Abstract
The diagnostic usefulness of an enzyme-linked immunosorbent assay (ELISA) using a purified recombinant ribosome recycling factor from Brucella melitensis (CP24 antigen) was tested in human and canine infections caused by smooth and rough Brucella species, respectively. Anti-CP24 antibodies were detected in 9 (43%) of 21 consecutive cases of canine brucellosis and in 8 (53%) of 15 dogs followed for 60 days after the diagnosis of acute brucellosis. Among eight patients with acute brucellosis, anti-CP24 antibodies were detected in four in the 10 weeks following diagnosis, but the remaining four were negative during the whole follow-up (22 weeks). The frequency of anti-CP24 antibodies was also low among 24 patients with subacute brucellosis and 23 patients with chronic illness (29 and 26%, respectively). While all patients positive for anti-CP24 antibodies were also positive for antibodies to total cytoplasmic proteins of Brucella (CP), five were negative for antibodies to another cytoplasmic protein, the Brucella lumazine synthase (BLS). When a larger sample of 35 human sera negative for anti-BLS antibodies was assayed, 85.7% were positive for anti-CP24 antibodies, suggesting that the combined measurement of both reactivities could yield a higher sensitivity than any test alone. To test this hypothesis, an ELISA combining both antigens was designed. The percentage of positive results among chronic cases was higher for this assay than for the individual measurement of anti-CP24 or anti-BLS antibodies (83 versus 26 and 65%, respectively) and was closer to the value obtained for anti-CP antibodies (91%). The frequency of anti-CP24 antibodies is low in both canine and human brucellosis. In the latter case, however, an ELISA combining CP24 and BLS is more sensitive than assays measuring anti-CP24 or anti-BLS antibodies separately and almost as sensitive as the ELISA using CP.
Brucellosis is a zoonosis with a worlwide distribution which affects cattle, sheep, goats, pigs, and dogs and is transmitted to human beings in several ways. Because of the low sensitivity of bacteriological methods, serological tests are generally preferred to establish the diagnosis in both humans and animals. According to the nature of their lipopolysaccharide (LPS) molecules, Brucella species are classified as rough or smooth. An important drawback of conventional serological techniques (i.e., agglutination tests) is that the bacterial suspension used to diagnose infections caused by smooth species (Brucella abortus, Brucella melitensis, and Brucella suis) are not useful for diagnosing infections caused by rough species (Brucella ovis and Brucella canis), and vice versa. Therefore, antigens shared by all Brucella species could be of special interest for diagnostic purposes. Many such antigens have been described in recent years. For example, we have shown that the Brucella lumazine synthase (BLS), an 18-kDa cytoplasmic protein present in all Brucella species, is useful in the diagnosis of human and canine brucellosis (3, 9). Another antigen found in all Brucella species is a homologue of the ribosome recycling factor (RRF) from Escherichia coli and other species. This Brucella protein, known as CP24, is detected mainly in the cytoplasmic fraction of smooth and rough brucellae (5). It has been shown that CP24 is recognized by sera from sheep experimentally infected with B. melitensis but not by sera from animals vaccinated with the B. melitensis attenuated strain Rev-1 (6). In addition, the CP24 protein expressed in recombinant form in E. coli was recognized by sera from Brucella-infected sheep (12). However, the diagnostic usefulness of recombinant CP24 (rCP24) has not been assayed by enzyme-linked immunosorbent assay (ELISA) and has not been tested in infections caused by other Brucella species or in hosts other than sheep. We have recently described the preparation of purified rCP24 and have shown that this protein elicits a vigorous antibody response in immunized mice (4a). In the study presented here, we have investigated the diagnostic usefulness of an ELISA using purified CP24 in human and canine infections caused by smooth and rough Brucella species, respectively.
MATERIALS AND METHODS
Serum samples. (i) Human sera.
