Abstract
Inflammatory bowel disease (IBD) is a chronic, debilitating disorder of uncertain and perhaps multiple etiologies. It is believed to be due in part to disregulation of the immune system. Neuroimmune interactions may be involved in induction or maintenance of IBD. In the present study, we examined the potential role of a neurotransmitter, substance P, in a mouse model of IBD. We found that binding sites for substance P, and more specifically, neurokinin-1 receptors, were upregulated in intestinal tissue of mice with IBD-like syndrome. Dosing of mice with LY303870, a neurokinin-1 receptor antagonist, reduced the severity of IBD, and treatment of mice with preexisting IBD allowed partial healing of lesions. We hypothesize that blocking the binding of substance P to the neurokinin-1 receptor interrupts the inflammatory cascade that triggers and maintains intestinal lesions of IBD.
Inflammatory bowel disease (IBD) affects up to 200 people per 100,000 in North America and Europe. This debilitating chronic illness results in a decreased quality of life for affected patients as well as increased health care costs (41). The predominant histological characteristic of this disease is a recurrent, chronic inflammation of segments of the gut, characterized by a dramatic influx of activated leukocytes in the lamina propria (10, 41). Unfortunately, although it is now widely recognized that an immune imbalance is at the root of this syndrome, the factors leading to the initiation of the imbalance, and the maintenance of the inflammatory response, are largely unknown (10, 41). While many studies have focused on the immune aspects of IBD, the contributions of the nervous system have not been extensively investigated.
In previous studies, we have used a mouse model to study the contributions of intestinal flora, and various subsets of lymphocytes to the pathogenesis of IBD (39, 45-47). Mombaerts et al. (35) reported the spontaneous development of a wasting syndrome in T-cell receptor alpha-deficient (TCRα−) mice characterized by development of weight loss, diarrhea, and chronic colitis and typhlitis in mice 4 to 12 months old. The intestinal lesions in these mice closely resemble those of IBD in humans (35). We have modified this system to establish a highly reproducible model of IBD-like disease (39, 45, 46). We reported that all TCRα− mice infected with Cryptosporidium parvum (an intracellular parasite that causes a transient diarrhea in many species, including humans [13]) became persistently infected with the parasite (44) and developed IBD-like lesions as early as 3 weeks postinfection (45). We are thus able to induce IBD-like lesions reliably and rapidly. This model is also interesting from a comparative point of view, since C. parvum is known to induce an acute relapse of IBD in patients with preexisting IBD, as was discovered during a large cryptosporidiosis outbreak in Milwaukee in 1993 (32).
Several studies have proposed that the nervous system can modulate the inflammatory response of the gut (23, 33). One factor mediating this interaction may be a neurotransmitter, substance P, one of a family of short peptides collectively known as neurokinins. Substance P has potent proinflammatory effects, including vasodilatation, plasma extravasation, leukocyte activation, and enhanced immunoglobulin production. Substance P exerts its effects on cells by activating cyclic AMP following binding to G-protein-linked receptors, which are characterized by seven highly conserved trans-membrane spanning domains with unique N- and C-terminal amino acid sequences (for recent reviews, see references 30 and 37). There are three major neurokinin receptors; substance P binds preferentially to the neurokinin-1 receptor (NK1-R), while neurokinin A (or substance K) and neurokinin B (or neuromedin K) preferentially bind to the neurokinin-2 and -3 receptors, respectively.
To determine whether substance P plays a role in the pathogenesis of IBD-like lesions in C. parvum-infected TCRα− mice, we studied the effects of an NK1-R antagonist, LY303870 (a kind gift of the Lilly Corporation) on the development of IBD-like lesions in these mice. LY303870 is a highly specific, potent, and long-lasting antagonist of substance P at the NK1-R (15, 16, 24). It is well absorbed orally and crosses the blood-brain barrier, effectively blocking both peripheral and central NK1-Rs.
In the present study, we demonstrated that receptors for substance P are upregulated in the intestinal lamina propria of C. parvum-infected TCRα− mice with IBD. Administration of LY303870 to mice prior to induction of IBD reduced the severity of lesions compared to that in nontreated control mice. Treatment with LY303870 also modulated lesions in mice with preexisting IBD. These data suggest that release of substance P contributes to the inflammatory response of the gut in IBD.
MATERIALS AND METHODS
Animals.
All animal procedures were approved by the Institutional Committee on Animal Use and Care at Iowa State University. Breeding pairs of TCRα− mice were purchased from Jackson Laboratories (Bar Harbor, Maine). A breeding colony was established and maintained at the Iowa State University Small Animal Facility, Ames, for the generation of mice used in the experiments. Breeding stocks of TCRα− mice were housed in HEPA-filtered hooded cages and received autoclaved and acidified tap water and autoclaved rodent chow (5L79 Purina rat & mouse 18% chow, manufactured for Charles River Laboratories; PMI Nutrition International Inc., Brentwood, Mo.). Breeding colonies were free of Helicobacter sp . infection. Mice used in experiments received the same diet as the breeding colonies.
Establishment of IBD in TCRα− mice.
Purified oocysts were isolated from feces collected from calves experimentally inoculated with C. parvum by a method previously described (19). Mice were infected with C. parvum by giving them 103 (autoradiography study) or 104 (LY303870 studies) oocysts suspended in 100 ml of 0.15 M phosphate-buffered saline solution. Oocysts were administered by oral gavage with a feeding needle at 1 week of age.
Detection of substance P receptors by autoradiography.
Mice from at least five different litters born on different dates were used in this study. One-week-old TCRα− mice were infected with 103 viable C. parvum oocysts as described above. All mice were euthanatized at 8 weeks of age. In previous studies, C. parvum-infected TCRα− mice had severe IBD by 8 weeks of age (45). The cecum and proximal colon were collected immediately after euthanasia, flushed clean with phosphate-buffered saline, immersed in embedding matrix (Tissue Tek O.T.C. Compound; Electron Microscopy Sciences), immediately frozen, and stored at −80°C. Tissue sections (20 μm thick) were cut on a cryostat, dehydrated, and stored at −80°C until use.
