Abstract
This article reviews cigarette smoking in patients with psychiatric disorders (PD) and substance use disorders (SUD). Rates of smoking are approximately 23% in the U.S. population but approximately two- to four-fold higher in patients with PD and SUD. Many remaining smokers have had repeated smoking cessation failures, possibly due to the presence of co-morbid PD and SUDs. There is modest, evidence-based support for effective treatment interventions for nicotine addiction in PD and SUD. Further research is needed to increase our understanding of nicotine addiction in PD and SUD and develop more effective treatment interventions.
Although smoking prevalence in the United States has decreased from 43.8% in 1965 to 23.3% in 2000,1 there are many cigarette smokers who have been unable to quit. An important subset of refractory smokers are those with psychiatric disorders (PD) and substance use disorders (SUD), among whom smoking rates exceed those in the general population by two- to fourfold.2 In a population-based study of smoking prevalence in the U.S., Lasser and colleagues found that smoking prevalence among persons with and without a psychiatric disorder were 41% and 22.5%, respectively.2 The highest prevalence (67.9%) was found among persons with drug abuse. Consistent with these results, Degenhardt and Hall3 reported similar findings in their study of smoking prevalence in Australia. The prevalence of smoking in various PD and SUD4 is presented in Fig. 1. Other studies have found that individuals with PD and SUD are at higher risk for many tobacco-related diseases, including cardiovascular illness, respiratory disease, and cancer, than individuals in the general population.5–8
FIGURE 1.
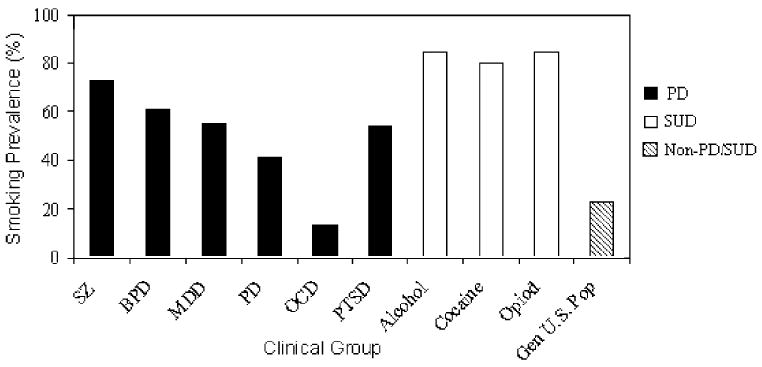
Prevalence of cigarette smoking in clinical samples of individuals with PD and SUD. Data were compiled from clinical studies of smoking prevalence in major PD and SUD.4 Abbreviations: SZ, schizophrenia; BPD, bipolar disorder; MDD, major depressive disorder; PD, panic disorder; OCD, obsessive-compulsive disorder; PTSD, post-traumatic stress disorder.
Among “ever smokers,” persons with PD or SUD are less likely to be former smokers than other smokers. Lasser et al.2 found that the quit rate among ever smokers with no history of PD or SUD was 42.5%. Significantly lower quit rates were associated with several other PD and SUD, including alcohol use disorder (16.9%), bipolar disorder (25.9%), major depression (26.0%), and post-traumatic stress disorder (23.2%). Clearly, improved treatments for nicotine addiction are needed for these populations.
Several explanations have been proposed for the high prevalence of smoking in individuals with PD and SUD. First, there may be intrinsic factors (eg, shared genes, abnormalities in brain reward pathways) that predispose individuals with PD and SUD to smoking. Second, nicotine may be used by PD and SUD patients to self-medicate psychiatric symptoms.9–12 Accordingly, concurrent presentation of PD and SUD (eg, “dual diagnosis”) is often associated with cigarette smoking.13 Furthermore, nicotine administration through cigarette smoking may modulate several neurotransmitter systems (eg, dopamine, glutamate) thought to be involved in the pathogenesis of PD and SUD.
This article reviews the existing literature on cigarette smoking in individuals with PD and SUD, with reference to neurobiology, clinical findings, and treatment approaches for smoking cessation in these populations. Recommendations for the treatment of nicotine addiction in these populations are also discussed, and gaps in our current knowledge are identified.
