Abstract
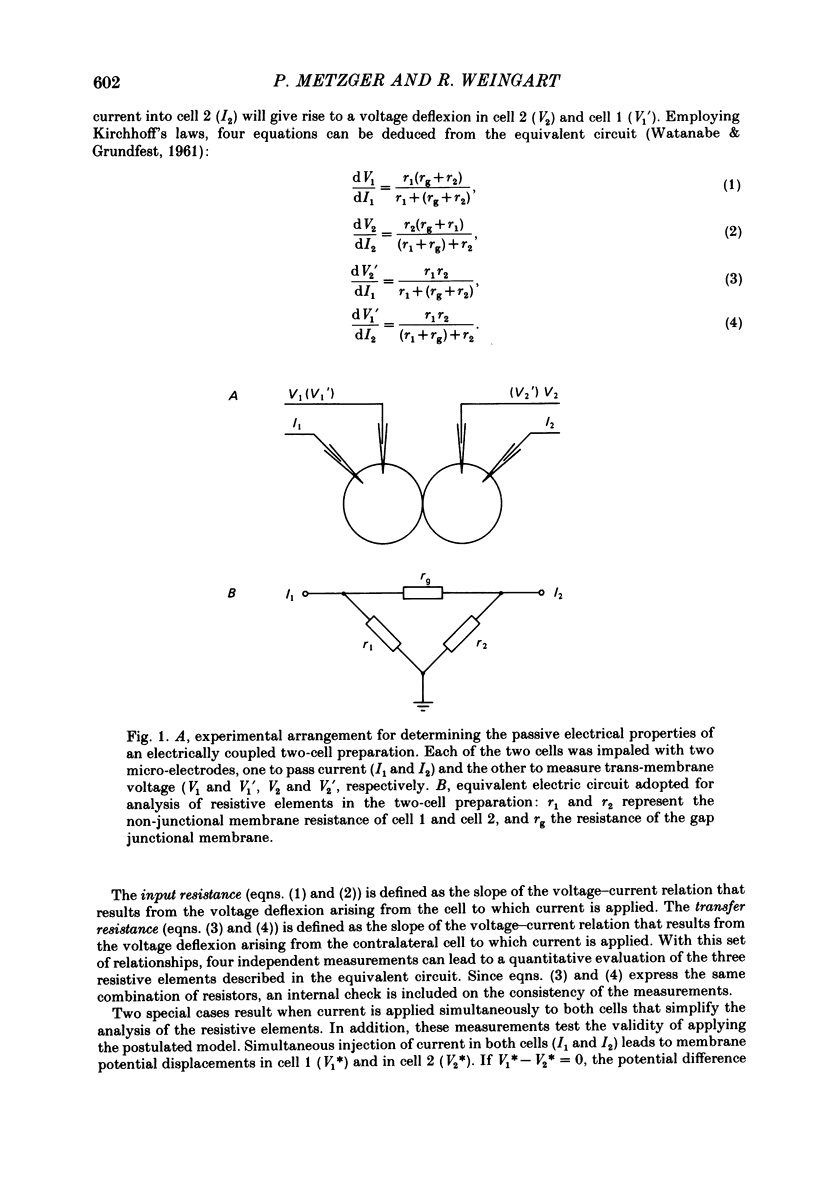
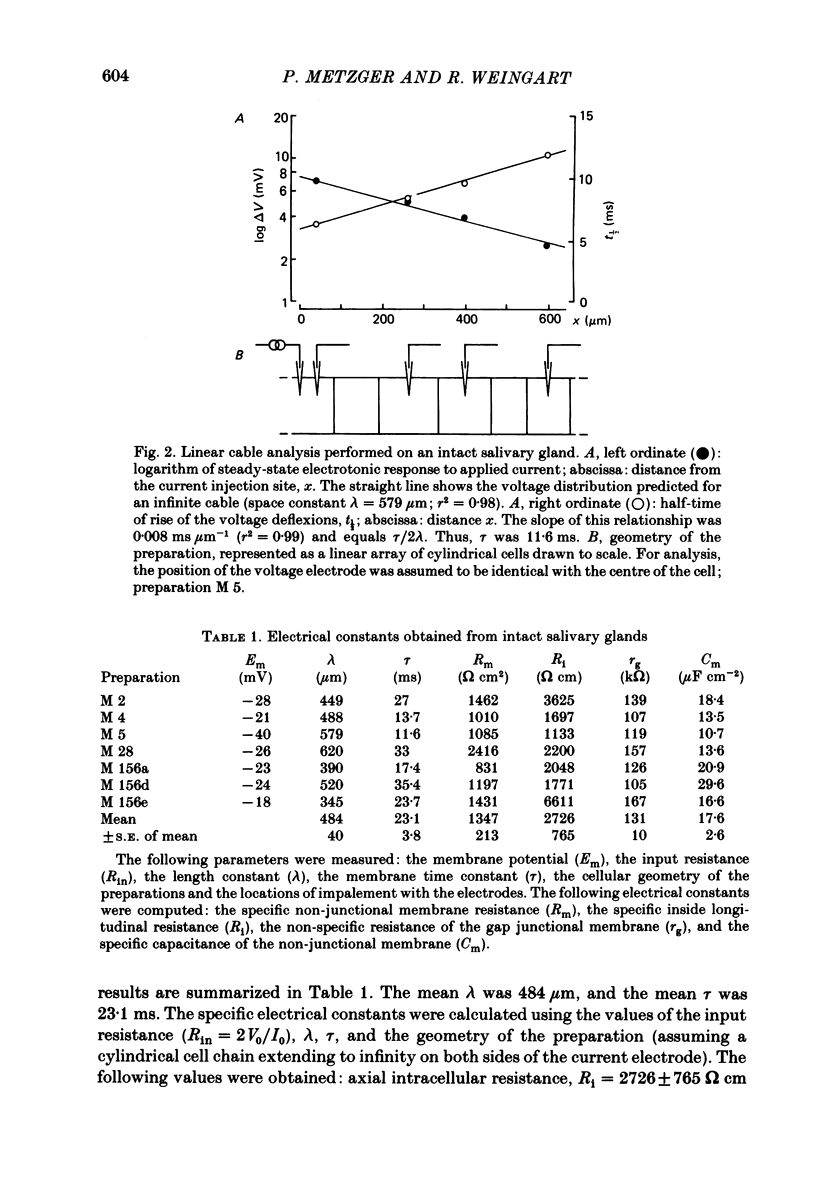
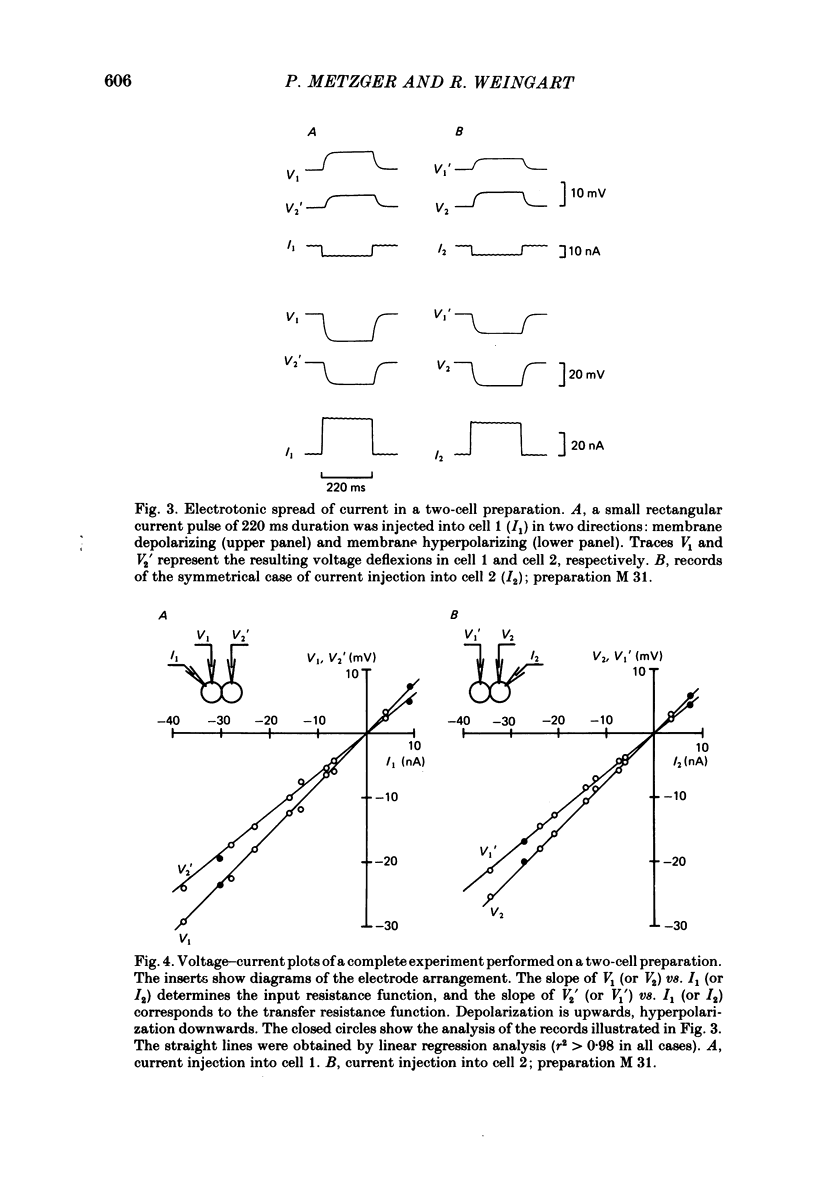
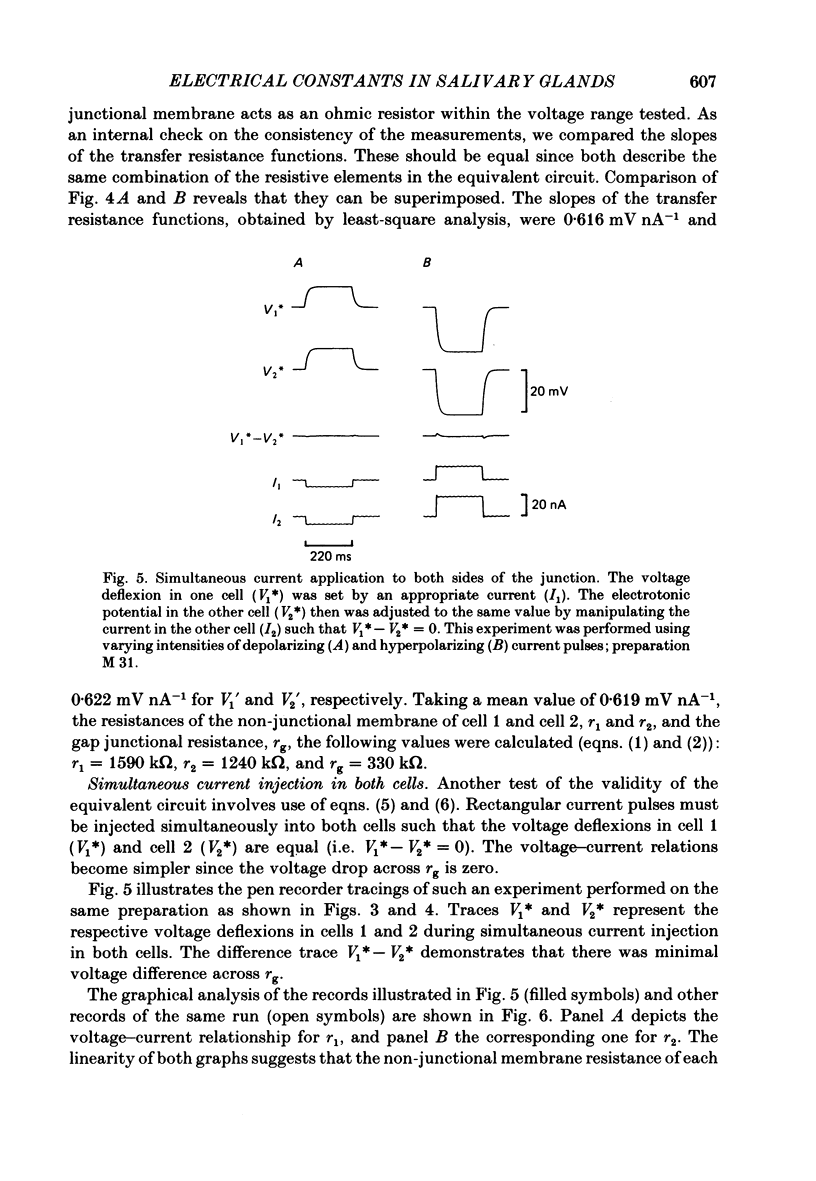
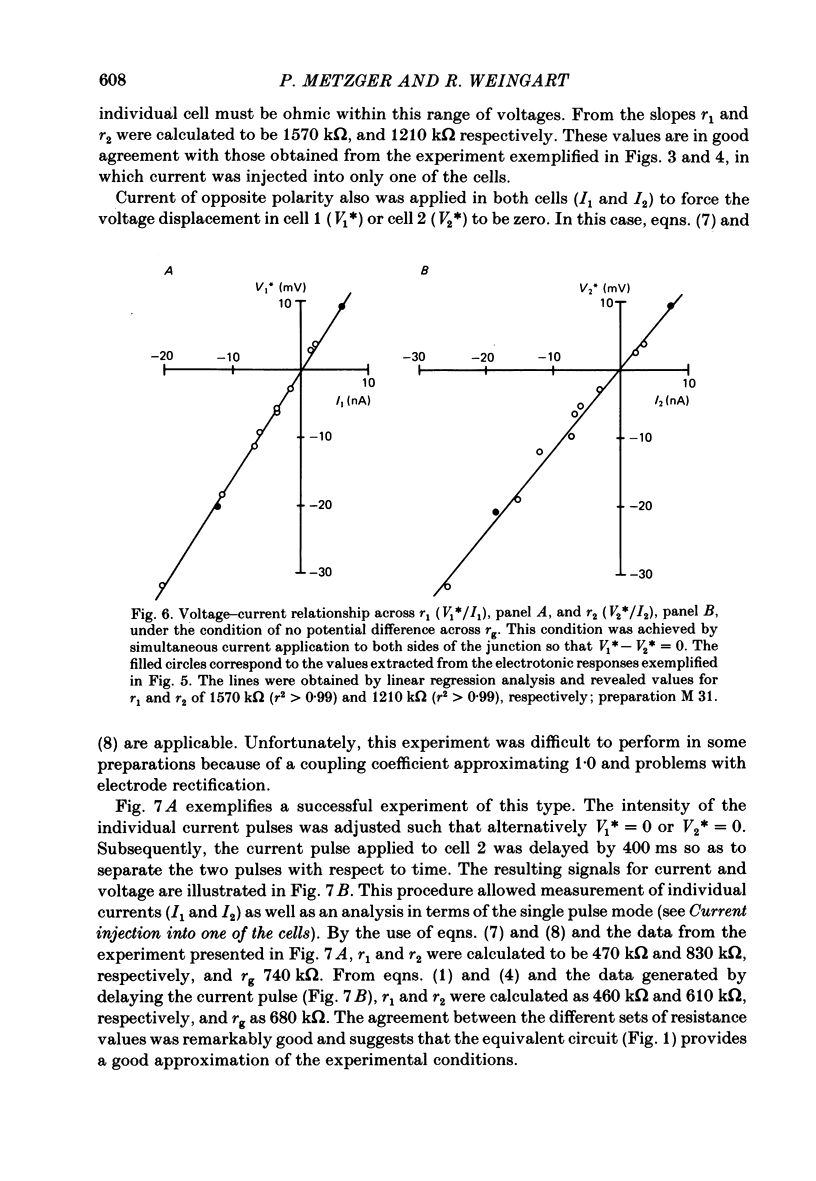
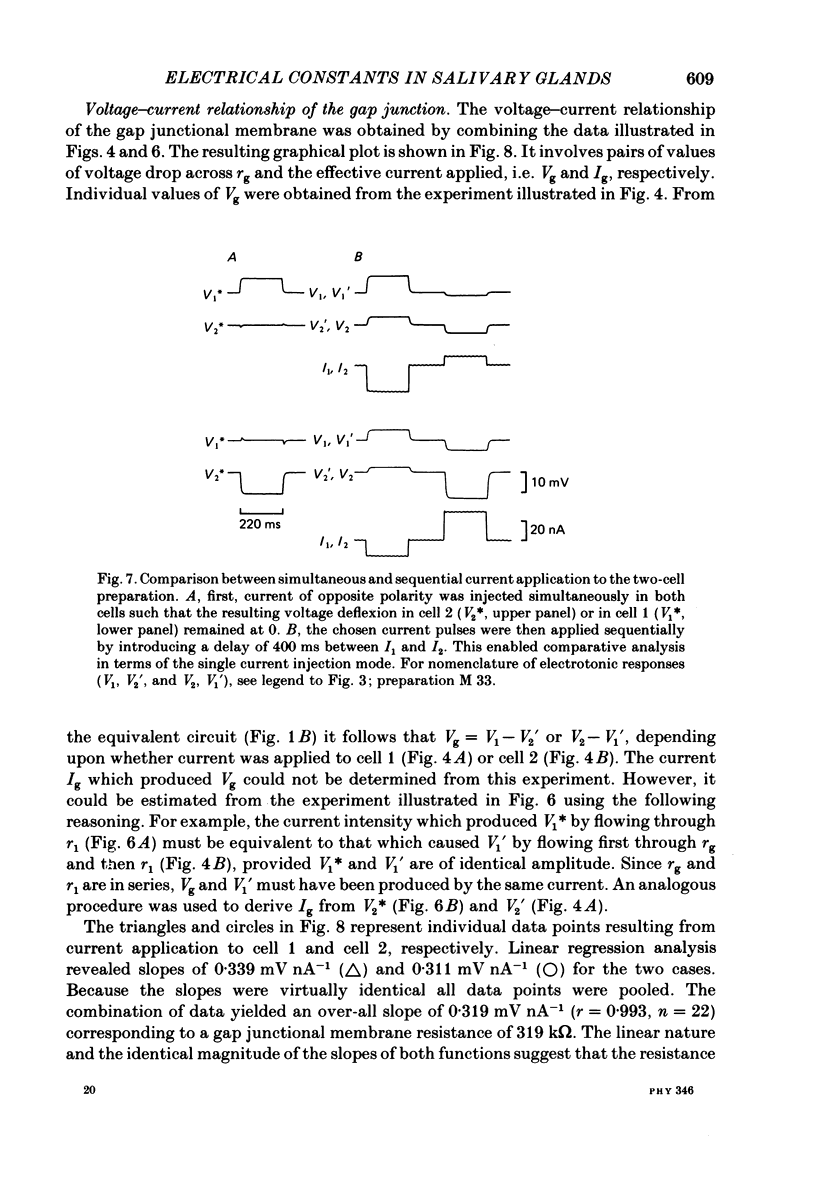
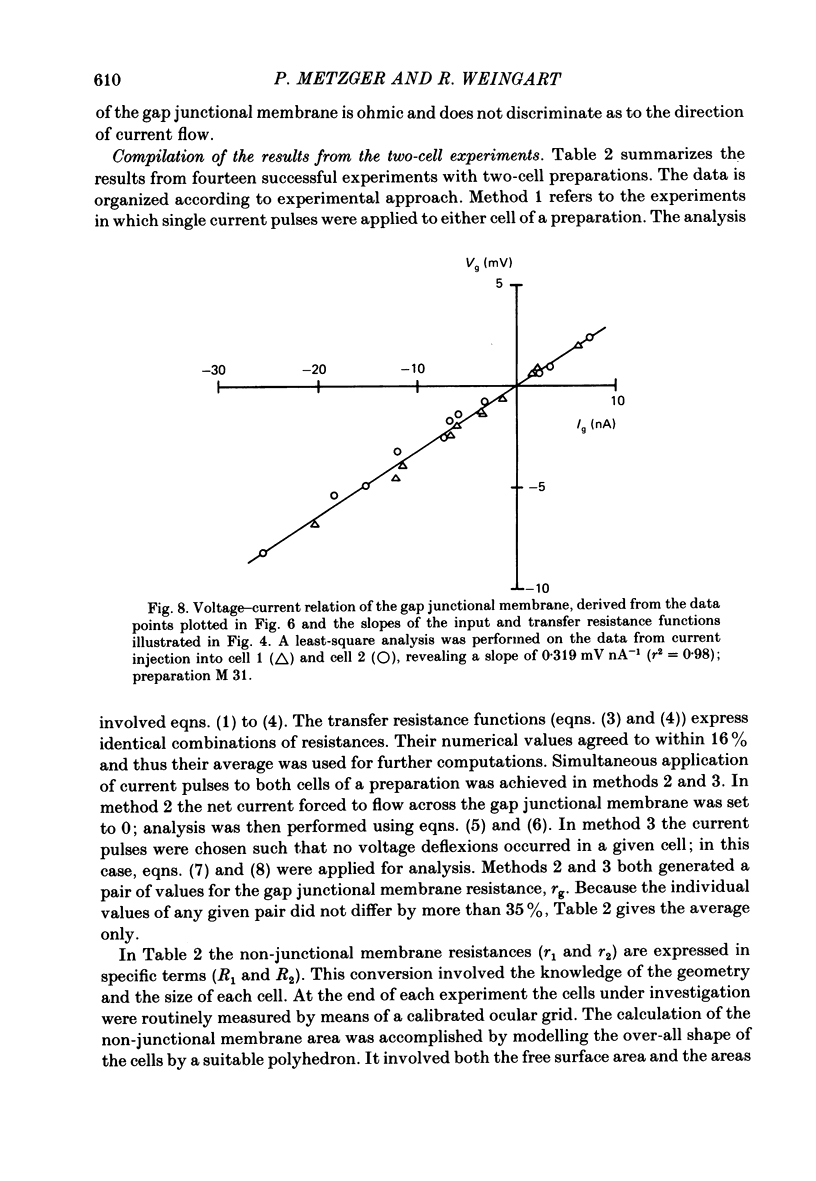
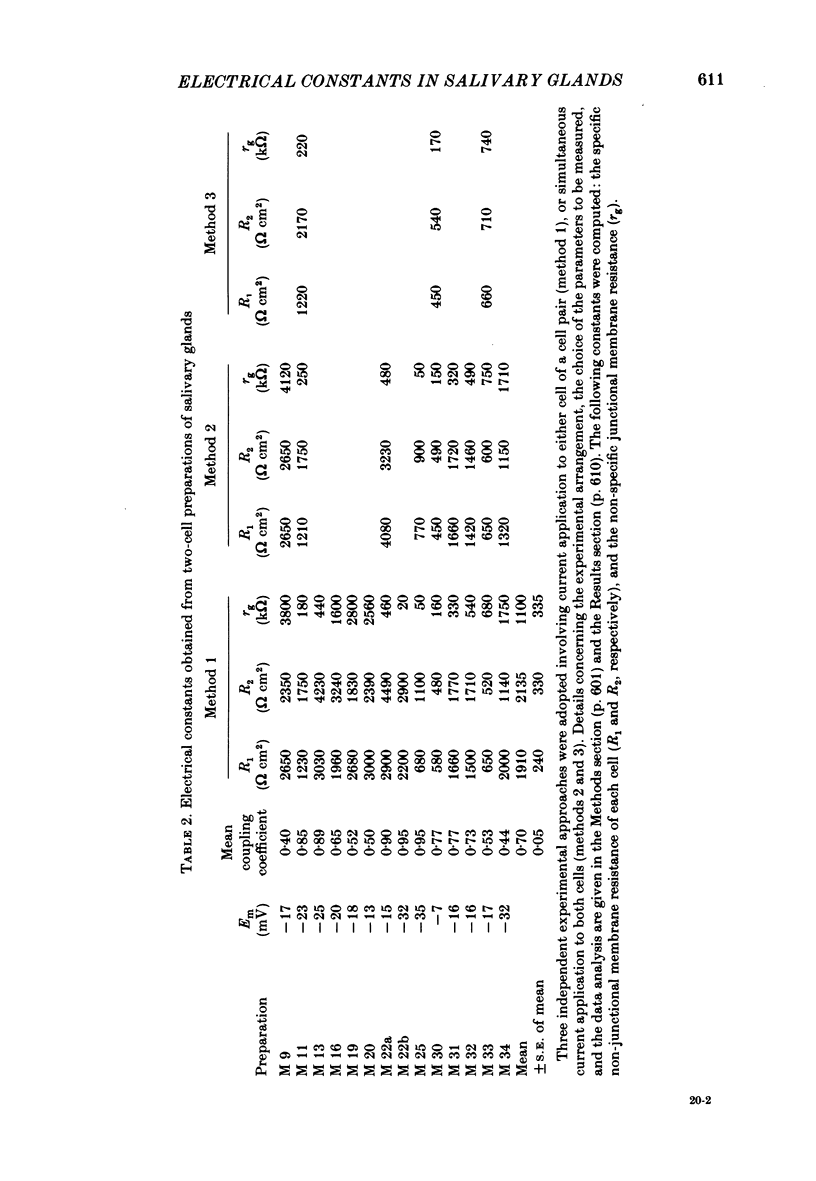
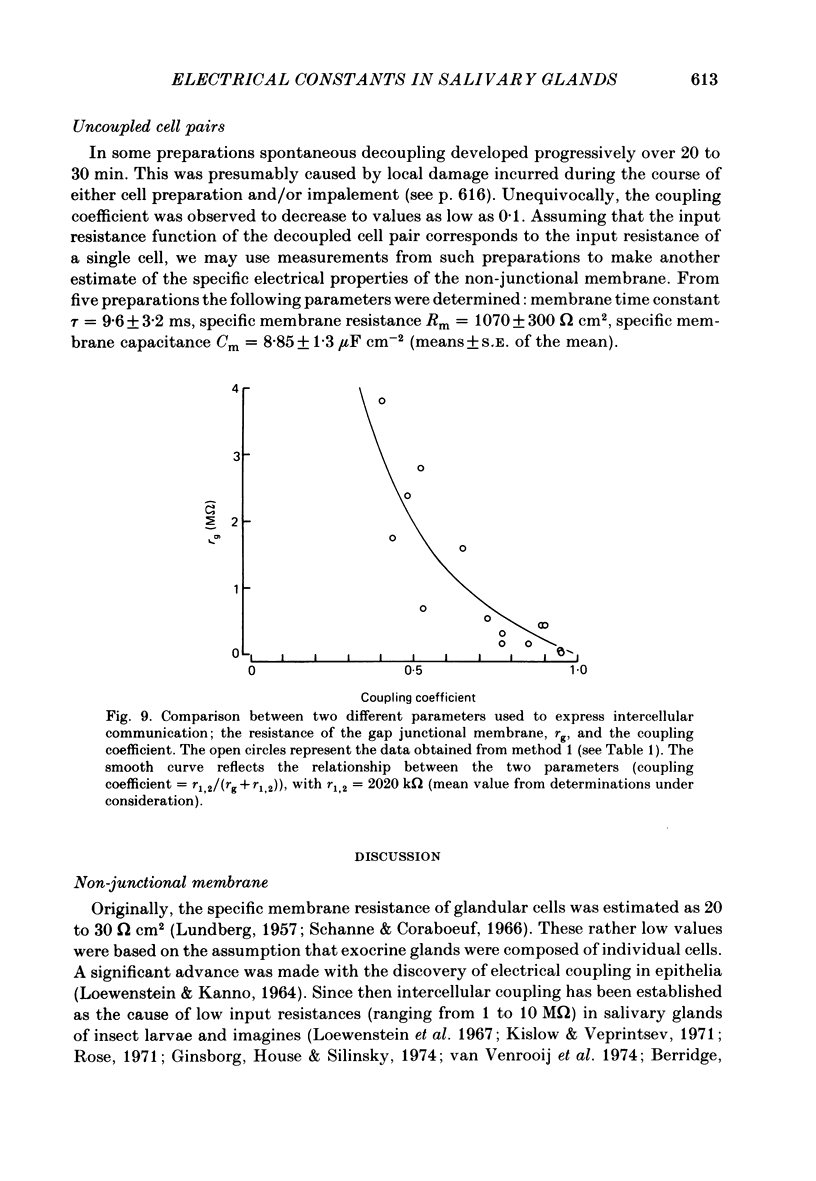
Conventional micro-electrode techniques were used to study the passive electrical properties of salivary glands from Chironomus nuditarsis insect larvae of the fourth instar stage. Linear cable analysis performed on intact glands revealed the following constants: axial intracellular resistance, Ri = 2730 omega cm; membrane resistance per unit apparent cylindrical area, Rm = 1350 omega cm2; membrane capacitance per unit apparent cylindrical area, Cm = 17.6 microF cm-2. The multicellular glands were reduced to intact two-cell preparations by destroying neighbouring cells mechanically. Each cell of a coupled cell pair was impaled with two micro-electrodes, one to pass rectangular current pulses and the other to monitor the resulting voltage deflexions. Internal consistency tests revealed that the experimental data under steady-state conditions may be described accurately by an equivalent circuit consisting of a delta configuration of three resistive elements: the resistances of the non-junctional membrane of cell 1 and cell 