A total of 83 sera from patients at different stages of brucellosis were assayed. The initial cohort included 55 patients (71 samples) and was defined on clinical grounds. According to classical criteria (10), patients were classified as having acute brucellosis when the duration of symptoms was up to 8 weeks (n = 8), as having subacute illness when symptoms were present for 9 to 52 weeks (n = 24), and as having chronic brucellosis when symptoms had lasted for more than 1 year (n = 23). All acute patients were infected by B. melitensis and at diagnosis had a duration of illness of up to 40 days. Anti-CP24 antibodies were assayed in samples obtained at diagnosis and 10 and 22 weeks later. At diagnosis, these patients were positive by standard tube agglutination (STA), immunoglobulin M (IgM) antibodies to LPS by ELISA, and IgM and/or IgG antibodies to total cytoplasmic proteins of Brucella (CP). For subacute cases, the time since onset of symptoms ranged from 3 to 12 months (mean ± standard deviation [SD], 7.4 ± 3.1 months). At the time of blood sampling, all these patients were positive by STA and anti-LPS IgG. Anti-CP IgG was positive in 23 cases, anti-LPS IgM was positive in 21 cases, and anti-BLS IgG was positive in 20 cases. Blood cultures were performed in 17 subacute cases, and Brucella was isolated in 9. For chronic cases, time since initial symptoms ranged from 30 to 180 months (mean ± SD, 85.4 ± 51.3 months). At the time of blood sampling, all these patients were positive by STA and for anti-LPS IgG. Anti-CP IgG was detected in 21 cases, anti-BLS IgG was detected in 15 cases, and anti-LPS IgM was detected in 11 cases. After the results of anti-CP24 and anti-BLS were compared (see Results), a subsequent sample of 35 sera negative for anti-BLS was chosen. All these sera were positive for anti-LPS IgG and also for anti-CP IgG. Fifty serum samples from healthy donors were used to establish the cutoff for ELISAs.
(ii) Canine sera.
A total of 69 serum samples from dogs infected with B. canis were assayed. One group comprised 21 sera from consecutive cases of canine brucellosis, most of which were positive for B. canis by blood culture. In addition, serial samples from diagnosis to 60 days postdiagnosis were obtained from 15 dogs involved in an outbreak of canine brucellosis. All 36 dogs had high titers of antibodies to B. canis strain M− by agglutination. Serum samples from 30 healthy dogs were assayed under the same conditions to establish the cutoff for the assay.
rCP24.
The open reading frame of CP24 was cloned in the Pet17b vector (Novagen, Madison, Wis.) as described previously (4a). Briefly, the sequence information previously reported by Vizcaíno et al. (12) was used to design specific primers for CP24. B. melitensis genomic DNA was purified and used as a template for PCR with Pfu DNA polymerase (Stratagene, La Jolla, Calif.). After ligation, the mix was used to transform E. coli DH5α competent cells (Invitrogen, Carlsbad, Calif.), and miniprep plasmid DNA was purified from overnight cultures. The plasmid DNA of a clone containing the insert in the right orientation was used to transform E. coli strain BL21(DE3) competent cells (Stratagene). rCP24 was successfully expressed after induction with IPTG (isopropyl-β-d-thiogalactopyranoside; 1 mM) in the soluble fraction of E. coli cells. This preparation was purified using a Mono Q column in a fast protein liquid chromatography apparatus (Pharmacia, Uppsala, Sweden). rCP24 was adsorbed with Sepharose-polymyxin B to eliminate LPS contamination. This preparation contained less than 0.05 endotoxin units per mg of protein, as assessed by a Limulus amebocyte lysate analysis kit (Sigma, St Louis, Mo.). The identity of rCP24 was confirmed by Western blotting revealed with the B12A15 anti-CP24 monoclonal antibody (MAb).
ELISA with CP24.
Maxisorp polystyrene plates (Nunc, Roskilde, Denmark) were sensitized with 0.5 μg of CP24 per well diluted in phosphate-buffered saline (PBS). The plates were blocked with 200 μl of PBS containing 3% skim milk/well. After a wash with PBS containing 0.05% Tween 20, sera diluted in PBS containing 0.05% Tween 20 and 1% skim milk were dispensed. Specific antibodies were detected with horseradish peroxidase-conjugated antibodies to human IgG (Jackson, West Grove, Pa.) or dog immunoglobulins (Sigma). The reaction was developed by adding ortho-phenylenediamine (2 μg μl−1 in 0.1 M citrate-phosphate buffer containing 0.03% H2O2) and was stopped with 4 N H2SO4. To establish the cutoff value of the assays, serum samples from uninfected controls were tested under the same conditions indicated above. The cutoff value for each ELISA system was calculated as the mean specific optical density (OD) of control sera plus 3 SDs.
Other serological methods.
Agglutination tests for human brucellosis (slide agglutination, STA, and 2-mercaptoethanol) were performed according to the methods of Alton et al. (1). The rapid slide agglutination test for canine brucellosis was performed using the B. canis M− strain as described by Carmichael and Joubert (4), and the reaction was rated from 1+ to 4+ according to the observer's experience. Antibodies to CP, BLS, and LPS in human samples and to BLS in canine samples were measured by ELISA as described previously (2, 3, 9).
RESULTS
Human sera.