The autoradiography protocol has been described in detail elsewhere (40). In brief, after washing to remove endogenous neurokinins, tissue sections were incubated for 1 h at room temperature (22 to 24°C) in a solution containing 20 pM [I125]Bolton-Hunter-labeled substance P (125I-BHSP) in a solution containing 50 mM Tris-HCl, 3 mM MnCl2, bovine serum albumin (200 mg/liter), chymostatin (2 mg/liter), leupeptin (4 mg/liter), and bacitracin (40 mg/liter). As controls for the specificity of binding, adjacent sections from the same tissues were incubated in the same solution of 125I-BHSP with the addition of an excess (1 mM) of either unlabeled substance P, the N terminus of substance P [SP(1-4)], the C terminus of substance P [SP(4-11)], or the specific NK1-R agonist Ac-[Arg, Sar, Met(O2)]-substance P(6-11) (Ac-SP). After incubation, sections were washed, air dried, and apposed to Hyperfilm-βmax (Amersham) in a radiographic cassette. Films were developed manually after a suitable exposure time. Tissue sections were then fixed in 4% phosphate-buffered paraformaldehyde, stained with hematoxylin and eosin, and covered with a coverslip. Sections were compared to the corresponding autoradiographs to determine the type of tissue(s) to which 125I-BHSP was bound. All tissues were processed identically, and representative slides from each group were exposed to the same sheets of film to eliminate any differences arising from processing.
Determination of NK1-R mRNA expression.
Mice from five different litters born on different dates were used in this study. Eight-week-old infected and uninfected TCRα− mice were killed, and the cecal tip, including the cecal tonsil, was removed, rinsed in phosphate-buffered saline, and stored in RNAlater (Ambion). Tissues were homogenized, and total RNA was extracted using an RNeasy mini kit (Qiagen) following the manufacturer's instructions, with a DNase digestion step. Isolated RNA was reverse transcribed using random hexamers and murine leukemia virus reverse transcriptase (Promega), and the resulting cDNA was quantified. All samples were then processed simultaneously, using the same master mix containing Taq polymerase (0.015 U/μl; Red Jump Start; Sigma), PCR buffer with1.5 mM MgCl2 (Sigma), a 0.2 mM concentration of each deoxynucleoside triphosphate (Sigma), 4 pM concentrations of murine NK1-R specific primers (9) (5′-CCA ACA CCT CCA CCA ACA CTT CTG-3′ and 5′-GCC ACA GCT GTC ATG GAG TAG AT-3′), and commercial 18S competimers and primers (at an 8:2 ratio; Quantum RNA 18S classic kit; Ambion). Each individual reaction tube thus contained 24 μl of the master mix and 1 μl (1 to 1.5 μg) of sample cDNA. PCR was performed using a Stratagene Robocycler Gradient 40 thermal cycler, using a modified touchdown procedure. The conditions for amplification were as follows: after an initial 3 min and 20 s at 94°C, all incubations lasted 1 min, with the exception of the last extension step (5 min). Annealing temperatures were held at 65°C for 2 cycles, followed by 2 cycles at 64°C, and 34 cycles at 63°C. All PCR products were electrophoresed simultaneously on a single 2% agarose gel, stained with SybrGold, and viewed under UV light, and the image was captured by a COHU high-performance charge-coupled device camera and digitized using Scion Image software on a Digital Imagers Gel Imaging system. The relative amounts of the NK1-R and 18S PCR products were quantified using Scion Image software (calculated by the formula optic density/pixel × number of pixels in band), and the ratios of NK1-R/18S product from the cecal tips of infected and uninfected TCRα− mice were compared.
Effects of LY303870 on the development of IBD.
Two litters of TCRα− mice born on different dates were used in this study. Two hours following oral inoculation of 1-week-old TCRα− mice with 104 C. parvum oocysts, one half of each litter of mice was given LY303870 (30 mg/kg of body weight) dissolved in sterile endotoxin-free water, by oral gavage. The remaining mice in each litter served as nontreated controls and received the same volume of sterile endotoxin-free water by oral gavage. All mice were given LY303870 or vehicle once daily by oral gavage for the remainder of the study. All mice were killed at 4 weeks of age, and tissues were examined for C. parvum infection and IBD lesions as described above.
Mice were monitored weekly for infection by examining feces for the presence of C. parvum. Fecal pellets were collected by placing individual mice into beakers until they defecated. Fresh pellets were then smeared onto glass slides, stained with carbol fuchsin, and examined for the presence of C. parvum oocysts. All mice were killed at the end of each study, and tissues were collected to determine C. parvum infection status and the development of IBD-like lesions (45). The ileum, cecum, and proximal colon were removed; fixed in 10% formalin; embedded in paraffin; and processed for light microscopic examination. Histological sections were cut at a thickness of 4 μm, stained with hematoxylin and eosin, and examined microscopically for C. parvum. Individual mice were scored as positive or negative for the presence of C. parvum; mice were considered positive if parasites were present in any of the three tissues. Slides were examined for IBD-like lesions in a coded fashion so the examiner was unaware of the treatments the mice had received. Cecal tissue from all mice and colon tissue from some mice were examined and scored for degree of lymphocytic infiltration in the lamina propria, gland dilatation, and epithelial cell hyperplasia. Each of these three factors was assigned a score from 0 (no significant lesions) to 5 (severe lesions); criteria are described in Table 1.
TABLE 1.
Scoring of intestinal lesionsa
| Score (lesion severity) | Lamina propria infiltration by lymphocytes and macrophages | Epithelial gland dilatation | Epithelial cell hyperplasia |
|---|---|---|---|
| 0 (normal) | Normal | Normal | Normal |
| 1 (minimal) | Low numbers of lymphocytes and macrophages within lamina propria, <5 cells/field of view | One or more glands dilated but empty, lined by normal epithelium | Focal area of glands lined by tightly packed enterocytes |
| 2 (mild) | 5-10 cells/field of view | One or more glands dilated, most empty or containing cellular debris, lined by normal epithelium | Multifocal areas of slightly elongated glands lined by tightly packed enterocytes |
| 3 (moderate) | 10-20 cells/field of view, mild expansion of space between glands by cellular infiltrate | Multifocal dilated glands, most containing cellular debris; multifocal glands lined by a flattened epithelium | Multifocal areas of elongate and slightly tortuous glands lined by tightly packed enterocytes with several mitotic figures |
| 4 (marked) | 20-50 cells/field of view, glands widely separated by expanded lamina propria due to cellular infiltrate | Multifocal dilated glands, most lined by flattened or denuded basement membrane and containing cellular debris | Multifocal, coalescent areas of elongate, tortuous glands, lined by tightly packed enterocytes with numerous mitotic figures |
| 5 (severe) | Inflammatory cells efface normal lamina propria | Diffuse gland dilatation with glands lined by flattened epithelium or denuded basement membrane and containing cellular debris | Diffuse elongate and irregular tortuous glands lined by tightly packed enterocytes with numerous mitotic figures |
Tissues examined and scored included the ileum, cecum, and colon of TCRα− mice. For lamina propria infiltration, sections were scored at a final magnification of ×200.