2 (r1 and r2), and the resistance of the gap junctional membrane connecting the two cells (rg). The current-voltage relation of the non-junctional membrane was found ohmic over a membrane potential ranging from -40 mV to + 10 mV. The mean value of Rm was 2020 omega cm2. The resistance function of the gap junctional membrane was also ohmic. There was no dependence of gap junctional resistance on voltage or direction of current flow, at least over the relatively narrow range of potentials tested (approximately +/- 10 mV). Individual values of rg varied from 20 to 3800 k omega, with an over-all mean of 1100 k omega. The lower values are thought to represent the physiological state of cellular coupling, whereas the higher ones may reflect partial uncoupling caused by local damage. The proposed cell pair is a suitable preparation for studying problems related to intercellular coupling.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett M. V. Physiology of electrotonic junctions. Ann N Y Acad Sci. 1966 Jul 14;137(2):509–539. doi: 10.1111/j.1749-6632.1966.tb50178.x. [DOI] [PubMed] [Google Scholar]
- Berger W. K., Uhrík B. Membrane junctions between salivary gland cells of Chironomus Thummi. Z Zellforsch Mikrosk Anat. 1972;127(1):116–126. doi: 10.1007/BF00582761. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Lindley B. D., Prince W. T. Membrane permeability changes during stimulation of isolated salivary glands of Calliphora by 5-hydroxytryptamine. J Physiol. 