Abstract
Convective mixing (CM) refers to the different transport mechanisms except Brownian diffusion that irreversibly transfer inspired air into resident air and can be studied using aerosol bolus inhalations. This paper provides a review of the present understanding of how each of these mechanisms contributes to CM. Original data of the combined effect of stretch and fold and gravitational sedimentation on CM are also presented. Boli of 0.5 μm-diameter particles were inhaled at penetration volumes (Vp) of 300 and 1200 ml in eight subjects. Inspiration was followed by a 10-s breath hold, during which small flow reversals (FR) were imposed, and expiration. There was no physiologically significant dependence in dispersion and deposition with increasing FR. The results were qualitatively similar to those obtained in a previous study in microgravity in which it was speculated that the phenomenon of stretch and fold occurred during the first breathing cycle without the need of any subsequent FR.
Keywords: Particle deposition, Aerosol bolus dispersion, Gravity
1. Introduction
Altshuler et al. (1959) pioneered the use of aerosols to study pulmonary ventilation. They recognized that a particle with minimal intrinsic mobility could act as a non-diffusing gas and closely follow the air streamlines. Particles could therefore be used to study pulmonary mixing that did not result from molecular diffusion. Using full inspirations of 0.5 μm-diameter particles, they showed that a significant number of particles were transported from the tidal air into the resident air during breathing. Such transport was the result of the mixing of the flow patterns without additional mixing by molecular diffusion. This was the first experimental evidence that convective transport plays an important role in pulmonary mixing.
Since this early work, aerosol bolus inhalations have been widely used to study mixing processes at various depths within the lung (Brand et al., 1997; Brown et al., 1995; Darquenne et al., 1998, 1999; Heyder et al., 1988; Rosenthal et al., 1992). This technique consists of inserting a small amount of aerosol (a bolus) at a predetermined point in the subject’s inspiratory volume and analyzing the distribution of the aerosol bolus in the subsequent exhalation. Such analysis always shows that the expired bolus is spread over a larger volume than the inspired bolus, showing that particles are irreversibly transferred from the bolus to the adjacent air. All the mechanisms of particle movements, except Brownian diffusion that are involved in this transfer are usually referred to as convective mixing. Factors contributing to convective mixing include non-reversibility of velocity profiles within the airspaces, airway and alveolar geometries asymmetries between inspiratory and expiratory flows (Scherer and Haselton, 1982), non-homogeneous ventilation of the lung (Darquenne et al., 1999; Rosenthal, 1993), cardiogenic mixing (Darquenne et al., 2000; Scheuch and Stahlhofen, 1991) and the phenomenon of “stretch and fold” (Butler and Tsuda, 1998; Darquenne and Prisk, 2004). It should be noted that in this context, the term convective mixing not only include mechanisms that are specific to convection itself but also mechanisms, such as inertia and gravitational sedimentation that result in particle crossing streamlines.
The concept of stretch and fold was introduced by Butler and Tsuda (1998) and Tsuda et al. (2002). These authors suggested that the mixing of convective streamlines in the airways was much more complex than previously thought. Using flow visualization techniques, they showed that the reciprocal motion of the air in the airways wraps the streamlines around each other during tidal breathing. As a consequence, initially close streamlines tend to diverge from one another while previously widely separated streamlines are brought into close apposition with each other. As such, geometrically long diffusion distances are greatly reduced and the effect is that of an increase in the apparent diffusion coefficient, potentially contributing to acinar mixing.
In a previous study by our group (Darquenne and Prisk, 2004), we performed a series of bolus studies with a protocol designed to induce a complex folding pattern within the confines of a controlled, invariant respiratory maneuver in an attempt to determine whether the phenomenon of stretch and fold was an operative mechanism in aerosol mixing in humans. Boluses were inhaled both at a shallow (Vp = 300 ml) and deep (Vp = 1200 ml) acinar depth with the expectation that the mechanism of stretch and fold would be more readily observed at the higher penetration volume. Such expectation was based on the fact that, as the size of the airways is smaller at deeper penetration volumes, the stretched-and-folded streamlines would be confined in a smaller space and the characteristic distance between streamlines would be reduced. As a consequence, we would expect that a smaller number of flow reversals would be necessary at deep than shallow acinar depth before diffusion became an effective mixing mechanism. This previous study was performed in microgravity to avoid the confounding effect of gravitational sedimentation. We hypothesized that with every additional flow reversal, aerosol mixing in the lung would slowly but steadily increase until a sharp transition in the extent of mixing would occur after which any additional flow reversal would only have minimal impact on the level of mixing. While the data were suggestive although not definitive of the concept, we speculated that the phenomenon of stretch and fold already occurred during one single flow reversal in the lung. This conclusion was consistent with the high degree of dispersion and deposition previously observed with single bolus inhalations in microgravity (Darquenne et al., 1998, 1999, 2000) and the complex mixing pattern seen in polymer filled rat lungs after a single breathing cycle (Tsuda et al., 2002).