Effect of LY303870 on preexisting IBD.
In a second study, TCRα− mice were infected with 104 C. parvum oocysts at 1 week of age and allowed to develop IBD. Two litters of TCRα− mice born on different dates were used in this study. At 6 weeks of age, one half of the mice in each litter began receiving daily doses of LY303870 as above, and the other half received water only. All mice were killed at 8 weeks of age, and tissues were examined for C. parvum infection and IBD lesions as described above.
Statistical evaluation.
The ratios of NK1-R/18S reverse transcription (RT)-PCR product optic densities were compared using a one-tailed rank sum test. The numbers of treated and nontreated mice infected with C. parvum were compared using Fisher's exact test. Differences between scores for severity of IBD based on histological results were evaluated using Student's t test (with Welch correction where appropriate) or the Mann-Whitney U test for nonparametric data. The results for each parameter were compared between treated and nontreated groups for cecum and colon individually. Composite scores comparing the means of all three parameters for each of the two tissues were also compared between treated and nontreated groups for cecum and colon. Results were considered significant when P was <0.05.
RESULTS
Autoradiography.
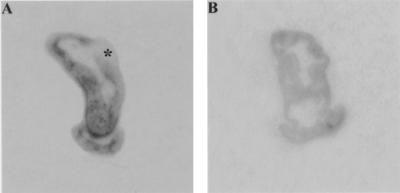
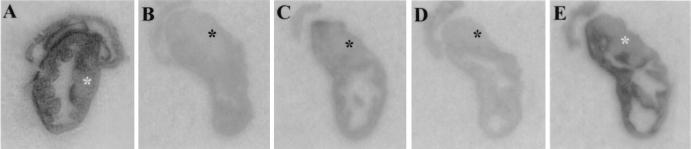
We first sought to determine whether enteric inflammation in our model of IBD was accompanied by an upregulation of substance P binding sites in enteric lymphoid aggregates. We examined cecal sections from 11 8-week-old C. parvum-infected TCRα− mice with IBD and 14 age-matched TCRα− noninfected mice without IBD. In noninfected TCRα− mice, binding sites for 125I-BHSP were most numerous in the lamina propria and smooth muscle layers, as previously reported (8). Binding sites for 125I-BHSP were similar in the ceca of nonaffected TCRα− mice and age-matched C57BL/6 mice (n = 3). Nonspecific binding was minimal in all cases. Figure 1 presents a representative comparison of binding sites for 125I-BHSP in cecal tissue from healthy and IBD-affected TCRα− mice. There were more binding sites for 125I-BHSP in tissue from IBD-affected compared with nonaffected TCRα− mice (compare areas of dark staining in Fig. 1A and B). These binding sites corresponded to areas of lymphocytic infiltration in the submucosa of the cecum and to the luminal side of the circular muscle. The increase in 125I-BHSP was most marked in the cecum, where IBD-like lesions first appear and are most severe in this model (34). As shown in Fig. 2, incubation with an excess of either nonlabeled substance P or an excess of Ac-SP (a specific NK1-R agonist) inhibited binding of 125I-BHSP (compare Fig. 2B and C to Fig. 2A). In addition, incubation with an excess of the C-terminal, but not the N-terminal, portion of nonlabeled substance P inhibited binding of 125I-BHSP (compare Fig. 2D and E).
FIG. 1.
Autoradiographs of cecal tissue from IBD-affected (A) and unaffected (B) 8-week-old TCRα− mice, showing the greater amount of binding sites for 125I-BHSP in the gut of the IBD-affected TCRα− mouse. Sites where 125I-BHSP was bound to the tissues are black or grey, with darker areas corresponding to areas of greater binding of 125I-BHSP. Sections were processed simultaneously and exposed to the same piece of autoradiographic film. (A) The cecal tonsil is indicated (∗); (B) the cecal tonsil is not visible.
FIG. 2.
Autoradiographs from adjacent cross-sections of the cecum from a TCRα− mouse with IBD. The sections were processed and exposed simultaneously to the same piece of autoradiographic film. The cecal tonsil is indicated by an asterisk. (A) Tissue exposed to 125I-BHSP. (B) Tissue exposed to 125I-BHSP and an excess of unlabeled substance P. (C) Tissue exposed to 125I-BHSP and an excess of Ac-SP, a specific NK1-R agonist. Note that Ac-SP prevents most but not all 125I-BHSP binding. (D) Tissue exposed to 125I-BHSP and an excess of SP(4-11), the C-terminal portion of SP. (E) Tissue exposed to 125I-BHSP and an excess of SP(1-4), the N-terminal portion of SP (which does not bind to neurokinin receptors).
Expression of NK1-R mRNA in infected and uninfected TCRα− mice.
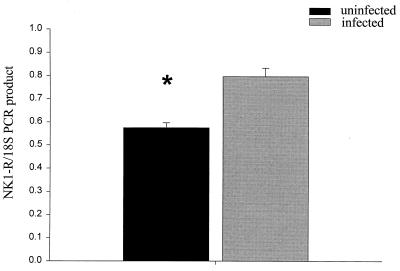
Relative RT-PCR was performed on RNA extracted from the cecal tips of five infected and five uninfected 8-week-old TCRα− mice. We were able to use 18S rRNA, which is expressed at a very steady rate across all tissues, as an internal control by decreasing the efficiency of its amplification with the addition of a commercially produced 18S competimer to the PCR. We compared the relative amplification of NK1-R mRNA to 18S rRNA in the same reaction for each sample and calculated the NK1-R/18S ratio of relative PCR product amounts. The cecal tips from infected TCRα− mice contained a statistically significant greater amount of NK-1R mRNA compared to the controls (Fig. 3), with an NK-1R/18S ratios (means ± standard errors of the mean) of 0.796 ± 0.037 for infected mice and of 0.574 ± 0.022 for uninfected mice (one-tailed rank sum test, P = 0.005).