1975 Jan;244(3):549–567. doi: 10.1113/jphysiol.1975.sp010812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mello W. C. Cell-to-cell communication in heart and other tissues. Prog Biophys Mol Biol. 1982;39(3):147–182. doi: 10.1016/0079-6107(83)90016-0. [DOI] [PubMed] [Google Scholar]
- FURSHPAN E. J., POTTER D. D. Transmission at the giant motor synapses of the crayfish. J Physiol. 1959 Mar 3;145(2):289–325. doi: 10.1113/jphysiol.1959.sp006143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallacher D. V., Petersen O. H. Electrophysiology of mouse parotid acini: effects of electrical field stimulation and ionophoresis of neurotransmitters. J Physiol. 1980 Aug;305:43–57. doi: 10.1113/jphysiol.1980.sp013348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsborg B. L., House C. R., Silinsky E. M. Conductance changes associated with the secretory potential in the cockroach salivary gland. J Physiol. 1974 Feb;236(3):723–731. doi: 10.1113/jphysiol.1974.sp010462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley R. D., Murphy A. D., Kater S. B. Ionic bases of resting and action potentials in salivary gland acinar cells of the snail Helisoma. J Exp Biol. 1980 Feb;84:213–225. doi: 10.1242/jeb.84.1.213. [DOI] [PubMed] [Google Scholar]
