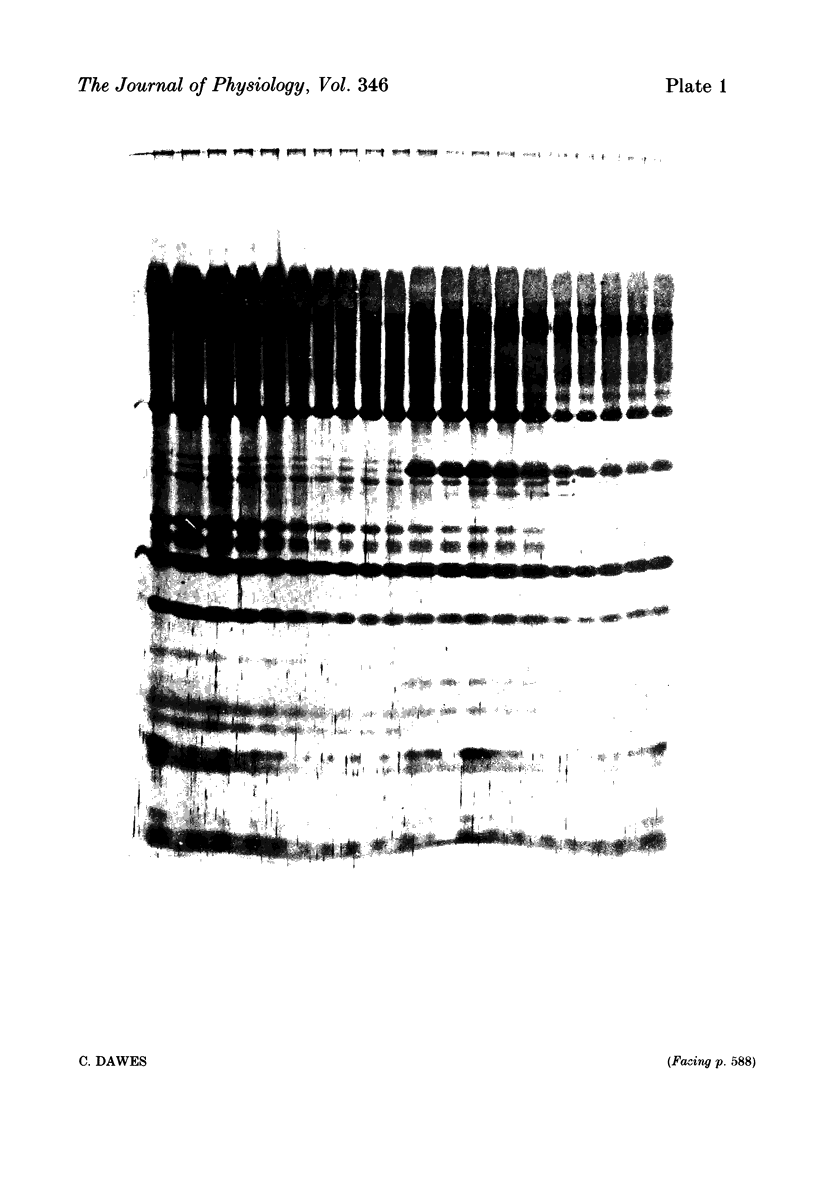
Abstract
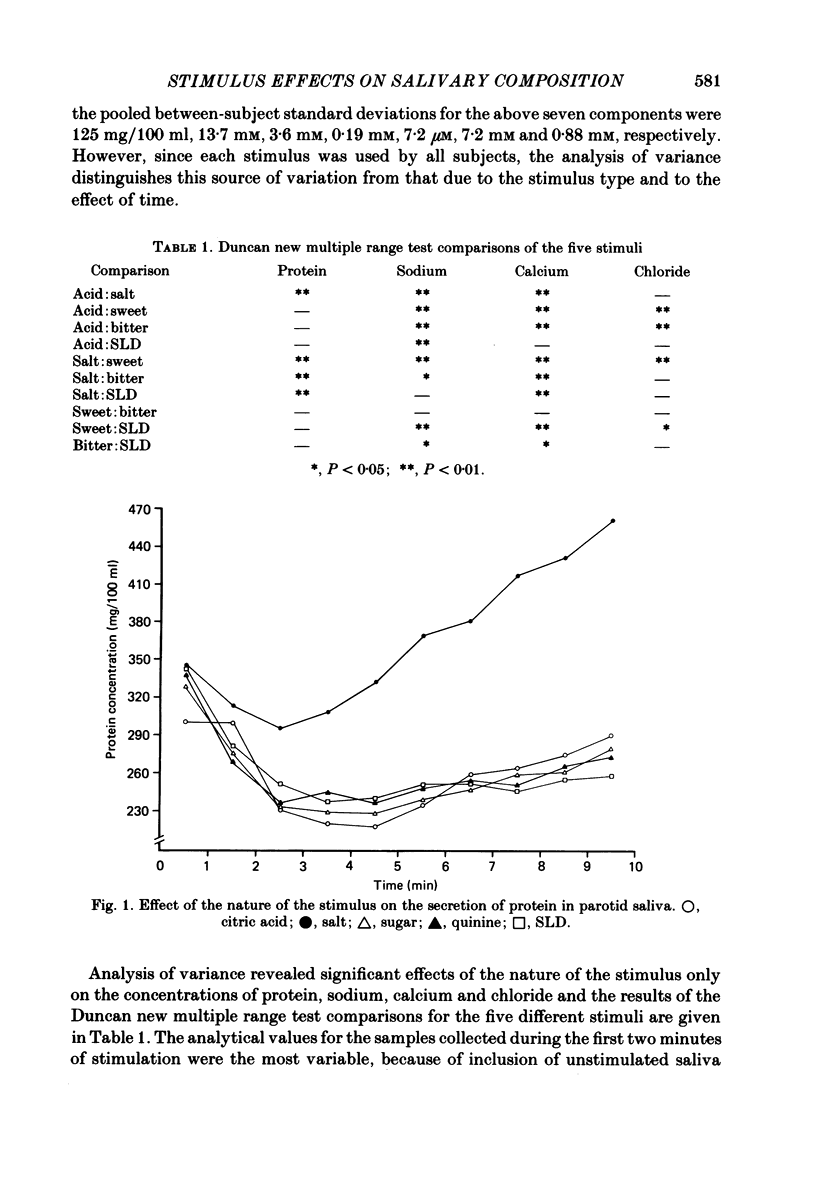
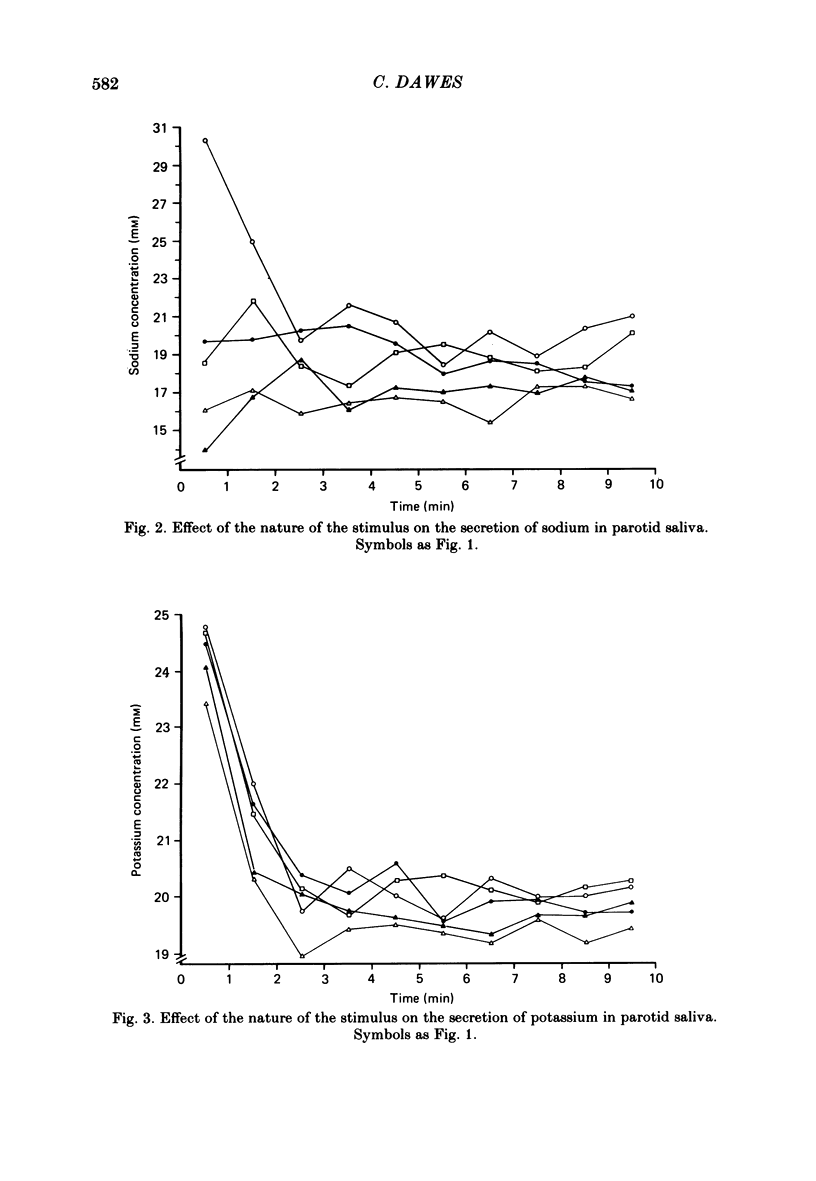
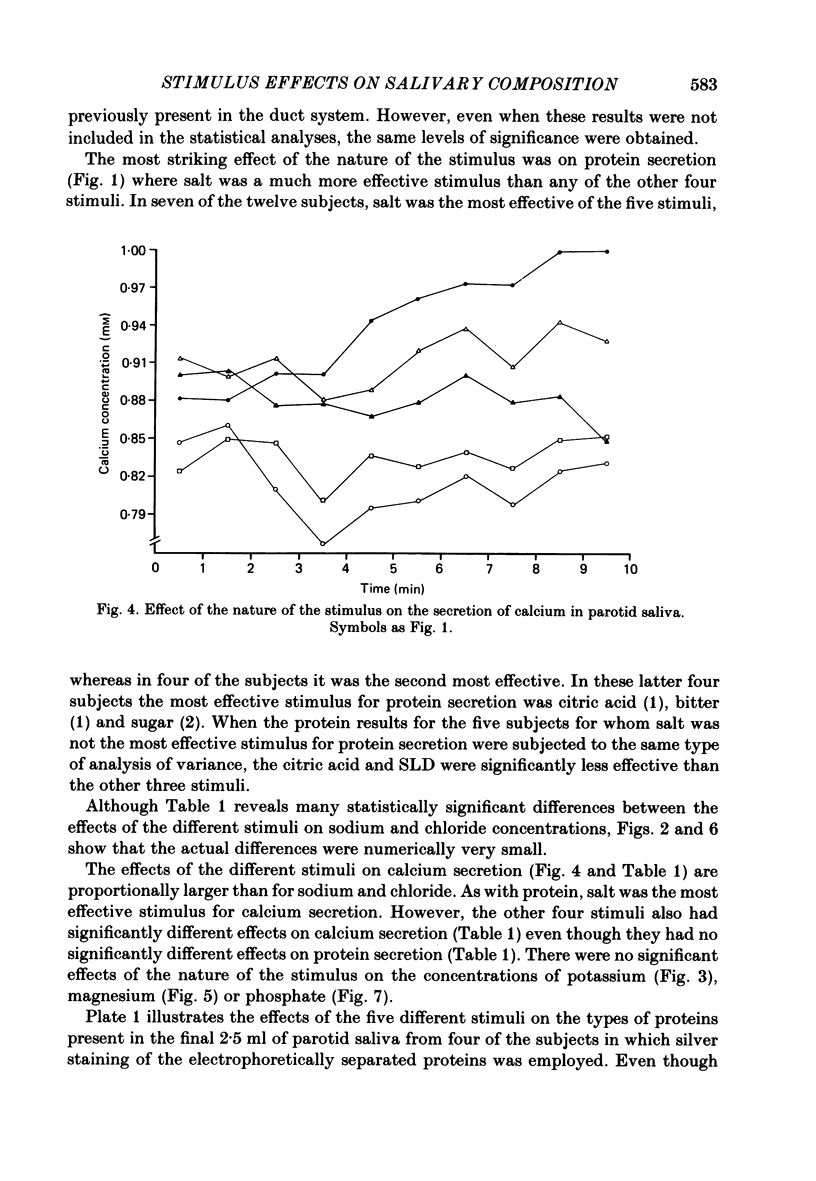
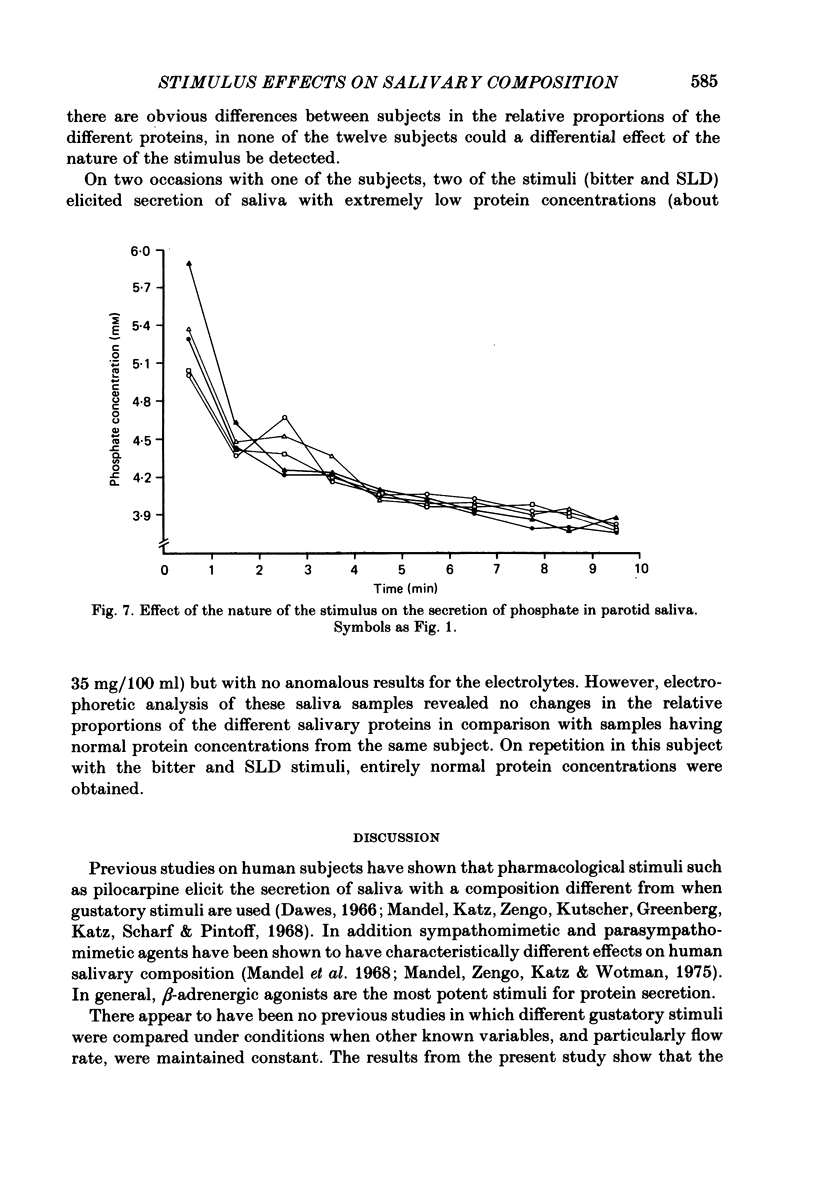
Twelve subjects collected ten 1 min samples and then a 2.5 ml sample of parotid saliva at a constant flow rate on five separate days with citric acid, salt, sugar, quinine sulphate, and sour lemon drops as gustatory stimuli. The ten 1 min samples were analysed for protein and electrolyte content and the final 2.5 ml sample was used for electrophoretic separation of the different salivary proteins. In most subjects, salt elicited the secretion of saliva with a much higher protein concentration than did the other stimuli, but none of the stimuli differentially influenced the relative proportions of the different proteins secreted. There were several small but statistically significant effects of the nature of the stimulus on the concentrations of sodium, calcium and chloride, but not on potassium, magnesium or phosphate. Since the nature of the gustatory stimulus can influence the composition of saliva, salivary composition could be influenced by the nature of the diet.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell C. Transmission from vasoconstrictor and vasodilator nerves to single smooth muscle cells of the guinea-pig uterine artery. J Physiol. 1969 Dec;205(3):695–708. doi: 10.1113/jphysiol.1969.sp008991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell C., Vogt M. Release of endogenous noradrenaline from an isolated muscular artery. Release of endogenous noradrenaline from an isolated muscular artery. J Physiol. 