NEUROBIOLOGY
Neuropharmacology
Nicotine modulates several neurotransmitter systems that are involved in the pathogenesis of PD and SUD, including dopamine (DA).4,9,14 the reinforcing effects of nicotine are primarily mediated through the activation of nicotinic acetylcholine receptors (nAChRs), located pre-synaptically on mesolimbic DA neurons.15,16 The role of mesolimbic DA neurons in mediating the reinforcing effects of nicotine is suggested by rodent studies demonstrating that lesions of the VTA reduce nicotine self-administration, as do local infusions of the nAChR antagonist mecamylamine into the VTA.17,18 It has also been observed that nicotine withdrawal leads to reductions in central DA in rodents19,20 and urinary catecholamine excretion in human smokers.21,22
Nicotine facilitates the release of other neurotransmitters, including acetylcholine (ACh), endogenous opioid peptides (EOPs), γ-aminobutyric acid (GABA), glutamate (Glu), norepinephrine (NE), and serotonin (5-HT), which are also involved in the pathogenesis of PD and SUD.16 These biochemical and pharmacological findings provide a conceptual link between cigarette smoking and putative neurobiological abnormalities underlying several PD and SUD. A summary of the neurotransmitter systems that may contribute to the higher rates of smoking in PD and SUDs is given in Table 1.
TABLE 1.
Neurotransmitter Systems of Relevance to the Co-Morbidity of Cigarette Smoking in PD and SUD
| Neurotransmitter | Brain Region Localization | PD/SUD Involved |
|---|---|---|
| Dopamine (DA) | VTA, NAc, SNc, PFC, ACC | Schizophrenia, bipolar disorder, alcohol and drug addiction |
| Norepinephrine (NE) | LC, PFC | Bipolar disorder, major depressive disorder, cocaine dependence |
| Serotonin (5-HT) | RN, PFC, OFC | Major depression, PTSD |
| Acetylcholine (ACh) | NBM, PPN, HIPP, PFC | Schizophrenia, major depression, nicotine dependence |
| Endogenous opioid peptides (EOPs) | PAG, VTA | Opioid and alcohol dependence |
| Glutamate | PFC, NAc, VTA, THAL | Schizophrenia, bipolar disorder, major depression |
| γ-aminobutyric acid (GABA) | PFC, NAc, VTA, THAL | Schizophrenia, major depressive disorder, cocaine dependence |
| Endocannabinoids (ECBs) | VTA, NAc, HIPP, CEREB | Cannabis and opioid dependence |
Abbreviations: VTA, ventral tegemental area; NAc, nucleus accumbens; SN, substantia nigra; PFC, prefrontal cortex; ACC, anterior cingulated cortex; LC, locus ceruleus; RN, raphe nucleus; OFC, orbitofrontal cortex; NBM, nucleus basalis of Meynert; PPN, pedunculopontine nucleus; HIPP, hippocampus; PAG, periaquaductal gray; THAL, thalamus; CEREB, cerebellum.
Genetics
Few studies have evaluated genetic factors as determinants of the high co-morbid rates of smoking in PD and SUD. Kendler and colleagues23 examined 1566 female twin pairs from the Virginia Twin Registry using a best-fitting bivariate twin model and found evidence for shared genetic factors to explain the association between smoking and major depression. Another study of 173 monozygotic and 183 dizygotic male twin pairs from the National Heart Lung and Blood Institute’s Twin Study suggested that the heritability of smoking may relate to common factors determining substance abuse.24
The genetic influence of alleles of several post-synaptic DA receptors (D1, D2, and D4) and the dopamine transporter (DAT), which have been studied as genetic determinants of PD and SUD (eg, schizophrenia and cocaine dependence), have been implicated in cigarette smoking behavior. D1 dopamine receptor (D1DR) alleles25 and D2DR gene polymorphisms26,27 have been associated with differences in smoking behavior, and polymorphisms in the D4DR gene have been associated with smoking and depression.28 In addition, polymorphisms in the DAT have been associated with lower rates of smoking and longer periods of smoking abstinence.29
Genetic variations in nAChRs and the enzymes involved in nicotine metabolism in PD and SUD may explain high rates of smoking in these disorders. For example, Freedman and colleagues30 have found evidence for an allelic variation in the α7 nAChR subunit gene in schizophrenic patients associated with auditory gating deficits, which may explain their high rates of smoking. The primary hepatic enzyme system involved in the metabolism of nicotine to cotinine is CYP 2A6. Recent work by Tyndale and colleagues31 suggests that individuals with one or more null alleles (that do not lead to active enzyme products) of the CYP 2A6 hepatic microsomal system smoke significantly fewer cigarettes per week, and smokers with such null genotypes have reduced expired breath carbon monoxide and plasma cotinine levels.32 However, there have been no studies describing abnormalities of CYP 2A6 expression or differences in the presence of null alleles in patients with PD or SUD as compared to controls. Interestingly, there is evidence that polymorphisms in CYP 2D6 can determine smoking cessation responses to bupropion SR,33,34 and thus allelic variations in candidate genes related to smoking in PDs and SUDs may have pharmacogenetic treatment implications.