- Hammer M. G., Sheridan J. D. Electrical coupling and dye transfer between acinar cells in rat salivary glands. J Physiol. 1978 Feb;275:495–505. doi: 10.1113/jphysiol.1978.sp012203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris A. L., Spray D. C., Bennett M. V. Kinetic properties of a voltage-dependent junctional conductance. J Gen Physiol. 1981 Jan;77(1):95–117. doi: 10.1085/jgp.77.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzberg E. L., Lawrence T. S., Gilula N. B. Gap junctional communication. Annu Rev Physiol. 1981;43:479–491. doi: 10.1146/annurev.ph.43.030181.002403. [DOI] [PubMed] [Google Scholar]
- Ito S., Sato E., Loewenstein W. R. Studies on the formation of a permeable cell membrane junction. II. Evolving junctional conductance and junctional insulation. J Membr Biol. 1974;19(4):339–355. doi: 10.1007/BF01869985. [DOI] [PubMed] [Google Scholar]
- Kagayama M., Nishiyama A. Membrane potential and input resistance in acinar cells from cat and rabbit submaxillary glands in vivo: effects of autonomic nerve stimulation. J Physiol. 1974 Oct;242(1):157–172. doi: 10.1113/jphysiol.1974.sp010699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameyama M. Electrical coupling between ventricular paired cells isolated from guinea-pig heart. J Physiol. 1983 Mar;336:345–357. doi: 10.1113/jphysiol.1983.sp014585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kater S. B., Galvin N. J. Physiological and morphological evidence for coupling in mouse salivary gland acinar cells. J Cell Biol. 1978 Oct;79(1):20–26. doi: 10.1083/jcb.79.