1971 Jun;215(2):509–520. doi: 10.1113/jphysiol.1971.sp009482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan J. A., Osher J. V. Distribution of norepinephrine released from adrenergic motor terminals in arterial wall. Eur J Pharmacol. 1970;13(1):55–58. doi: 10.1016/0014-2999(70)90182-2. [DOI] [PubMed] [Google Scholar]
- Biamino G., Kruckenberg P. Synchronization and conduction of excitation in the rat aorta. Am J Physiol. 1969 Aug;217(2):376–382. doi: 10.1152/ajplegacy.1969.217.2.376. [DOI] [PubMed] [Google Scholar]
- COTLOVE E., TRANTHAM H. V., BOWMAN R. L. An instrument and method for automatic, rapid, accurate, and sensitive titration of chloride in biologic samples. J Lab Clin Med. 1958 Mar;51(3):461–468. [PubMed] [Google Scholar]
- Caldwell R. C., Pigman W. Changes in protein and glycoprotein concentrations in human submaxillary saliva under various stimulatory conditions. Arch Oral Biol. 1966 Apr;11(4):437–450. doi: 10.1016/0003-9969(66)90108-7. [DOI] [PubMed] [Google Scholar]
- Casteels R., Kitamura K., Kuriyama H., Suzuki H. Excitation-contraction coupling in the smooth muscle cells of the rabbit main pulmonary artery. J Physiol. 1977 Sep;271(1):63–79. doi: 10.1113/jphysiol.1977.sp011990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung D. W. Spontaneous and evoked excitatory junction potentials in rat tail arteries. J Physiol. 1982 Jul;328:449–459. doi: 10.1113/jphysiol.1982.sp014276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWES C., JENKINS G. N. THE EFFECTS OF DIFFERENT STIMULI ON THE COMPOSITION OF SALIVA IN MAN. J Physiol. 1964 Jan;170:86–100. doi: 10.1113/jphysiol.1964.sp007315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes C., Chebib F. S. The influence of previous stimulation and the day of the week on the concentrations of protein and the main electrolytes in human parotid saliva. Arch Oral Biol. 1972 Sep;17(9):1289–1301. doi: 10.1016/0003-9969(72)90162-8. [DOI] [PubMed] [Google Scholar]