FIG. 3.
Comparison of NK1-R/18S PCR products (mean ± standard error of the mean [error bar]) from the cecum of IBD-affected and healthy TCRα− mice. The asterisk indicates a statistically significant difference (P = 0.005).
Effects of LY303870 on the development of IBD.
To determine whether blocking substance P receptor sites with LY303870 would modulate the development of IBD, we compared parasite colonization and intestinal lesions in TCRα− mice treated or not treated with the compound. Following challenge of 1-week-old TCRα− mice with C. parvum, eight mice were treated orally with LY303870 and seven mice received water as described in Materials and Methods. All mice were killed at 4 weeks of age, and tissues were examined for C. parvum infection and IBD lesions. All TCRα− mice in both groups became infected with C. parvum and remained so at 3 weeks after inoculation, consistent with our previous findings (44).
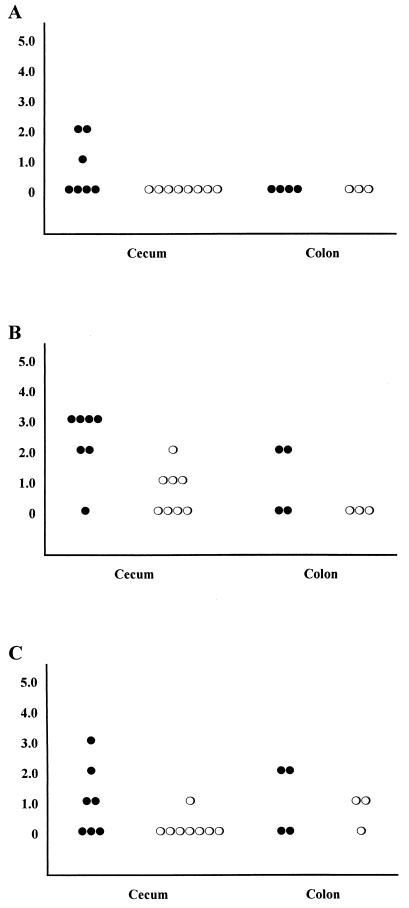
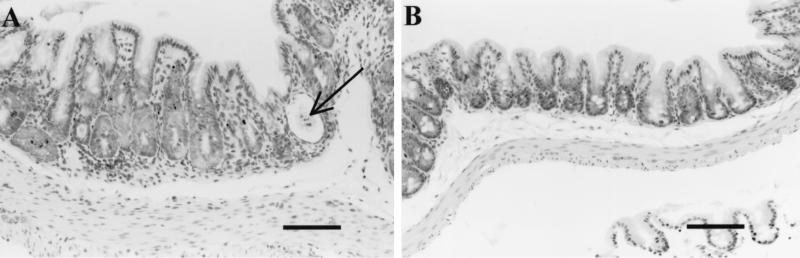
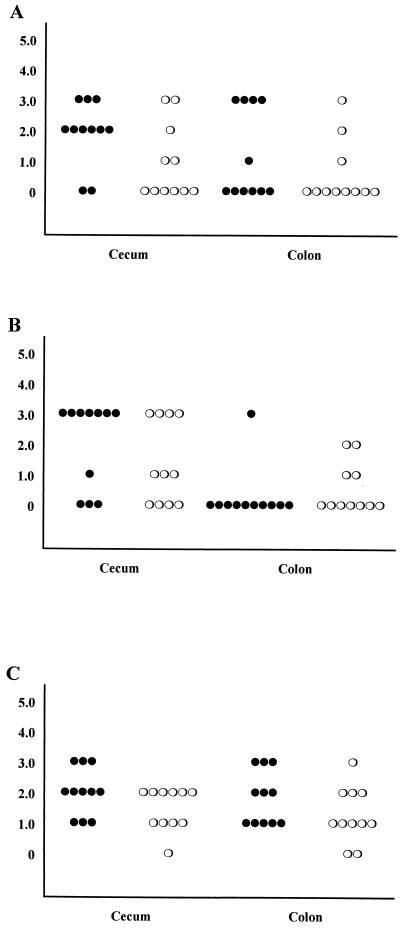
Figure 4 shows the intensity scores for IBD-like lesions in intestines of treated and nontreated TCRα− mice 3 weeks after inoculation with C. parvum. Three of seven nontreated mice had lymphocytic infiltration in the cecal lamina propria, whereas none was seen in the treated group. No mice examined from either group had lymphocytic infiltration in the colon (Fig. 4A). Cecal gland dilatation was present in tissues from six of seven nontreated mice and four of eight treated mice. Intensity scores (means ± standard errors) were significantly (P < 0.01) higher in the nontreated compared to treated mice (mean 2.29 ± 0.42 versus 0.63 ± 0.26, respectively). In colon tissue, two of four nontreated mice examined had gland dilatation in the colon, and none of three treated mice examined showed gland dilatation (Fig. 4B). Four of seven nontreated mice and one of eight treated mice had epithelial cell hyperplasia in the cecum. Two of four nontreated and two of three treated mice examined had epithelial cell hyperplasia in colon (Fig. 4C). When the mean intensity scores for all three parameters from mice in each group were compared, nontreated mice had a higher mean score in the cecum than did treated mice (1.33 ± 0.27 versus 0.25 ± 0.11, respectively) and the difference was statistically significant (P < 0.001). Combined colon scores were also higher in nontreated compared with treated mice (0.80 ± 0.33 versus 0.22 ± 0.15, respectively); however, the difference was not statistically significant (P > 0.05), probably due to the low number of samples. Representative histological sections are shown in Fig. 5, emphasizing the increased expression of IBD-like lesions in nontreated compared with treated mice (compare Fig. 5A and B). Note that in most cases, tissue from treated mice was indistinguishable from tissue from uninfected age-matched TCRα− mice (not affected with IBD; compare Fig. 5B and C).
FIG. 4.