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kater S. B., Rued J. R., Murphy A. D. Propagation of action potentials through electrotonic junctions in the salivary glands of the pulmonate mollusc, Helisoma trivolvis. J Exp Biol. 1978 Feb;72:77–90. doi: 10.1242/jeb.72.1.77. [DOI] [PubMed] [Google Scholar]
- Klevets M. Iu, Shuba M. F. Elektrychni kharakterystyky plasmatychnoï membrany klityn slynnoï zalozy vynohradnoho slymaka. Fiziol Zh. 1974 Jul-Aug;20(4):540–542. [PubMed] [Google Scholar]
- Kloetzel J. A., Laufer H. A fine-structural analysis of larval salivary gland function in Chironomus thummi (Diptera). J Ultrastruct Res. 1969 Oct;29(1):15–36. doi: 10.1016/s0022-5320(69)80053-5. [DOI] [PubMed] [Google Scholar]
- LOEWENSTEIN W. R., KANNO Y. STUDIES ON AN EPITHELIAL (GLAND) CELL JUNCTION. I. MODIFICATIONS OF SURFACE MEMBRANE PERMEABILITY. J Cell Biol. 1964 Sep;22:565–586. doi: 10.1083/jcb.22.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUNDBERG A. The mechanism of establishment of secretory potentials in sublingual gland cells. Acta Physiol Scand. 1957 Sep 17;40(1):35–58. doi: 10.1111/j.1748-1716.1957.tb01476.x. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R. Junctional intercellular communication: the cell-to-cell membrane channel. Physiol Rev. 1981 Oct;61(4):829–913. doi: 10.1152/physrev.1981.61.4.829. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R., Nakas M., Socolar S. J. Junctional membrane uncoupling. Permeability transformations at a cell membrane junction. J Gen Physiol. 1967 Aug;50(7):1865–1891. doi: 10.1085/jgp.50.7.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein W. R. Permeable junctions. Cold Spring Harb Symp Quant Biol. 1976;40:49–63. doi: 10.1101/sqb.1976.040.01.008. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R., Socolar S. J., Higashino S., Kanno Y., Davidson N. Intercellular Communication: Renal, Urinary Bladder, Sensory, and Salivary Gland Cells. Science. 1965 Jul 16;149(3681):295–298. doi: 10.1126/science.149.3681.295. [DOI] [PubMed] [Google Scholar]
- Mangos J. A. Morphological and functional characterization of isolated human parotid acinar cells. J Dent Res. 1979 Oct;58(10):2028–2035. doi: 10.1177/00220345790580101601. [DOI] [PubMed] [Google Scholar]
- NOBLE D. The voltage dependence of the cardiac membrane conductance. Biophys J. 1962 Sep;2:381–393. doi: 10.1016/s0006-3495(62)86862-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A., Petersen O. H. Membrane potential and resistance measurement in acinar cells from salivary glands in vitro: effect of acetylcholine. J Physiol. 1974 Oct;242(1):173–188. doi: 10.1113/jphysiol.1974.sp010700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson O. H., Iwatsuki N. The role of calcium in pancreatic acinar cell stimulus-secretion coupling: an electrophysiological approach. Ann N Y Acad Sci. 1978 Apr 28;307:599–617. doi: 10.1111/j.1749-6632.1978.tb41984.x. [DOI] [PubMed] [Google Scholar]
- Quissell D. O., Redman R. S. Functional characteristics of dispersed rat submandibular cells. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2789–2793. doi: 10.1073/pnas.76.6.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose B., Loewenstein W. R. Permeability of a cell junction and the local cytoplasmic free ionized calcium concentration: a study with aequorin. J Membr Biol. 1976 Aug 27;28(1):87–119. doi: 10.1007/BF01869692. [DOI] [PubMed] [Google Scholar]
- Rose B., Rick R. Intracellular pH, intracellular free Ca, and junctional cell-cell coupling. J Membr Biol. 1978 Dec 29;44(3-4):377–415. doi: 10.1007/BF01944230. [DOI] [PubMed] [Google Scholar]
- Satir P., Gilula N. B. The fine structure of membranes and intercellular communication in insects. Annu Rev Entomol. 1973;18:143–166. doi: 10.1146/annurev.en.18.010173.001043. [DOI] [PubMed] [Google Scholar]
- Schanne O., Coraboeuf E. Potential and resistance measurements of rat liver cells in situ. Nature. 1966 Jun 25;210(5043):1390–1391. doi: 10.1038/2101390a0. [DOI] [PubMed] [Google Scholar]