- Dawes C., Dowse C. M., Knull H. R. Stop-flow effects on human salivary composition and hydrostatic pressures. Arch Oral Biol. 1980;25(4):251–256. doi: 10.1016/0003-9969(80)90030-8. [DOI] [PubMed] [Google Scholar]
- Dawes C. The composition f human saliva secreted in response to a gustatory stimulus and to pilocaprine. J Physiol. 1966 Mar;183(2):360–368. doi: 10.1113/jphysiol.1966.sp007870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes C. The effects of exercise on protein and electrolyte secretion in parotid saliva. J Physiol. 1981 Nov;320:139–148. doi: 10.1113/jphysiol.1981.sp013940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes C. The effects of flow rate and duration of stimulation on the condentrations of protein and the main electrolytes in human parotid saliva. Arch Oral Biol. 1969 Mar;14(3):277–294. doi: 10.1016/0003-9969(69)90231-3. [DOI] [PubMed] [Google Scholar]
- Dawes C. The secretion of magnesium and calcium in human parotid saliva. Caries Res. 1967;1(4):333–342. doi: 10.1159/000259533. [DOI] [PubMed] [Google Scholar]
- Denniss A. R., Schneyer L. H., Sucanthapree C., Young J. A. Actions of adrenergic agonists on isolated excretory ducts of submandibular glands. Am J Physiol. 1978 Dec;235(6):F548–F556. doi: 10.1152/ajprenal.1978.235.6.F548. [DOI] [PubMed] [Google Scholar]
