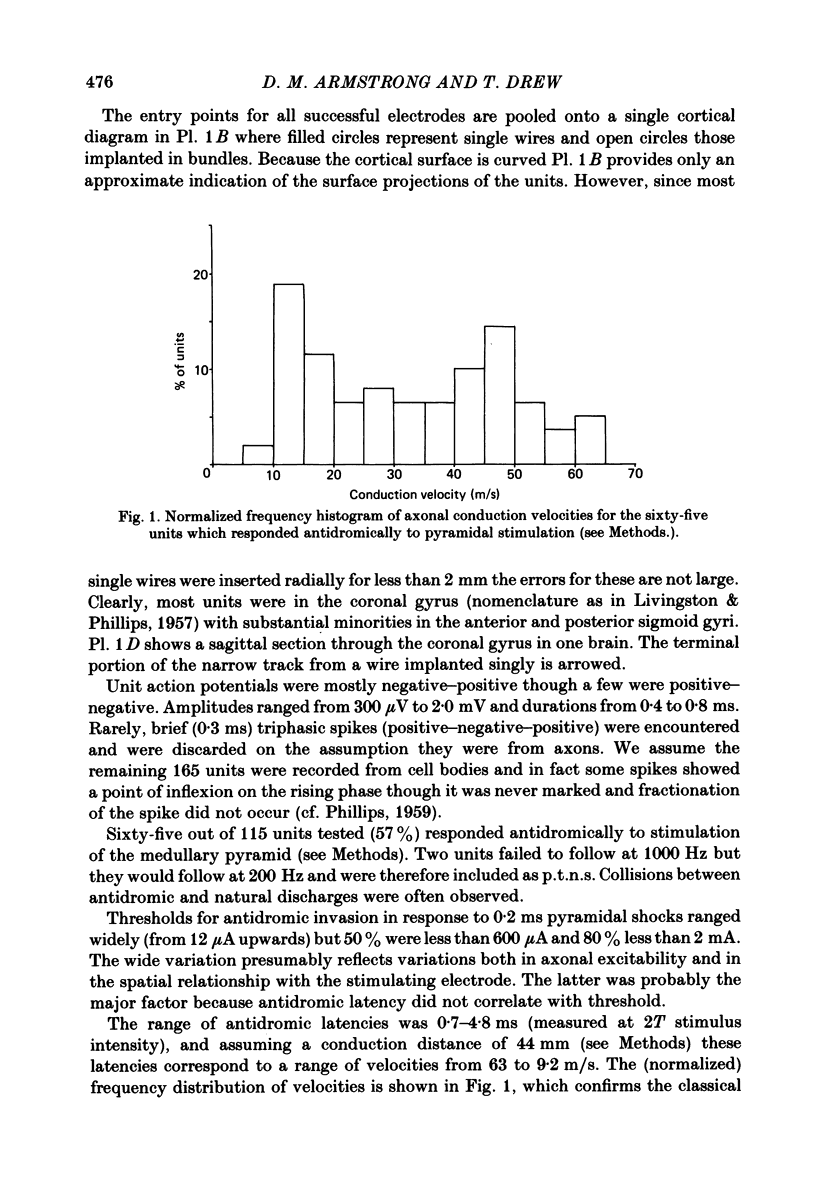
- EVARTS E. V. RELATION OF DISCHARGE FREQUENCY TO CONDUCTION VELOCITY IN PYRAMIDAL TRACT NEURONS. J Neurophysiol. 1965 Mar;28:216–228. doi: 10.1152/jn.1965.28.2.216. [DOI] [PubMed] [Google Scholar]
- Eidelberg E. Consequences of spinal cord lesions upon motor function, with special reference to locomotor activity. Prog Neurobiol. 1981;17(3):185–202. doi: 10.1016/0301-0082(81)90013-7. [DOI] [PubMed] [Google Scholar]
- Evarts E. V. Relation of pyramidal tract activity to force exerted during voluntary movement. J Neurophysiol. 1968 Jan;31(1):14–27. doi: 10.1152/jn.1968.31.1.14. [DOI] [PubMed] [Google Scholar]
- Grillner S. Locomotion in vertebrates: central mechanisms and reflex interaction. Physiol Rev. 1975 Apr;55(2):247–304. doi: 10.1152/physrev.1975.55.2.247. [DOI] [PubMed] [Google Scholar]
- Illert M., Lundberg A., Tanaka R. Integration in descending motor pathways controlling the forelimb in the cat. 1. Pyramidal effects on motoneurones. Exp Brain Res. 1976 Dec 22;26(5):509–519. doi: 10.1007/BF00238824. [DOI] [PubMed] [Google Scholar]
- LANCE J. W., MANNING R. L. Origin of the pyramidal tract in the cat. J Physiol. 1954 May 28;124(2):385–399. doi: 10.1113/jphysiol.1954.sp005115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIVINGSTON A., PHILLIPS C. G. Maps and thresholds for the sensorimotor cortex of the cat. Q J Exp Physiol Cogn Med Sci. 1957 Apr;42(2):190–205. doi: 10.1113/expphysiol.1957.sp001250. [DOI] [PubMed] [Google Scholar]
- LUNDBERG A., VOORHOEVE P. Effects from the pyramidal tract on spinal reflex arcs. Acta Physiol Scand. 1962 Nov-Dec;56:201–219. doi: 10.1111/j.1748-1716.1962.tb02498.x. [DOI] [PubMed] [Google Scholar]
- Nieoullon A., Rispal-Padel L. Somatotopic localization in cat motor cortex. Brain Res. 1976 Apr 9;105(3):405–422. doi: 10.1016/0006-8993(76)90590-4. [DOI] [PubMed] [Google Scholar]
- Orlovsky G. N. Activity of rubrospinal neurons during locomotion. Brain Res. 1972 Nov 13;46:99–112. doi: 10.1016/0006-8993(72)90008-x. [DOI] [PubMed] [Google Scholar]