In the present study, we performed a similar series of bolus inhalations with flow reversals in normal gravity. Data were compared to those previously obtained in microgravity to determine the combined effect of flow reversals and gravity on convective mixing. The paper also includes a detailed review of the different mechanisms that are thought to contribute to convective mixing in the acinus.
2. Methods
2.1. Equipment
Aerosol bolus data were collected with the equipment shown in Fig. 1. The system is similar to that used in our previous study in microgravity (Darquenne and Prisk, 2004). Briefly, the system allowed the injection of an aerosol bolus with a half-width of ~70 ml at a given point in the inhalation by switching computer-controlled pneumatic valves. Following the initial bolus inhalation maneuver, a piston assembly connected to the sliding valve SV2 could be used to produce flow reversal maneuvers as described in Section 2.3. The measurement of the aerosol concentration and the flow rate were provided by a photometer (model 993000, PARI, GmbH) (Westenberger et al., 1992) and a pneumotachograph (Fleisch #1, OEM medical), respectively. The photometer, the pneumotachograph and the valves were heated to body temperature to prevent water condensation. A diffusion dryer was located between the photometer and the mouthpiece. It removed the water vapor from the exhaled air to avoid condensation on the lenses of the photometer.
Fig. 1.
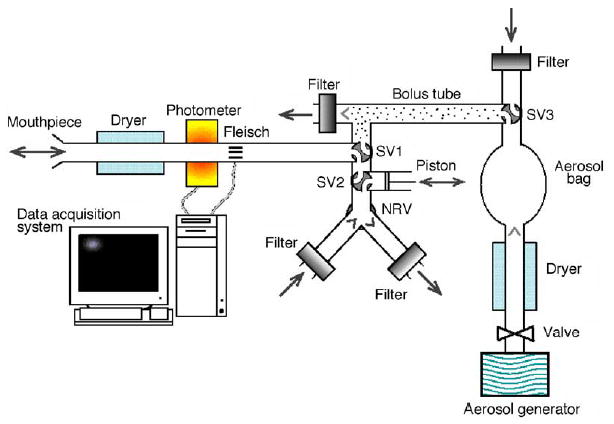
Schematic representation of experimental system. One configuration of sliding valves (SV) SV1 and SV2 allowed the subject to breathe air from the room through a two-way non-rebreathing valve (NRV) equipped with filters. In the other configuration of the valve SV1, subject inspired aerosol bolus located between SV1 and SV3. A syringe assembly to produce the flow reversal maneuvers was connected to one port of SV2. The breath hold in the absence of flow reversals was accomplished by maintaining the piston in a constant position. Measurement of aerosol concentration and flow rate was provided by a photometer and a pneumotachograph (Fleisch no. 1), respectively.
A laptop computer equipped with a 12-bit multifunction I/O card (National Instrument, DAQPad 6020E) was used for data acquisition. Signals from the photometer and the pneumotachograph were sampled at 100 Hz. We used the same custom software as in our previous study (Darquenne and Prisk, 2004) for the data acquisition.
2.2. Aerosol generation
The bolus tube was filled with aerosol containing monodisperse polystyrene latex particles (Duke Scientific). The particles were supplied in suspension (water) and the concentrate was diluted and dispensed via 2 Acorn II nebulizers (Marquest Medical Products, Inc). Before entering the bolus tube, the aerosol flowed through a heated hose and a diffusion dryer to remove water droplets so that the resulting aerosol was made of dry latex particles of uniform size.
The size of the particle used in this study was, as provided by the manufacturer, 0.505 ± 0.010 (S.D.) μm. For convenience, the particles are referred to as 0.5 μm-diameter particles. Aerosol concentration was ~104 particles/ml of gas. Previous size analysis (Darquenne et al., 1999) have shown that the number of doublets in the aerosol was <4.5% for this particle size.
2.3. Subjects and protocol
Eight healthy subjects participated in the study. They were the same subjects that participated in our previous study in microgravity (Darquenne and Prisk, 2004) and we retained their subject numbers for comparison purposes. The relevant anthropometric data of the subjects are listed in Table 1.
Table 1.
Anthropometric data
| Subject no. | Sex | Age (years) | Height (cm) | Weight (kg) | FVC (%pred) | FEV1/FVC (%pred) |
|---|---|---|---|---|---|---|
| 1 | F | 36 | 164 | 56 | 117 | 96 |
| 2 | F | 33 | 163 | 67 | 105 | 100 |
| 3 | M | 54 | 191 | 99 | 131 | 102 |
| 4 | M | 46 | 185 | 116 | 104 | 106 |
| 6 | M | 34 | 188 | 110 | 91 | 93 |
| 7 | M | 37 | 185 | 100 | 114 | 77 |
| 8 | F | 39 | 175 | 82 | 89 | 107 |
| 9 | F | 26 | 167 | 61 | 89 | 109 |
M, male; F, female; FVC, forced vital capacity; FEV1, forced expiratory volume in 1 sec; %pred, % predicted.