- Garland C. J., Keatinge W. R. Constrictor actions of acetylcholine, 5-hydroxytryptamine and histamine on bovine coronary artery inner and outer muscle. J Physiol. 1982 Jun;327:363–376. doi: 10.1113/jphysiol.1982.sp014236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham J. M., Keatinge W. R. Differences in sensitivity to vasoconstrictor drugs within the wall of the sheep carotid artery. J Physiol. 1972 Mar;221(2):477–492. doi: 10.1113/jphysiol.1972.sp009763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Neild T. O. Localization of specialized noradrenaline receptors at neuromuscular junctions on arterioles of the guinea-pig. J Physiol. 1981;313:343–350. doi: 10.1113/jphysiol.1981.sp013669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Neild T. O. Some properties of spontaneous excitatory junction potentials recorded from arterioles of guinea-pigs. J Physiol. 1980 Jun;303:43–60. doi: 10.1113/jphysiol.1980.sp013269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D. Neuromuscular transmission in arterioles of guinea-pig submucosa. J Physiol. 1977 Dec;273(1):263–275. doi: 10.1113/jphysiol.1977.sp012093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman M. E., Surprenant A. M. Some properties of the excitatory junction potentials recorded from saphenous arteries of rabbits. J Physiol. 1979 Feb;287:337–351. doi: 10.1113/jphysiol.1979.sp012663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keatinge W. R. Electrical and mechanical response of arteries to stimulation of sympathetic nerves. J Physiol. 1966 Aug;185(3):701–715. doi: 10.1113/jphysiol.1966.sp008011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keatinge W. R., Torrie C. Action of sympathetic nerves of inner and outer muscle of sheep carotid artery, and effect of pressure on nerve distribution. J Physiol. 