Intensity scores of IBD-like lesions for individual mice killed at 4 weeks of age. Scores are indicated as follows: 0, no significant lesions; 1, minimal lesions; 2, mild lesions; 3, moderate lesions; 4, marked lesions; 5, severe lesions. (A) Lamina propria infiltration; (B) epithelial gland dilatation; (C) epithelial cell hyperplasia. Symbols: closed circles, control mice; open circles, LY303870-treated mice.
FIG. 5.
Paraffin-embedded histological sections of the cecal tips from 4-week-old TCRα− mice, stained with hematoxylin and eosin. The bar in each figure corresponds to 100 μm. (A) Mouse infected at 1 week of age with C. parvum oocysts and treated with water only. Note the epithelial hyperplasia, the gland dilatation, and the presence of debris in the dilated gland indicated by an arrow. Lymphocytic infiltrates are present in the lamina propria. (B) Littermate infected at 1 week of age with C. parvum oocysts and treated with LY303870. The appearance of the cecal mucosa is indistinguishable from the uninfected control (C).
Effects of LY303870 on preexisting IBD.
In a second study, we examined the ability of LY303870 to modulate preexisting IBD. Twenty-two TCRα− mice were inoculated with 104 C. parvum organisms at 1 week of age. At 6 weeks of age (by which time mice in this model have developed IBD), 11 of the mice began receiving daily treatments of LY303870 and 11 mice received water. All mice were killed at 8 weeks of age, and tissues were examined for C. parvum infection and IBD-like lesions. Eleven of 11 treated and 10 of 11 nontreated mice were infected with C. parvum at the time of death, based on histology The difference in infection rate between treated and nontreated groups was not significant (P > 0.05). Figure 6 shows the intensity of IBD-like lesions. Nine of 11 nontreated and 5 of 11 treated mice had lymphocytic infiltration in cecal lamina propria (Fig. 6A). Mean intensity scores were higher in nontreated than in treated mice, although the difference was not statistically significant (P > 0.05). Cecal gland dilatation was seen in 8 of 11 nontreated and 7 of 11 treated mice (Fig. 6B); differences in intensity were not statistically significant (P > 0.05). While most mice in both groups had epithelial cell hyperplasia in cecal tissue, intensity scores were higher in nontreated than in treated mice (Fig. 6C); however, the difference was not statistically significant (P > 0.05). When the three parameters were combined for cecal tissue, the mean score was significantly (P < 0.01) higher in nontreated than in treated mice (1.97 ± 0.19 versus 1.24 ± 0.19, respectively). There were no significant differences in colon tissue for any of the three parameters individually or combined.
FIG. 6.
Intensity scores of IBD-like lesions for individual mice killed at 8 weeks of age. Scores are indicated as follows: 0, no significant lesions; 1, minimal lesions; 2, mild lesions; 3, moderate lesions; 4, marked lesions; 5, severe lesions. (A) Lamina propria infiltration; (B) epithelial gland dilatation; (C) epithelial cell hyperplasia. Symbols: closed circles, control mice; open circles, LY303870-treated mice.
DISCUSSION
It has become apparent that substance P is an important, if poorly understood, part of the body's defense mechanisms, which can have beneficial or harmful effects. Substance P is a potent vasodilator; constricts most smooth muscle; and stimulates salivary, tracheobronchial, and enteric secretion (21, 22). It has numerous effects on the immune system, as recently reviewed by Maggi (29). These effects include upregulating gamma interferonγ (4, 20), tumor-necrosis-factor alpha (2, 11, 12), interleukin-12 (27), adhesion factor production or expression (43), and mast cell activation and degranulation (38, 43).
Ablation of substance P-containing afferent nerves of the central nervous system (6) or administration of specific antagonists (26) dramatically exacerbates the lesions caused by Salmonella sp. or Mycoplasma pulmonis. Conversely, the administration of an NK1-R antagonist or deletion of the NK1-R gene completely prevents Clostridium difficile toxin A-induced enteritis (9, 36) and immune complex-mediated pulmonary disease (7). Similarly, systemic administration of an anti-substance P antibody dramatically reverses the enteric inflammation due to Trichinella spiralis in mice (1).
Substance P is contained in afferent nerves of the central nervous system and in many of the neurons (sensory, inter-, and motor neurons) of the intrinsic nervous system of the gut, a large and complex network that controls enteric function (14, 21, 22). Intrinsic afferent nerves of the gut respond to numerous chemical and mechanical stimuli, including serotonin, acid and basic solutions, by releasing substance P (14, 21, 22). Inflammation and the accompanying damage to the gut activate both intrinsic and extrinsic afferent nerves (14, 21, 22).
Substance P release is believed to be increased in IBD, although results of studies measuring substance P content in IBD are inconsistent (3, 28), perhaps due to the rapid removal of this peptide by degradation or internalization. There is other evidence to implicate the neurokinins in the pathogenesis of IBD. Binding sites for substance P are dramatically upregulated in lymphoid aggregates and small vessels in gut affected with IBD. These binding sites were shown to correspond to the NK1-R (17, 31).
In the present study, specific 125I-BHSP binding in the cecum and colon of healthy TCRα− mice resembled that previously reported for mice and rats using autoradiographic methods (8). The distribution of these binding sites corresponded to the areas where the NK1-R has been detected (42) using immunohistochemical methods. We further showed that specific 125I-BHSP binding sites are dramatically upregulated in the IBD-affected gut of TCRα− mice; this upregulation closely resembles that detected in the gut of IBD-affected humans (31). Because 125I-BHSP binding was saturable, i.e., blocked by an excess of a specific NK1-R agonist and unaffected by the presence of the N-terminal fragment of substance P, the binding sites seen in the inflamed gut most likely represented NK1-Rs and were not due to a direct intercalation of the N terminus of substance P into cell membranes (25). These data suggest that most 125I-BHSP binding sites in the lamina propria of the cecum and colon of IBD-affected TCRα− mice are NK1-Rs, although the simultaneous upregulation of neurokinin-2 or -3 receptors cannot be ruled out by these studies, since all three types of receptors are present in the rodent gut (18).