After a few normal breaths, the seated subject exhaled to residual volume (RV) to ensure a known lung volume starting point. The test breath consisted of an inspiration from RV to 1l above functional residual capacity (FRC) at a flow rate of ~0.45 l/s, a 10 s breath hold, and an expiration to RV, also at a flow rate of ~0.45 l/s. A flowmeter provided visual feedback to the subject who was well-trained in the maneuver. During the inspiration, an aerosol bolus of ~70 ml was introduced at two different target penetration volumes (Vp = 300 and 1200 ml). The penetration volume was defined as the volume of air inhaled from the mode of the aerosol bolus to the end of the inhalation.
During the breath hold, a variable number of small identical flow reversals were imposed on the subject using the piston assembly. Each flow reversal consisted of a small inspiratory maneuver immediately followed by an expiratory maneuver, resulting in no overall change in lung volume. The flow reversals were performed using a crank system so that volume changes were approximately sinusoidal in nature. The volume fluctuations generated by the flow reversals were chosen such that the aerosol bolus was forced to traverse at least one generation of alveolated ducts, an important point since non-reversibility of the flow at bifurcations is believed to greatly contribute to the mechanism of stretch and fold (Tsuda et al., 1999). For a penetration volume of 300 ml, the piston produced a 100 ml inspiratory maneuver followed by a 100 ml expiratory maneuver in 1 s, providing a flow of ~200 ml/s in each direction. For a penetration volume of 1200 ml, the piston produced a 500 ml inspiratory maneuver followed by a 500 ml expiratory maneuver in 2 s, providing a flow of ~500 ml/s in each direction. These flows were chosen to maintain a low Reynolds number in the lung region reached by the bolus. Zero, one, four, and seven flow reversals were imposed for Vp = 300 ml and 0, 1, 3, and 4 flow reversals for Vp = 1200 ml, matching the protocol used in previous parabolic flight experiments (Darquenne and Prisk, 2004).
The protocol was repeated three times for each penetration volume and each number of flow reversals. The protocol was approved by the Human Research Protection Program at the University of California, San Diego. Subjects signed a statement of informed consent.
2.4. Data analysis
For each bolus test, we calculated the aerosol deposition (DE) and the aerosol bolus dispersion (H). Calculations were performed in a similar manner as in our previous studies (Darquenne et al., 1998, 1999, 2000; Darquenne and Prisk, 2004). Briefly, aerosol deposition was calculated using the following equation,
| (1) |
where Nin and Nex are the number of particles in the inspired and expired bolus, respectively. Nin and Nex were calculated from the integration of the aerosol concentration multiplied by the instantaneous flow rate. The integration was only done when the concentration exceeded 5% of the maximal expired concentration in order to reduce error due to the noise of the signal (Darquenne et al., 1998).
On a graph of aerosol concentration as a function of the respired volume, we computed the bolus half-width defined as the difference in volume (ml) between the two points of one-half the maximum concentration of the bolus. The change in half-width H reflects the aerosol dispersion and was obtained by the following equation,
| (2) |
where Hin and Hex are the half-width of the inspired and expired boluses, respectively.
2.5. Statistical analysis
The same type of statistical analysis as in our previous study (Darquenne and Prisk, 2004) was used. Briefly, for each experimental condition (penetration volume and number of flow reversals) and for each subject, one single value for DE and H was determined as described below and used in the statistical analysis. For each target penetration volume, there was some variability in the measured Vp. Therefore, DE and H were determined at each target Vp by linear regression of the repeated measurements as a function of measured Vp. Linear regressions were performed separately for each subject and each experimental condition. By doing so, we eliminated the confounding effect of variations in penetration volume on DE and H. Using a previously published data set of bolus inhalations performed with no breath hold in normal gravity (Darquenne et al., 1999), we estimated that a variation of ± 100 ml around at a penetration volume of 300 and 1200 ml would lead to a difference in deposition of respectively, ± 15 and ± 9% when normalized to deposition measured at the target penetration volume.
For each penetration volume, a one-way analysis of variance (ANOVA) for correlated samples was then performed to test for differences in deposition and dispersion between flow reversals. Post-ANOVA pair-wise comparisons using Tukey HSD test was performed for tests showing significant F ratios. Significant differences were accepted at the p < 0.05 level.
3. Results
We collected data on eight subjects with 0.5 μm-diameter particles at both penetration volumes. However, exhaled boli could not be identified in five of the subjects at Vp = 1200 ml because of high deposition values. Therefore, data are reported for eight subjects at Vp = 300 ml and for three subjects (S3, S4 and S7) at Vp = 1200 ml. Averaged over all the tests performed, measured penetration volumes were (314 ± 97) ml (mean ± S.D.) and (1238 ± 98) ml for Vp = 300 and 1200 ml, respectively.