- Orlovsky G. N. Activity of vestibulospinal neurons during locomotion. Brain Res. 1972 Nov 13;46:85–98. doi: 10.1016/0006-8993(72)90007-8. [DOI] [PubMed] [Google Scholar]
- Orlovsky G. N. The effect of different descending systems on flexor and extensor activity during locomotion. Brain Res. 1972 May 26;40(2):359–371. doi: 10.1016/0006-8993(72)90139-4. [DOI] [PubMed] [Google Scholar]
- PHILLIPS C. G. Actions of antidromic pyramidal volleys on single Betz cells in the cat. Q J Exp Physiol Cogn Med Sci. 1959 Jan;44(1):1–25. doi: 10.1113/expphysiol.1959.sp001364. [DOI] [PubMed] [Google Scholar]
- Palmer C. A microwire technique for recording single neurons in unrestrained animals. Brain Res Bull. 1978 May-Jun;3(3):285–289. doi: 10.1016/0361-9230(78)90129-6. [DOI] [PubMed] [Google Scholar]
- Porter R. Relationship of the discharges of cortical neurones to movement in free-to-move monkeys. Brain Res. 1972 May 12;40(1):39–43. doi: 10.1016/0006-8993(72)90103-5. [DOI] [PubMed] [Google Scholar]
- Russell D. F., Zajac F. E. Effects of stimulating Deiters' nucleus and medial longitudinal fasciculus on the timing of the fictive locomotor rhythm induced in cats by DOPA. Brain Res. 1979 Nov 30;177(3):588–592. doi: 10.1016/0006-8993(79)90478-5. [DOI] [PubMed] [Google Scholar]
- Schmidt E. M., Bak M. J., McIntosh J. S. Long-term chronic recording from cortical neurons. Exp Neurol. 1976 Sep;52(3):496–506. doi: 10.1016/0014-4886(76)90220-x. [DOI] [PubMed] [Google Scholar]
- Takahashi K. Slow and fast groups of pyramidal tract cells and their respective membrane properties. J Neurophysiol. 1965 Sep;28(5):908–924. doi: 10.1152/jn.1965.28.5.908. [DOI] [PubMed] [Google Scholar]
- Udo M., Oda Y., Tanaka K., Horikawa J. Cerebellar control of locomotion investigated in cats: discharges from Deiters' neurones, EMG and limb movements during local cooling of the cerebellar cortex. Prog Brain Res. 1976;44:445–459. doi: 10.1016/S0079-6123(08)60751-7. [DOI] [PubMed] [Google Scholar]