To verify whether NK1-R expression was upregulated in IBD-affected TCRα− mice, we performed RT-PCR, which allowed us to compare the relative amounts of NK1-R mRNA in the ceca of healthy and IBD-affected TCRα− mice. There was a dramatic upregulation of NK1-R mRNA expression in IBD-affected TCRα− mice compared to age-matched controls, confirming the findings of our autoradiographic study. A similar upregulation of NK1-R mRNA has been detected in the intestine of IBD-affected patients (17). In humans, the distributions of NK1-R mRNA and protein in both healthy and IBD-affected intestine were similar: they were found on lamina propria mononuclear cells, lymphoid follicles, mucosal epithelium, enteric smooth muscle, small submucosal vessels, and enteric neurons. Upregulation of NK1-R mRNA and protein was attributed to either increased numbers of NK1-R expressing inflammatory cells, or upregulation of NK1-R expression on cells already expressing the NK1-R in healthy intestine (17). Although our studies do not allow us to draw any conclusions as to the type of cells expressing NK1-R in our rodent model of IBD, we predict that NK1-R expression in C. parvum infected TCRα− would follow the same general pattern as seen in human IBD, given the strong similarities between our experimental model of IBD and the clinical disease.
To determine whether substance P plays an important role in the pathogenesis of IBD-like lesions in C. parvum-infected TCRα− mice, we studied the effects of a NK1-R antagonist, LY303870, on the development of IBD-like lesions in these mice. LY303870 is a highly specific, potent, and long-lasting antagonist of substance P at the NK1-R (15, 16, 24). It is well absorbed orally and crosses the blood-brain barrier, effectively blocking both peripheral and central NK1-Rs. Oral administration to rodents and guinea pigs at doses up to 50 mg/kg causes no significant side effects (24).
LY303870 treatment had a marked effect on the development of histological IBD-like lesions in 4-week-old TCRα− mice infected at 1 week of age with C. parvum. All but one of the nontreated mice had some histological evidence of IBD-like lesions, ranging from mild to moderate in severity. In contrast, mice treated with LY303870, which would be expected to modulate the effects of substance P by competing for NK1-R sites, had consistently less-severe lesions. Intensity scores for cecal gland dilatation were significantly higher in nontreated compared to treated mice. Scores for lamina propria infiltration and epithelial cell hyperplasia in cecum were also higher in control compared to treated mice, although differences were not statistically significant. When the mean intensity scores for all three parameters of IBD (lamina propria infiltration, gland dilatation, and epithelial cell hyperplasia) in the cecum were compared, nontreated mice had significantly higher scores than did treated mice. In sum, these data indicate that substance P plays a role in the induction of IBD-like lesions, since a specific neurokinin-l receptor antagonist reduced the severity of lesions in the intestines of treated mice. The lack of effect of LY303870 treatment on C. parvum infection suggests that reduction of IBD-like lesions was not due to a reduction in C. parvum infection, which is the stimulus for development of IBD in this model. Rather, the NK-1R antagonist may act by blocking the inflammatory events that follow interaction of substance P with its receptor, such as gamma interferon, interleukin-12, and tumor necrosis factor alpha upregulation; macrophage activation; and immunoglobulin production (2, 4, 11, 12, 20, 22, 27).
We also tested whether treatment with LY303870 would have an effect on preexisting lesions in this model. We treated TCRα− mice with established IBD for 2 weeks with LY303870 and then compared lesions to those seen in age-matched, nontreated mice. As in the previous study, the mice in both groups remained infected with C. parvum, confirming the lack of effect of LY303870 on infection with the parasite. Intensity of lesions in the intestines was consistently greater in nontreated mice compared with mice treated with LY303870. When the mean intensity scores for all three parameters of IBD (lamina propria infiltration, gland dilatation, and epithelial cell hyperplasia) in the cecum were compared, nontreated mice had significantly higher scores than did treated mice. There were no apparent differences in the intensity of lesions in the colons of nontreated versus treated mice. These results suggest that, at least in the cecum, blocking the action of substance P allows partial healing of IBD-like lesions. Again, we hypothesize that these effects are due to the reduction or interruption of the cascade of host inflammatory mediators, since no direct effects were seen on C. parvum infection.
In summary, we have shown that a dramatic upregulation of substance P binding sites occurs over lamina propria lymphoid aggregates in TCRα− mice with IBD-like lesions, similar to what is seen in human patients with IBD. Based on the results of our autoradiographic and RT-PCR studies, the upregulation of substance P binding sites in our model corresponds to an upregulation of NK1-R, as is seen in clinical IBD (17, 31). Further, oral dosing with a specific NK1-R antagonist both reduces development and alleviates severity of preexisting IBD-like lesions in C. parvum-infected TCRα− mice. This system presents a unique and highly reproducible model in which to investigate the contributions of substance P to the development and maintenance of chronic IBD. Because this murine model of IBD so closely resembles human IBD, it affords the opportunity to gain important insights into neuroimmune regulation of this clinically significant disease.
Acknowledgments
This study was funded by an InterInstitutional Seed Grant from the College of Medicine, University of Iowa, and the College of Veterinary Medicine, Iowa State University.