Fig. 2 shows the aerosol bolus dispersion (H, mean ± S.D.) and deposition (DE, mean ± S.D.) of 0.5 μm-diameter particles as a function of the number of flow reversals (FR) performed during the 10-s breath hold at a penetration volume of 300 ml (closed symbols). Dispersion varied between 366 ± 69 ml for FR = 0 to 455 ± 111 ml for FR = 7 (Fig. 2A). Significant differences in dispersion were only found between FR = 0 and FR = 7 (p < 0.05), although the increase was small. Deposition was unaffected by the presence of flow reversals (Fig. 2B) and averaged 57 ± 13% over all the tests. The data were then compared to those previously obtained in the same subjects in the absence of gravity aboard the NASA Microgravity Research Aircraft (Darquenne and Prisk, 2004). Data obtained in microgravity are shown in Fig. 2 by open symbols. Comparison shows that deposition was significantly higher in normal gravity compared to microgravity for all flow reversals (p < 0.01, Fig. 2B). In sharp contrast, there was no significant difference in dispersion between the gravity levels (Fig. 2A).
Fig. 2.
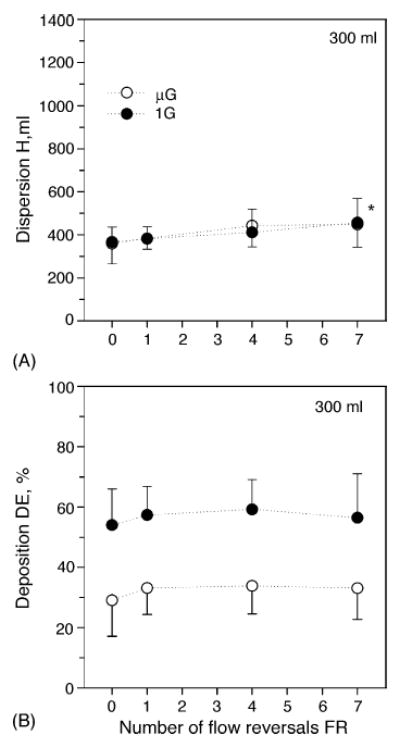
Data averaged over all subjects (mean ± S.D.; n = 8) for a penetration volume Vp of 300 ml. Data are plotted as a function of flow reversals. (•) Normal gravity; (○) microgravity. A, Dispersion; B, Deposition. *Significantly different from FR = 0. Data in microgravity are from Darquenne and Prisk (2004).
Data obtained at Vp = 1200 ml are shown in Fig. 3 in the same format as in Fig. 2. In normal gravity (closed symbols), there were no significant effects on either dispersion or deposition based on the number of flow reversals in normal gravity, a situation matching that seen in microgravity. Averaged over all flow reversals, dispersion was 1067 ± 134 ml and deposition was 79 ± 10%. Comparison between gravity levels shows that both dispersion and deposition were significantly higher in normal gravity compared to microgravity for all flow reversals (p < 0.01). Note that data in microgravity refer to the same three subjects as those for which identifiable exhaled boli could be obtained in normal gravity.
Fig. 3.
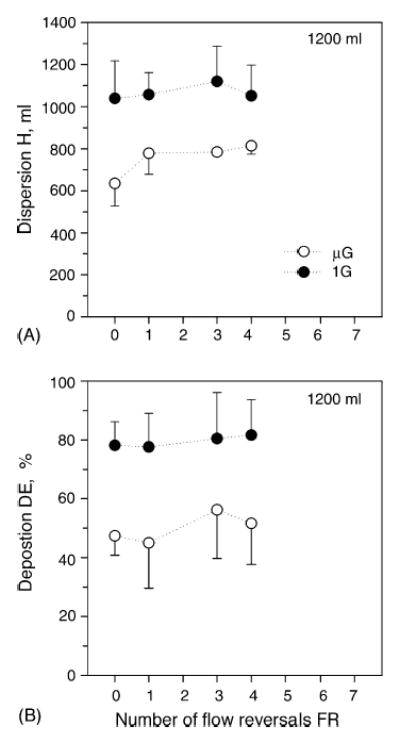
Data averaged over three subjects (mean ± S.D.; S3, S4 and S7) for a penetration volume Vp of 1200 ml. Data are plotted as a function of flow reversals. (•), Normal gravity; (○), microgravity. A, Dispersion; B, Deposition. Data in microgravity are from the same three subjects (Darquenne and Prisk, 2004).
When deposition reaches values above ~90%, the signal to noise ratio is such that accurate determination of both deposition and bolus dispersion becomes very difficult. Data for subjects with such high values were not reported. As a consequence, data presented at Vp = 1200 ml in normal gravity represent a lower bound for both deposition and bolus dispersion. Even so, there were significant differences between data measured in normal gravity and microgravity (on the same three subjects). Therefore, we do not think that the removal of five of our eight subjects in our data set at Vp = 1200 ml has introduced a significant bias in our analysis.