- Shimono M., Yamamura T., Fumagalli G. Intercellular junctions in salivary glands: freeze-fracture and tracer studies of normal rat sublingual gland. J Ultrastruct Res. 1980 Sep;72(3):286–299. doi: 10.1016/s0022-5320(80)90065-9. [DOI] [PubMed] [Google Scholar]
- Vozhkova V. P., Kovalev S. A., Mittel'man L. A., Shilianskaia E. N. Izmeneniia provodimosti mezhkletochnykh kontaktov v protsesse differentsirovki kletok sliunnoi zhelezu lichinok Drosophila virilis na 3-i stadii razvitiia. Tsitologiia. 1970 Sep;12(9):1110–1115. [PubMed] [Google Scholar]
- WATANABE A., GRUNDFEST H. Impulse propagation at the septal and commissural junctions of crayfish lateral giant axons. J Gen Physiol. 1961 Nov;45:267–308. doi: 10.1085/jgp.45.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIDMANN S. The electrical constants of Purkinje fibres. J Physiol. 1952 Nov;118(3):348–360. doi: 10.1113/jphysiol.1952.sp004799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIENER J., SPIRO D., LOEWENSTEIN W. R. STUDIES ON AN EPITHELIAL (GLAND) CELL JUNCTION. II. SURFACE STRUCTURE. J Cell Biol. 1964 Sep;22:587–598. doi: 10.1083/jcb.22.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Venrooij G. E., Hax W. M., van Dantzig G. F., Prijs V., van der Deiner J. J., van der Gon Model approaches for the evaluation of electrical cell coupling in the salivary gland of the larva of Drosophila hydei. The influence of lysolecithin on the electrical coupling. J Membr Biol. 1974;19(3):229–252. doi: 10.1007/BF01869980. [DOI] [PubMed] [Google Scholar]