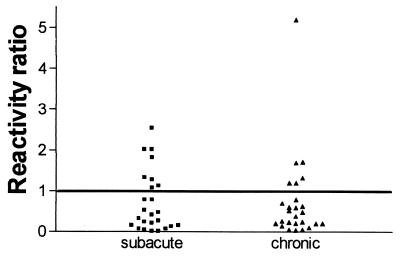
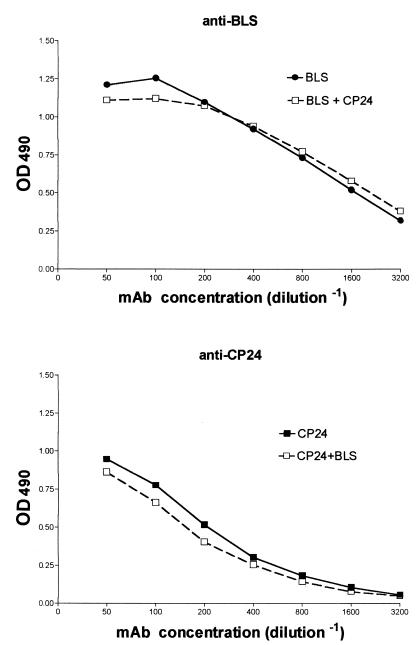
Anti-CP24 antibodies were first assayed in serial samples from patients with acute brucellosis (duration of illness, up to 40 days). At diagnosis, these antibodies were detected in only two of the eight patients, whose durations of illness were 30 and 15 days. The ratios between the OD of each sample and the cutoff for the assay (reactivity ratios) were 4.56 and 1.21, respectively. Both patients had low titers of anti-CP IgG (100 and 200, respectively), and the second patient was also positive for anti-BLS IgG. For both patients, the anti-CP24 reactivity ratios had decreased in the samples obtained 10 weeks later (1.67 and 0.17, respectively). For two other patients, anti-CP24 antibodies were first detected in the sample obtained 10 weeks after diagnosis, but the reactivity ratios were low in both cases (1.14 and 1.06, respectively). Overall, no correlation was found between the anti-CP24 results and the duration of illness (means, 25 and 20 days for anti-CP24-positive and -negative patients, respectively). Similarly, no correlation was found between anti-CP24 status and initial titers of anti-LPS, anti-CP, or anti-BLS antibodies at diagnosis. Anti-CP24 antibodies were also assayed in patients with subacute brucellosis or chronic brucellosis in order to assess whether the responses in these clinical stages differ. However, anti-CP24 antibodies were equally frequent in both stages, since they were detected in seven (29%) subacute cases and in six (26%) chronic cases. The reactivity ratios of positive samples ranged from 1.08 to 2.53 among subacute cases and from 1.20 to 2.78 among chronic cases (Fig. 1). Within each group, no correlation was found between anti-CP24 status and the duration of symptoms. Interestingly, while all patients positive for anti-CP24 antibodies were also positive for anti-CP antibodies, five were negative for anti-BLS antibodies. Thus, we decided to assess the frequency of anti-CP24 reactivity among a larger group of brucellosis patients who were negative for anti-BLS antibodies but positive for anti-CP antibodies. Among 35 samples tested, 30 (85.7%) were positive for anti-CP24 antibodies, with reactivity ratios ranging from 1.05 to 7.14 (mean ± SD, 2.20 ± 1.39). These results suggested that almost all anti-CP-positive cases could be detected by the combined measurement of anti-BLS and anti-CP24 antibodies. To test whether both reactivities could be measured simultaneously in the same well, ELISA plates were coated with both antigens mixed at concentrations equal to those used in the individual assays. To assess whether each antigen was coated and recognized as efficiently as in the individual ELISA, the wells were reacted with MAbs specific for CP24 and BLS. As shown in Fig. 2, the reactivity of each MAb was essentially the same in wells coated with the homologous antigen and in wells coated with both antigens. When the 35 sera negative for anti-BLS were assayed in the combined ELISA (after the determination of the cutoff specific for this test), 23 (66%) yielded positive results. Six sera positive for anti-BLS antibodies, included as controls, were also positive in the combined assay. The serum samples from subacute and chronic cases that had been assayed for anti-CP24 antibodies were also tested in the combined ELISA. As shown in Table 1, the percentage of positive results was higher for the combined test than for any of the independent assays (CP24 or BLS), especially in the chronic cases.
FIG. 1.
Analysis of anti-CP24 antibodies in patients with subacute or chronic brucellosis. Antibodies were determined by indirect ELISA. The reactivity ratio is the ratio between the OD of the sample and the cutoff value for the assay.
FIG. 2.