4. Discussion
4.1. Mechanisms contributing to convective mixing in the acinus
Convective mixing refers to all the mechanisms except Brownian diffusion that contribute to the transfer of inhaled particles to the resident air. It has long been assumed that flow-induced mixing was only significant in proximal airways (Ultman et al., 1978) and that acinar mixing was largely governed by molecular diffusion with minor effects of convection. This concept was challenged by the work Heyder et al. (1988) who performed aerosol bolus inhalations at different depths in the lung. Heyder et al. (1988) used aerosol bolus dispersion as a measure of convective mixing. They showed that dispersion increased linearly with increasing penetration volume. These results provided strong evidence that convective mixing occurs beyond the central airways. Even more interestingly, the linearity of the increase in bolus dispersion with increasing bolus penetration strongly suggests that convective mixing processes in the periphery of the lung have mixing coefficients that are similar to those representative of mixing in the central airways, although, given the difference in geometry and in Reynolds number between the central airways and the acinus, acinar mixing likely results from different mechanisms than those occurring in the central airways.
One of the factors that have been proposed to significantly contribute to convective acinar mixing is the non-homogeneous ventilation of the lung. It is well known that ventilation inhomogeneities are highly sensitive to gravity (Guy et al., 1994; Prisk et al., 1995; Verbanck et al., 1996). Because the lung distorts under its own weight, the alveoli at the base of the lung are relatively compressed compared with the apical alveoli and because poorly expanded alveoli are more compliant, ventilation is greater near the bottom of the lung and becomes progressively lower near the top. Changes in gravity level, and thus in lung weight, will therefore affect the distribution of ventilation and as a consequence convective mixing. It should however be noted that any inhomogeneity in the topographical distribution of ventilation per se will not result in any increase in convective mixing without the presence of flow asynchrony.
Darquenne et al. (1999) performed a series of aerosol bolus inhalations in microgravity, normal gravity and hypergravity and showed that aerosol bolus dispersion was gravity-dependent with the greatest dispersion occurring for the highest gravity level. In particular, when particles with minimal intrinsic motion properties were used (~0.5 μm), the difference between dispersion in microgravity and normal gravity mainly reflects the increase in ventilatory inhomogeneities from microgravity to normal gravity. Darquenne et al. (1999) determined that the slope of the linear regression between dispersion and penetration volume almost doubled between microgravity and normal gravity, suggesting that non-gravitational convective ventilatory inhomogeneities are at least as large as gravitational convective ventilatory inhomogeneities, a result consistent with studies of gas mixing (Verbanck et al., 1996). These results also demonstrated the presence of significant gravity-independent convective ventilatory inhomogeneity. Such direct observation of convective inhomogeneity in the acinar region of the lung cannot be made when using gases because convective effects cannot be separated from the diffusive effects that dominate gas transport in the acinus.
Differences in velocity profiles between inspiration and expiration are also likely to contribute to convective mixing. Such differences result from the branching structure of the bronchial tree that imparts a directional asymmetry to the flow field. During inspiration, airflow is more rapid at the center of the airways. Therefore, particles in the center of the airways reach more distal regions of the lung than do particles located near the walls of the airways, where velocities are smaller. During expiration, the velocity profile is more blunted and particles at the center of the airways travel a slower rate than during inspiration, whereas particles near the walls travel faster, preventing the aerosol bolus from recovering its original shape. This mechanism is however not expected to be significant in the acinar region of the lung where Stokes flow dominates. Therefore, the difference in dispersion we measured between boluses inhaled to shallow and deep acinar depth cannot be explained by this mechanism. Indeed, for such flow, parabolic velocity profiles are established in each alveolated duct very rapidly within a distance that is less than 2% of the duct length (Darquenne, 2001). Using a two-dimensional model of a six-generation structure of alveolated ducts, Darquenne and Prisk (2003) studied the dispersion undergone by an aerosol bolus during its transport in the alveolar region of the lung and showed that the minor differences between inspiratory and expiratory velocity profiles resulted in only a very small effect on simulated dispersion. It should however be noted that, as their model did not include the rhythmical motions of the alveolar space, they could not simulate any potential differences in the particle trajectories between inspiration and expiration that could lead to increased dispersion.
Other authors (Heyder et al., 1988; Scheuch and Stahlhofen, 1991) have also suggested that cardiogenic mixing contributes to the dispersion of an aerosol bolus. This effect can be differentiated from the mixing resulting from inhaled and exhaled flows by looking at the effect of breath-holds on overall dispersion. Scheuch and Stahlhofen (1991) performed such experiments on a subject at rest and after exercise when the heart rate was increased by more than a factor of two. They showed that the motion of the heart influenced aerosol dispersion with a more pronounced effect at shallow penetrations. Darquenne et al. (2000) performed similar bolus inhalations on resting subjects in microgravity so that the effect of cardiogenic mixing could be studied without the confounding effect of gravitational sedimentation. Their data also suggested that cardiogenic mixing contributes to aerosol dispersion and that, although limited, the effect was more pronounced in the central airways than in the periphery of the lung.