1976 Jun;257(3):699–712. doi: 10.1113/jphysiol.1976.sp011393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mandel I. D., Katz R., Zengo A., Kutscher A. H., Greenberg R. A., Katz S., Scharf R., Pintoff A. The effect of pharmacologic agents on salivary secretion and composition in man. I. Pilocarpine, atropine and anticholinesterases. J Oral Ther Pharmacol. 1967 Nov;4(3):192–199. [PubMed] [Google Scholar]
- Mekata F. Current spread in the smooth muscle of the rabbit aorta. J Physiol. 1974 Oct;242(1):143–155. doi: 10.1113/jphysiol.1974.sp010698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekata F. Electrical current-induced contraction in the smooth muscle of the rabbit aorta. J Physiol. 1981 Aug;317:149–161. doi: 10.1113/jphysiol.1981.sp013818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekata F. Electrophysiological properties of the smooth muscle cell membrane of the dog coronary artery. J Physiol. 1980 Jan;298:205–212. doi: 10.1113/jphysiol.1980.sp013076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekata F. Electrophysiological studies of the smooth muscle cell membrane of the rabbit common carotid artery. J Gen Physiol. 1971 Jun;57(6):738–751. doi: 10.1085/jgp.57.6.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekata F., Nagatsu I. Electrophysiology and innervation of the smooth muscle of dog inferior vena cava. J Physiol. 1982 Dec;333:201–211. doi: 10.1113/jphysiol.1982.sp014449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekata F., Niu H. Biophysical effects of adrenaline on the smooth muscle of the rabbit common carotid artery. J Gen Physiol. 1972 Jan;59(1):92–102. doi: 10.1085/jgp.59.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekata F. Rectification in the smooth muscle cell membrane of rabbit aorta. J Physiol. 