Reactivities of specific MAbs against CP24 or BLS by ELISA using wells coated with the homologous antigen or with both antigens.
TABLE 1.
Diagnostic performance of combined ELISA with CP24 and BLS in patients with subacute or chronic brucellosis
| Cases | % Samples positive for antibodies to:
|
|||
|---|---|---|---|---|
| BLS | CP24 | BLS + CP24a | CP | |
| Subacute (n = 24) | 83 | 29 | 87.5 | 96 |
| Chronic (n = 23) | 65 | 26 | 83 | 91 |
Combined ELISA with both antigens in the same well.
Canine sera.
Only 9 (43%) of the 21 serum samples from consecutive cases of canine brucellosis were positive by ELISA against CP24, with reactivity ratios that varied from 1.13 to 5.27 (mean, 2.26). No differences were found between CP24-positive and -negative samples regarding their reactivities against the BLS protein (mean reactivity ratios, 4.50 and 4.95, respectively). All but two dogs lacking anti-CP24 antibodies had B. canis M− agglutination rated 3+ or 4+. Among the 15 dogs involved in an outbreak of brucellosis and followed for 60 days after diagnosis, 7 (47%) were negative for anti-CP24 antibodies during the whole follow-up, 4 were always positive, 3 were positive from day 0 to day 15, and 1 dog initially negative became positive at 60 days. In all these cases, anti-BLS antibodies were first detected at diagnosis or at day 15. As with the consecutive cases, no correlation was found between the presence of anti-CP24 antibodies and the reactivity ratio of anti-BLS antibodies.
DISCUSSION
In this study, antibodies to the CP24 cytoplasmic antigen of Brucella were assessed in two different host species infected with rough or smooth Brucella strains and having a wide range of duration of illness. In spite of this variety of status, a common factor was evident: CP24 is recognized by a low percentage of the individuals that have had contact with Brucella. This finding is surprising in view of the good immunogenicity of rCP24 in mice (4a). One possible explanation for the low prevalence of anti-CP24 antibodies in naturally infected hosts is that the protein may have a low expression in Brucella cells. In any case, this expression level seems to affect the inmmune responses to smooth and rough strains of Brucella similarly, since anti-CP24 antibodies were as prevalent in human brucellosis as in canine brucellosis. Alternatively, CP24 may be significantly expressed or recognized only during some stages of the infection. This possibility seems unlikely, however, since the frequencies of anti-CP24 antibodies were similar in patients with acute, subacute, and chronic brucellosis. Moreover, most patients who tested negative during the acute phase of the disease did not develop anti-CP24 antibodies during the subacute phase. Conceivably, the anti-CP24 response may depend on multiple factors, including a favorable match between the major histocompatibility complex of the host and the antigenic epitopes of the protein. The absence of a uniform response to CP24 among infected humans and dogs is in line with the results obtained by Debbarh et al. in infected sheep (6). By immunoblotting with a cytosoluble fraction of B. melitensis H38, less than 50% of the sera from ewes naturally infected with B. melitensis reacted with the CP24 protein. In contrast, this antigen was recognized by all the ewes experimentally infected with B. melitensis H38. These results would tend to favor the hypothesis of a low level of expression of CP24 in Brucella cells, which would be offset by the large inoculum usually employed in experimental infections.
In spite of the low prevalence of anti-CP24 antibodies in infected hosts, this response was detected in almost all the patients who had antibodies to an extract of CP but lacked antibodies to a particular component of CP (BLS). The reasons for this combination of results are unknown. Notably, the simultaneous measurement of anti-CP24 and anti-BLS antibodies yielded positive results for 91% of the patients positive for anti-CP antibodies. This suggests that an ELISA combining a discrete array of recombinant cytoplasmic proteins of Brucella could be more sensitive than those based on a unique cytoplasmic antigen and could approach the sensitivity of the assay performed with the CP antigen. The latter antigen is obtained from Brucella cells by sequential disruption, ultracentrifugation, and LPS removal by immunoaffinity. As with other complex antigens obtained from whole bacteria by extractive methods, such as brucellin and hot saline (8, 11), the properties of CP may vary according to the strain used, the culture conditions, and the efficiency of the extraction. In the case of brucellin, for example, the variation in the protein content of the batches has been suggested as a possible cause of heterogeneity in the results of diagnostic tests (7). The development of an ELISA combining several recombinant proteins would not only simplify the preparation of the antigens but would also allow a better standardization of the assay. Our results suggest that this combined ELISA is feasible and would be almost as sensitive as the assay based on native antigens.
Acknowledgments
We thank Jorge Wallach for providing human sera and María M. Wanke for providing canine samples.