Finally, it has been proposed that the phenomenon of stretch and fold might be another mechanism that contributes to convective mixing (Butler and Tsuda, 1998). Stretch and fold addresses the mechanism of complex mixing that is thought to occur in the acinus as a result of the rhythmical expansion and contraction of complex alveolar structures. Because of the reciprocal motion of the air in the airways, the streamlines fold back on each other as tidal breathing continues. As such, previously long geometrical distances that could not be covered by diffusive transport are greatly reduced and the effect is that of an increase in the apparent diffusion coefficient. Butler and Tsuda (1998) and Tsuda et al. (2002) first reported the existence of such complex stretch and fold mixing patterns in a rat lung filled with white silicone oil and ventilated with blue silicone oil for several tidal breaths. The major finding of their studies was that the presence of even modest alveolar flow irreversibility leads to a sudden increase in mixing that results from the coupling of diffusion with the mechanism of stretch and fold (Butler and Tsuda, 1998).
4.2. Effect of flow reversals and gravity on convective mixing
In a previous study by our group (Darquenne and Prisk, 2004), we performed a series of bolus inhalations in the absence of gravity with a protocol designed to examine the influence of the phenomenon of stretch and fold on aerosol mixing in the acinar region of the lung. We hypothesized that with each additional flow reversal, dispersion would slowly but steadily increase until a sharp transition in the extent of mixing would occur when the slowly increasing diffusive length scale approximately matched that of the rapidly decreasing convective length scale associated with the kinetics of folding. The same protocol was repeated in this study in normal gravity so that the influence of stretch and fold in conjunction with the effects of gravitational sedimentation could be examined. Gravity causes particles to sediment and cross streamlines while stretch and fold moves streamlines relative to each other. Therefore, there is a potential for interaction between the two mechanisms, although the effect of such an interaction was unclear to us from a theoretical viewpoint. To our knowledge, the only attempt to address this potential interaction was made by Haber et al. (2003) in a numerical model of a single expanding and contracting alveolus. In that model, they predicted that the combined effect of gravitational sedimentation and rhythmic alveolar expansion substantially enhanced convective mixing compared to mixing resulting from the alveolar expansion alone.
The same subjects participated in both studies allowing for direct comparison of the results. Our data show that the effect of flow reversals on aerosol dispersion and deposition in normal gravity is qualitatively similar to that in microgravity (Figs. 2 and 3). The microgravity data have been extensively described previously (Darquenne and Prisk, 2004). Briefly, these previous data showed that increasing the number of flow reversals had almost no effect on aerosol dispersion and deposition. There were two main potential interpretations to those results: either the phenomenon of stretch and fold did not occur within the number of flow reversals performed in the experiment, or the phenomenon had already occurred during the one breathing cycle that is included in the basic maneuver, without the need for any subsequent additional flow reversals. While neither of these two suggestions could be fully supported by previous published data, we speculated that the phenomenon of stretch and fold occurred within one breathing cycle included in the basic maneuver. Such a conclusion was consistent with the high degree of dispersion and deposition observed previously in microgravity (Darquenne et al., 1997, 1998, 1999, 2000). Further, in a study using silicone filled rat lungs, Tsuda et al. (2002) showed direct evidence of complex mixing after only one breathing cycle and complete mixing within as few as three to five mechanical oscillations. Because the effect of flow reversals we observed in normal gravity is qualitatively similar to that measured in microgravity, it is very likely that the same conclusion applies to our results in normal gravity.
Because of the small effect of flow reversals on both dispersion and deposition at each gravity level, data are pooled by penetration volume and gravity levels for the remaining of the discussion. The pooled data are summarized in Table 2. The increases in both deposition and dispersion as a function of gravity level were qualitatively similar in the distal region of the acini (Vp = 1200 ml) while there was a clear dissociation between deposition and dispersion in the vicinity of the acinar entrance (Vp = 300 ml). Gravity clearly increased deposition while it had no effect on dispersion.
Table 2.
Effect of gravity level and penetration volume on aerosol bolus dispersion (H) and deposition (DE)
|
Vp = 300 ml
|
Vp = 1200 ml
|
|||
|---|---|---|---|---|
| μG | 1 G | μG | 1 G | |
| H (ml) | 403 ± 90 | 403 ± 92 | 753 ± 97* | 1067 ± 134 |
| DE (%) | 33 ± 9* | 57 ± 11 | 50 ± 12* | 79 ± 10 |
Values are mean ± S.D. Vp, penetration volume; μG, microgravity; 1 G, normal gravity.
Significantly different from 1 G data (p < 0.001).