REFERENCES
- 1.Agro, A., and A. M. Stanisz. 1993. Inhibition of murine intestinal inflammation by anti-substance P antibody. Regional Immunol. 5:120-126. [PubMed] [Google Scholar]
- 2.Ansel, J. C., J. R. Brown, D. G. Payan, and M. A. Brown. 1993. Substance P selectively activates TNF alpha gene expression in murine mast cells. J. Immunol. 150:4478-4485. [PubMed] [Google Scholar]
- 3.Bernstein, C. N., M. E. Robert, and V. E. Eysselein. 1993. Rectal substance P concentrations are increased in ulcerative colitis but not in Crohn's disease. Am. J. Gastroenterol. 88:908-913. [PubMed] [Google Scholar]
- 4.Blum, A. M., A. Metwali, D. Elliot, M. Sandor, R. Lynch, and J. V. Weinstock. 1996. Substance P receptor antagonist inhibits murine IgM expression in developing schistosome granulomas by blocking the terminal differentiation of intragranuloma B cells. J. Neuroimmunol. 66:1-10. [DOI] [PubMed] [Google Scholar]
- 5.Blum, A. M., A. Metwali, M. Kim-Miller, J. Li, K. Qadir, D. E. Elliott, B. Lu, Z. Fabry, N. Gerard, J. V. Weinstock. 1999. The substance P receptor is necessary for a normal granulomatous response in murine Schistosomiasis mansoni. J. Immunol. 162:6080-6085. [PubMed] [Google Scholar]
- 6.Bowden, J. J., P. Baluk, P. M. Lefevre, T. R. Schoeb, J. R. Lindsey, and D. M. McDonald. 1996. Sensory denervation by neonatal capsaicin treatment exacerbates Mycoplasma pulmonis infection in rat airways. Am. J. Physiol. 270:L393-L403. [DOI] [PubMed] [Google Scholar]
- 7.Bozic, C. R., B. Lu, U. E. Hopken, C. Gerard, and N. P. Gerard. 1996. Neurogenic amplification of immune complex inflammation. Science 273:1722-1725. [DOI] [PubMed] [Google Scholar]
- 8.Burcher, E., S. H. Buck, W. Lovenberg, and T. L. O'Donohue. 1986. Characterization and autoradiographic localization of multiple tachykinin binding sites in gastrointestinal tract and bladder. J. Pharmacol. Exp. Ther. 236:819-831. [PubMed] [Google Scholar]
- 9.Castagliuolo, I., M. Riegler, A. Pasha, S. Nikulasson, B. Lu, C. Gerard, N. P. Gerard, and C. Pothoulakis. 1998. Neurokinin-1 (NK-1) receptor is required in Clostridium difficile-induced enteritis. J. Clin. Investig. 101:1547-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chadwick, V. S., and R. P. Anderson. 1992. Microorganisms and their products in inflammatory bowel disease, p. 241-258. In R. P. McDermott and W. F. Stenson (ed.), Inflammatory bowel disease. Elsevier, New York, N.Y.
- 11.Cocchiara, R., A. Bongiovanni, G. Albeggianai, A. Azzolina, N. Lampiasi, F. D. Blasi, and D. Geraci. 1997. Inhibitory effect of neuraminidase on SP-induced histamine release and TNF alpha in rat mast cells: evidence of a receptor-independent mechanism. J. Neuroimmunol. 75:9-18. [DOI] [PubMed] [Google Scholar]
- 12.Dickerson, C., B. Undem, B. Bullock, and R. A. Winchurch. 1998. Neuropeptide regulation of proinflammatory cytokine responses. J. Leukoc. Biol. 63:602-605. [DOI] [PubMed] [Google Scholar]
- 13.Fayer, R., C. A. Speer, and J. P. Dubey. 1990. General biology of Cryptosporidium, p. 1-29. In J. P. Dubey, C. A. Speer, and R. Fayer (ed.), Cryptosporidiosis of man and animals. CRC Press, Boca Raton, Fla.
- 14.Furness, J. B., W. A. A. Kunze, P. P. Bertrand, N. Clerc, and J. C. Bornstein. 1998. Intrinsic primary afferent neurons of the intestine. Progress Neurobiol. 54:1-18. [DOI] [PubMed] [Google Scholar]
- 15.Gehlert, D. R., D. A. Schober, P. A. Hipskind, B. D. Gitter, and J. J. Howbert. 1996. [3H]LY303870, a novel nonpeptide radioligand for the NK-1 receptor. J. Neurochem. 66:1095-1102. [DOI] [PubMed] [Google Scholar]
- 16.Gitter, B. D., R. F. Bruns, J. J. Howbert, C. D. Waters, P. G. Threlkeld, L. M. Cox, J. A. Nixon, K. L. Lobb, N. R. Mason, P. W. Stengel, S. L. Cockerham, S. A. Silbaugh, D. R. Gelhert, D. A. Schober, S. Iyengar, D. O. Calligaro, D. Regoli, and P. A. Hipskind. 1995. Pharmacological characterization of LY303870: a novel, potent and selective nonpeptide substance P (neurokinin-1) receptor antagonist. J. Pharmacol. Exp. Ther. 275:737-744. [PubMed] [Google Scholar]
- 17.Goode, T., J. O'Connell, P. Anton, H. Wong, J. Reeve, G. C. O'Sullivan, J. K. Collins, and F. Shanahan. 2000. Neurokinin-1 receptor expression in inflammatory bowel disease: molecular quantitation and localisation. Gut 47:387-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grady, E. F., P. Baluk, S. Bohm, P. D. Gamp, H. Wong, D. G. Payan, J. Ansel, A. L. Portbury, J. B. Furness, D. M. McDonald, and N. W. Bunnett. 1996. Characterization of antisera specific to NK1, NK2, and NK3 neurokinin receptors and their utilization to localize receptors in the rat gastrointestinal tract. J. Neurosci. 16:6975-6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harp, J. A., W. Chen, and A. G. Harmsen. 1992. Resistance of severe combined immunodeficient mice to infection with Cryptosporidium parvum: The importance of intestinal microflora. Infect. Immun. 60:3509-3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hart, R., H. Dancygier, F. Wagner, C. Lersch, and M. Classen. 1990. Effect of substance P on immunoglobulin and interferon-gamma secretion of human cultured duodenal mucosa. Immunol. Lett. 23:199-204. [DOI] [PubMed] [Google Scholar]
- 21.Holzer, P., and U. Holzer-Petsche. 1997. Tachykinins in the gut. Part I. Expression, release and motor function. Pharmacol. Ther. 73:173-217. [DOI] [PubMed] [Google Scholar]
- 22.Holzer, P., and U. Holzer-Petsche. 1997. Tachykinins in the gut. Part II. Roles in neural excitation, secretion and inflammation. Pharmacol. Ther. 73:219-263. [DOI] [PubMed] [Google Scholar]
- 23.Ilnyckyj, A., F. Shanahan, P. A. Anton, M. Cheang, and C. N. Bernstein. 1997. Quantification of the placebo response in ulcerative colitis. Gastroenterology 112:1854-1858. [DOI] [PubMed]
- 24.Iyengar, S., P. A. Hipskind, D. R. Gehlert, D. Schober, K. L. Lobb, J. A. Nixon, D. R. Helton, M. J. Kallman, S. Boucher, R. Couture, D. L. Li, and R. M. A. Simmons. 1997. LY303870, a centrally active neurokinin-1 antagonist with a long duration of action. J. Pharmacol. Exp. Ther. 280:774-785. [PubMed] [Google Scholar]
- 25.Kavelaars, A., F. Jeurissen, and C. J. Heijnen. 1994. Substance P receptors and signal transduction in leukocytes. Immunomethods 5:41-48. [DOI] [PubMed] [Google Scholar]
- 26.Kincy-Cain, T., and K. L. Bost. 1996. Increased susceptibility of mice to Salmonella infection following in vivo treatment with the substance P antagonist, spantide II. J. Immunol. 157:255-264. [PubMed] [Google Scholar]
- 27.Kincy-Cain, T., and K. L. Bost. 1997. Substance P-induced IL-12 production by murine macrophages. J. Immunol. 158:2334-2339. [PubMed] [Google Scholar]
- 28.Koch, T. R., J. A. Carney, and V. L. W. Go. 1987. Distribution and quantitation of gut neuropeptides in normal intestine and inflammatory bowel diseases. Dig. Dis. Sci. 32:369-376. [DOI] [PubMed] [Google Scholar]
- 29.Maggi, C. A. 1997. The effects of tachykinins on inflammatory and immune cells. Reg. Peptides 70:75-90. [DOI] [PubMed] [Google Scholar]
- 30.Maggi, C. A. 1998. Tachykinin antagonists in asthma and inflammation, p. 545-587. In S. I. Said (ed.), Proinflammatory and antiinflammatory peptides. Marcel Dekker, New York, N.Y.