This work was supported by grant PICT 05-06324 from the Agencia Nacional de Promoción Científica y Tecnológica (ANPCYT). C.A.V. and J.C. were supported by fellowships from CONICET. M.V.D. was supported by a fellowship from ANPCYT. P.C.B and C.A.F are members of the Research Career of CONICET. C.A.F. is also a member of the Facultad de Ciencias Exactas, Universidad Nacional de La Plata.
REFERENCES
- 1.Alton, G. G., L. M. Jones, R. D. Angus, and J. M. Verger. 1988. Techniques for the brucellosis laboratory. Institut National de la Recherché Agronomique, Paris, France.
- 2.Baldi, P. C., S. E. Miguel, C. A. Fossati, and J. C. Wallach. 1996. Serological follow-up of human brucellosis by measuring IgG antibodies directed to LPS and cytoplasmic proteins of Brucella. Clin. Infect. Dis. 22:446-455. [DOI] [PubMed] [Google Scholar]
- 3.Baldi, P. C., M. M. Wanke, M. E. Loza, N. Monachesi, and C. A. Fossati. 1997. Diagnosis of canine brucellosis by detection of IgG antibodies against an 18 kDa cytoplasmic protein of Brucella spp. Vet Microbiol. 57:273-281. [DOI] [PubMed] [Google Scholar]
- 4.Carmichael, L. E., and J. C. Joubert. 1987. A rapid slide agglutination test for the serodiagnosis of Brucella canis infection that employs a variant (M-) organism as antigen. Cornell Vet. 77:3-12. [PubMed] [Google Scholar]
- 4a.Cassataro, J., C. A. Velikovsky, G. H. Giambartolomei, S. Estein, L. Bruno, A. Clockaert, R. Bowden, M. Spitz, and C. A. Fossati. Immunogenicity of the Brucella melitensis recombinant ribosome recycling factor-homologous protein and its cDNA. Vaccine, in press. [DOI] [PubMed]
- 5.Cloeckaert, A., H. S. Debbarh, M. S. Zygmunt, and G. Dubray. 1996. Production and characterisation of monoclonal antibodies to Brucella melitensis cytosoluble proteins that are able to differentiate antibody responses of infected sheep from Rev.1 vaccinated sheep. J. Med. Microbiol. 45:206-213. [DOI] [PubMed] [Google Scholar]
- 6.Debbarh, H. S., A. Cloeckaert, M. S. Zygmunt, and G. Dubray. 1995. Identification of sero-reactive Brucella melitensis cytosoluble proteins which discriminate between antibodies elicited by infection and Rev.1 vaccination in sheep. Vet. Microbiol. 44:37-48. [DOI] [PubMed] [Google Scholar]
- 7.Denoel, P. A., T. K.-O. Vo, V. E. Weynants, A. Tibor, D. Gilson, M. S. Zygmunt., J. N. Limet and J.-J. Letesson. 1997. Identification of the major T-cell antigens present in the Brucella melitensis B115 protein preparation, Brucellergene OCB. J. Med. Microbiol. 46:801-806. [DOI] [PubMed] [Google Scholar]
- 8.Gamazo, C., A. J. Winter, I. Moriyón, J. I. Riezu-Boj, J. M. Blasco, and R. Díaz. 1989. Comparative analyses of proteins extracted by hot saline or released spontaneously into outer membrane blebs from field strains of Brucella ovis and Brucella melitensis. Infect. Immun. 57:1419-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldbaum, F. A., J. Leoni, J. C. Wallach, and C. A. Fossati. 1993. Characterization of an 18-kilodalton Brucella cytoplasmic protein which appears to be a serological marker of active infection of both human and bovine brucellosis. J. Clin. Microbiol. 31:2141-2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gotuzzo, E., and C. Cellillo. 1992. Brucella, p. 1513-1521. In S. L. Gorbach, J. G. Bartlett, and N. R. Blacklow (ed.), Infectious diseases. W. B. Saunders Company, Philadelphia, Pa.
- 11.Jones, L. M., R. Diaz, and A. G. Taylor. 1973. Characterization of allergens prepared from smooth and rough strains of Brucella melitensis. Br. J. Exp. Pathol. 54:492-508. [PMC free article] [PubMed] [Google Scholar]
- 12.Vizcaíno, N., A. Cloeckaert, G. Dubray, and M. S. Zygmunt. 1996. Cloning, nucleotide sequence, and expression of the gene coding for a ribosome releasing factor-homologous protein of Brucella melitensis. Infect. Immun. 64:4834-4837. [DOI] [PMC free article] [PubMed] [Google Scholar]