Our experimental design allowed us to probe the lung both near the acinar entrance (Vp = 300 ml) and deep in the periphery of the acinus (Vp = 1200 ml). Gas mixing inhomogeneity in these two distinct regions has been widely studied using single-breath (SBW) and multi-breath (MBW) washins of tracer gases with largely differing diffusivities (Crawford et al., 1985; Engel et al., 1979). Inhomogeneity of ventilation distribution can be attributed to both convection-dependent inhomogeneity (CDI) and to diffusion- and convection-dependent inhomogeneity (DCDI). CDI is thought to take place proximal to the acinar entrance while DCDI is an effective mixing mechanism in the periphery of the lung where convective and diffusive processes are of the same order of magnitude. It is likely that CDI also occur in the periphery of the lung, however, such a mechanism cannot be detected with gases because of their high diffusivity. Indeed, while convective inhomogeneity might create differences in gas concentrations, these differences will be completely abolished by diffusion. The use of particles, which have a diffusivity several orders of magnitude smaller than any gas, is therefore well-suited to probe convective ventilatory inhomogeneities in the periphery of the lung.
Our data showed that at shallow acinar depth, there were no significant differences in dispersion between gravity levels, suggesting that gravitationally-induced CDI is negligible up to the vicinity of the acinar entrance. This is consistent with observations of Prisk et al. (1995) who showed that, in contrast to measurements performed during vital capacity maneuvers, microgravity resulted in only small changes in CDI when measurements were made with near-normal tidal volumes from FRC. Our dispersion data at Vp = 300 ml are therefore in agreement with the previous gas studies as these aerosol boli were probing lung volumes near FRC. In the more peripheral region of the lung, dispersion was significantly higher in normal gravity than in microgravity, suggesting that significant convective ventilatory inhomogeneities exist in the periphery of the lung. An alternative explanation may be that, in the periphery of the lung, crossing of the streamlines due to gravitational sedimentation significantly affects aerosol dispersion, whereas more centrally such a mechanism is not significant. However, the effect of such a mechanism is likely to be small for 0.5 μm-diameter particles. Indeed, a particle of this size would only sediment ~9 μm in 1 s; half the distance, the same particle would be covered by diffusion in the same amount of time (Darquenne et al., 1999). While the presence of CDI in the periphery of the lung is of little interest in gas transport (indeed, any gradient in gas concentration would quickly equilibrate by molecular diffusion), such mechanism may have important implications in the deposition patterns of inhaled particles.
Acknowledgments
This work was supported by National Institute of Environmental Health Sciences grant 1 RO1 ES11184.
References
- Altshuler B, Palmes ED, Yarmus L, Nelson N. Intrapulmonary mixing of gases studied with aerosols. J Appl Physiol. 1959;14:321–327. doi: 10.1152/jappl.1959.14.3.321. [DOI] [PubMed] [Google Scholar]
- Brand P, Rieger C, Schulz H, Beinert T, Heyder J. Aerosol bolus dispersion in healthy subjects. Eur Respir J. 1997;10:460–467. doi: 10.1183/09031936.97.10020460. [DOI] [PubMed] [Google Scholar]
- Brown JS, Gerrity TR, Bennett WD, Kim CS, House DE. Dispersion of aerosol boluses in the human lung: dependence on lung volume, bolus volume, and gender. J Appl Physiol. 1995;79:1787–1795. doi: 10.1152/jappl.1995.79.5.1787. [DOI] [PubMed] [Google Scholar]
- Butler JP, Tsuda A. Effect of convective stretching and folding on aerosol mixing deep in the lung, assessed by approximate entropy. J Appl Physiol. 1998;83:800–809. doi: 10.1152/jappl.1997.83.3.800. [DOI] [PubMed] [Google Scholar]
- Crawford ABH, Makowska M, Paiva M, Engel LA. Convection-and diffusion-dependent ventilation maldistribution in normal subjects. J Appl Physiol. 1985;59:838–846. doi: 10.1152/jappl.1985.59.3.838. [DOI] [PubMed] [Google Scholar]
- Darquenne C. A realistic two-dimensional model of aerosol transport and deposition in the alveolar zone of the human lung. J Aerosol Sci. 2001;32:1161–1174. [Google Scholar]