- 31.Mantyh, C. R., S. R. Vigna, J. E. Maggio, P. W. Mantyh, R. R. Bollinger, and T. N. Pappas. 1994. Substance P binding sites on intestinal lymphoid aggregates and blood vessels in inflammatory disease correspond to authentic NK-1 receptors. Neurosci. Lett. 178:255-259. [DOI] [PubMed] [Google Scholar]
- 32.Mathey, M. W., A. B. Ross, and K. H. Soergel. 1997. Cryptosporidiosis and inflammatory bowel disease. Dig. Dis. Sci. 42:1580-1586. [DOI] [PubMed] [Google Scholar]
- 33.Meyers, S., and H. D. Janowitz. 1984. Natural history of Crohns disease. An analytical review of the placebo lesson. Gastroenterology 87:1189-1192. [PubMed] [Google Scholar]
- 34.Mizoguchi, A., E. Mizoguchi, C. Chiba, and A. K. Bhan. 1996. Role of the appendix in the development of inflammatory bowel disease in TCR alpha mutant mice. J. Exp. Med. 184:707-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mombaerts, P., E. Mizoguchi, M. J. Grusby, L. H. Glimcher, A. K. Bhan, and, S. Tonegawa. 1993. Spontaneous development of inflammatory bowel disease in T cell receptor mutant mice. Cell 75:275-282. [DOI] [PubMed] [Google Scholar]
- 36.Pothoulakis, C., I. Gastagliuolo, J. T. LaMont, A. Jaffer, J. C. O'Keane, R. M. Snider, and S. E. Leeman. 1994. CP 96,345, a substance P antagonist, inhibits rat intestinal responses to Clostridium difficile toxin A but not cholera toxin. Proc. Natl. Acad. Sci. USA 91:947-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Regoli, D., P. Geppetti, and G. Calo. 1998. The tachykinin family of peptides and their receptors, p. 147-162. In S. I. Said (ed.), Proinflammatory and antiinflammatory peptides. Marcel Dekker, New York, N.Y.
- 38.Repke, H., and M. Bienert. 1987. Mast cell activation: a receptor-independent mode of substance P action? Fed. Eur. Biochem. Soc. 221:236-240. [DOI] [PubMed] [Google Scholar]
- 39.Sacco, R. E., J. S. Haynes, J. A. Harp, W. R. Waters, and M. J. Wannemuehler. 1998. Cryptosporidium parvum initiates inflammatory bowel disease in germfree T cell receptor-alpha-deficient mice. Am. J. Pathol. 153:1717-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sonea, I. M., R. M. Bowker, and N. E. Robinson. 1997. Tachykinin receptors in the equine pelvic flexure. Equine Vet. J. 29:306-312. [DOI] [PubMed] [Google Scholar]
- 41.Stenson, W. F. 1995. Inflammatory bowel disease, p. 1748-1806. In T. Yamada (ed.), Textbook of gastroenterology, 2nd ed. Lippicott Co., Philadelphia, Pa.
- 42.Sternini, C., D. Su, P. D. Gamp, and N. W. Bunnett. 1995. Cellular sites of expression of the neurokinin-1 receptor in the rat gastrointestinal tract. J. Comp. Neurol. 358:531-540. [DOI] [PubMed] [Google Scholar]
- 43.Suzuki, H., S. Miura, Y. Y. Liu, M. Tsuchiya, and H. Ishii. 1995. Substance P induces degranulation of mast cells and leukocyte adhesion to venular endothelium. Peptides 16:1447-1452. [DOI] [PubMed] [Google Scholar]
- 44.Waters, W. R., and J. A. Harp. 1996. Cryptosporidium parvum infection in T-cell receptor (TCR)-alpha- and TCR-delta-deficient mice. Infect. Immun. 64:1854-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Waters, W. R., M. V. Palmer, M. R. Ackermann, and J. A. Harp. 1997. Accelerated inflammatory bowel disease of TCR-alpha-deficient mice persistently infected with Cryptosporidium parvum. J. Parasitol. 83:460-464. [PubMed] [Google Scholar]
- 46.Waters, W. R., M. V. Palmer, M. J. Wannemuehler, R. E. Sacco, and J. A. Harp. 2000. B cells are required for the induction of intestinal inflammatory lesions in TCR alpha-deficient mice persistently infected with Cryptosporidium parvum. J. Parasitol. 86:1073-1077. [DOI] [PubMed] [Google Scholar]
- 47.Waters, W. R., M. J. Wannemuehler, R. E. Sacco, M. V. Palmer, J. S. Haynes, B. A. Pesch, and J. A. Harp. 1999. Cryptosporidium parvum-induced inflammatory bowel disease of TCR-beta × TCR-delta-deficient mice. J. Parasitol. 85:1100-1105. [PubMed] [Google Scholar]