1976 Jun;258(2):269–278. doi: 10.1113/jphysiol.1976.sp011419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merril C. R., Dunau M. L., Goldman D. A rapid sensitive silver stain for polypeptides in polyacrylamide gels. Anal Biochem. 1981 Jan 1;110(1):201–207. doi: 10.1016/0003-2697(81)90136-6. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Rees P. M. The distribution of biogenic amines in the carotid bifurcation region. J Physiol. 1967 Nov;193(2):245–253. doi: 10.1113/jphysiol.1967.sp008355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SU C., BEVAN J. A., URSILLO R. C. ELECTRICAL QUIESCENCE OF PULMONARY ARTERY SMOOTH MUSCLE DURING SYMPATHOMIMETIC STIMULATION. Circ Res. 1964 Jul;15:26–27. doi: 10.1161/01.res.15.1.20. [DOI] [PubMed] [Google Scholar]
- Speirs R. L., Herring J., Cooper W. D., Hardy C. C., Hind C. R. The influence of sympathetic activity and isoprenaline on the secretion of amylase from the human parotid gland. Arch Oral Biol. 1974 Sep;19(9):747–752. doi: 10.1016/0003-9969(74)90161-7. [DOI] [PubMed] [Google Scholar]
- Surprenant A. A comparative study of neuromuscular transmission in several mammalian muscular arteries. Pflugers Arch. 1980 Jul;386(1):85–91. doi: 10.1007/BF00584192. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P. M., Verbeuren T. J., Webb R. C. Local modulation of adrenergic neuroeffector interaction in the blood vessel well. Physiol Rev. 1981 Jan;61(1):151–247. doi: 10.1152/physrev.1981.61.1.151. [DOI] [PubMed] [Google Scholar]
- Windeler A. S., Jr, Shannon I. L. The effect of flow rate on parotid fluid calcium and protein levels. Arch Oral Biol. 1966 Oct;11(10):1043–1045. doi: 10.1016/0003-9969(66)90205-6. [DOI] [PubMed] [Google Scholar]