- Darquenne C, Paiva M, Prisk GK. Effect of gravity on aerosol dispersion and deposition in the human lung after periods of breath-holding. J Appl Physiol. 2000;89:1787–1792. doi: 10.1152/jappl.2000.89.5.1787. [DOI] [PubMed] [Google Scholar]
- Darquenne C, Paiva M, West JB, Prisk GK. Effect of microgravity and hypergravity on deposition of 0.5 to 3 μm-diameter aerosol in the human lung. J Appl Physiol. 1997;83:2029–2036. doi: 10.1152/jappl.1997.83.6.2029. [DOI] [PubMed] [Google Scholar]
- Darquenne C, Prisk GK. Effect of gravitational sedimentation on simulated aerosol dispersion in the human acinus. J Aerosol Sci. 2003;34:405–418. doi: 10.1016/s0021-8502(02)00187-8. [DOI] [PubMed] [Google Scholar]
- Darquenne C, Prisk GK. Effect of small flow reversals on aerosol mixing in the alveolar region of the huma lung. J Appl Physiol. 2004;97:2083–2089. doi: 10.1152/japplphysiol.00588.2004. [DOI] [PubMed] [Google Scholar]
- Darquenne C, West JB, Prisk GK. Deposition and dispersion of 1 μm aerosol boluses in the human lung: effect of micro- and hypergravity. J Appl Physiol. 1998;85:1252–1259. doi: 10.1152/jappl.1998.85.4.1252. [DOI] [PubMed] [Google Scholar]
- Darquenne C, West JB, Prisk GK. Dispersion of 0.5–2 μm aerosol in micro- and hypergravity as a probe of convective inhomogeneity in the human lung. J Appl Physiol. 1999;86:1402–1409. doi: 10.1152/jappl.1999.86.4.1402. [DOI] [PubMed] [Google Scholar]
- Engel LA, Paiva M, Siegler DIM, Fukuchi Y. Dual tracer single-breath studies of gas transport in the lung. Respir Physiol. 1979;36:103–119. doi: 10.1016/0034-5687(79)90018-5. [DOI] [PubMed] [Google Scholar]
- Guy HJB, Prisk GK, Elliott AR, Deutschman RAI, West JB. Inhomogeneity of pulmonary ventilation during sustained microgravity as determined by single-breath washouts. J Appl Physiol. 1994;76:1719–1729. doi: 10.1152/jappl.1994.76.4.1719. [DOI] [PubMed] [Google Scholar]
- Haber S, Yitzhak D, Tsuda A. Gravitational deposition in a rhytmically expanding and contracting alveolus. J Appl Physiol. 2003;95:657–671. doi: 10.1152/japplphysiol.00770.2002. [DOI] [PubMed] [Google Scholar]
- Heyder J, Blanchard JD, Feldman HA, Brain JD. Convective mixing in human respiratory tract: estimates with aerosol boli. J Appl Physiol. 1988;64:1273–1278. doi: 10.1152/jappl.1988.64.3.1273. [DOI] [PubMed] [Google Scholar]
- Prisk GK, Guy HJB, Elliott AR, Paiva M, West JB. Ventilatory inhomogeneity determined from multiple-breath washouts during sustained microgravity on Spacelab SLS-1. J Appl Physiol. 1995;78:597–607. doi: 10.1152/jappl.1995.78.2.597. [DOI] [PubMed] [Google Scholar]
- Rosenthal FS. The effect on non-uniform ventilation on the dispersion of inspired aerosol boluses: a modeling study. J Aerosol Med. 1993;6:177–197. [Google Scholar]
- Rosenthal FS, Blanchard JD, Anderson PJ. Aerosol bolus dispersion and convective mixing in human and dog lungs and physical models. J Appl Physiol. 1992;73:862–873. doi: 10.1152/jappl.1992.73.3.862. [DOI] [PubMed] [Google Scholar]
- Scherer PW, Haselton FR. Convective exchange in oscillatory flow through bronchial-tree models. J Appl Physiol. 1982;53:1023–1033. doi: 10.1152/jappl.1982.53.4.1023. [DOI] [PubMed] [Google Scholar]
- Scheuch G, Stahlhofen W. Effect of heart rate on aerosol recovery and dispersion in human conducting airways after periods of breath-holding. Exp Lung Res. 1991;17:763–787. doi: 10.3109/01902149109062877. [DOI] [PubMed] [Google Scholar]
- Tsuda A, Otani O, Butler JP. Acinar flow irreversibility caused by perturbations in reversible alveolar wall motion. J Appl Physiol. 1999;86:977–984. doi: 10.1152/jappl.1999.86.3.977. [DOI] [PubMed] [Google Scholar]
- Tsuda A, Rogers RA, Hydon PE, Butler JP. Chaotic mixing deep in the lung. Proc Nat Acad Sci USA. 2002;99:10173–10178. doi: 10.1073/pnas.102318299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ultman JS, Doll BE, Spiegel R, Thomas MW. Longitudinal mixing in pulmonary airways-normal subjects respiring at a constant flow. J Appl Physiol. 1978;44:297–303. doi: 10.1152/jappl.1978.44.2.297. [DOI] [PubMed] [Google Scholar]
- Verbanck S, Linnarsson D, Prisk GK, Paiva M. Specific ventilation distribution in microgravity. J Appl Physiol. 1996;80:1458–1465. doi: 10.1152/jappl.1996.80.5.1458. [DOI] [PubMed] [Google Scholar]
- Westenberger S, Gebhart J, Jaser S, Knoch M, Köstler R. A novel device for the generation and recording of aerosol micropulses in lung diagnostic. J Aerosol Sci. 1992;23:S449–S452. [Google Scholar]