SMOKING AND PSYCHIATRIC DISORDERS (PD)
While higher rates of smoking in patients with PD have been well-described, most published studies of PD have not addressed the role of nicotine and tobacco use as a confounding variable on study outcomes.35 The failure to account for co-morbid smoking in these studies is an important issue to consider in interpreting such study results. For example, most studies do not control for time of last cigarette, which could create potential artifacts in outcome measures due to varying levels of withdrawal or the misinterpretation of withdrawal symptoms from nicotine as being due to mental illness.
Hypotheses accounting for the high rates of smoking in PD9,36 include:
shared genetic factors that determine vulnerability to both smoking and PD
self-medication by cigarette smoking of clinical symptoms, medication side effects, and cognitive deficits associated with PD
common environmental factors such as stress that can increase expression of smoking behavior and the onset of psychiatric symptoms.
An overview of relevant clinical studies relating smoking with a number of PD is presented in this section.
Schizophrenia
Patients with schizophrenia have higher rates of smoking (45–88%) compared to the general population in both clinical4,37 and population-based2 samples. In clinical samples, smoking rates are higher in inpatient (81.5%)38–41 compared to outpatient (68.4%)10,42–49 settings, which is consistent with higher smoking rates in institutional settings. A preliminary report found high smoking rates (92%) in first-episode schizophrenic patients with no history of using anti-psychotic medications. These findings suggest that smoking in this population is related to pathophysiological features of the illness and not to an iatrogenic effect of antipsychotic treatment (eg, an effort to alleviate side effects of medication by smoking).48 The temporal sequence of smoking preceding the onset of psychotic symptoms is unlikely to suggest a causal connection between smoking and the onset of schizophrenias because a large percentage patients with schizophrenia are non-smokers (8–42%), based on clinic-based smoking prevalence surveys,4 and smoking cessation in schizophrenics does not appear to lead to significant changes (either a worsening or improvement) in psychotic symptoms.50–52
Cross-sectional studies have examined the associations between cigarette smoking and psychotic symptoms in patients with schizophrenia.42,49,53 Goff and colleagues42 found that schizophrenic smokers had higher Brief Psychiatric Rating Scale (BPRS) total scores than non-smokers and higher levels of positive and negative symptoms. Ziedonis and colleagues49 found increased positive symptoms but reduced negative symptoms in schizophrenic smokers versus non-smokers. Heavy smokers had the highest positive and lowest negative symptom scores. While their sample was confounded by diagnostic heterogeneity, Hall and colleagues53 found that chronically mentally ill patients (87% with schizophrenia or schizoaffective disorder) who were former smokers had fewer negative symptoms (on BPRS) than current mentally ill smokers. However, recent controlled laboratory studies of tobacco abstinence,54,55 along with data from four controlled smoking treatment trials, have found no evidence for significant changes in psychotic symptoms with smoking cessation50,51,56 or smoking reduction52 in schizophrenic patients. Thus, controlled prospective studies have not confirmed the effects of smoking on clinical symptoms in schizophrenia as observed in cross-sectional studies. This suggests that other differences (eg, trait factors) between schizophrenic smokers and non-smokers may explain the results from cross-sectional studies.
The most compelling evidence for a pathophysiologically-based vulnerability to smoking in schizophrenic patients relates to well-defined deficits in psychophysiological measures (eg, P50 auditory gating) and neuropsychological performance deficits that appear to be improved or normalized by nicotine administration or cigarette smoking. For example, nicotine and smoking transiently normalize P50 gating and smooth-pursuit eye movement (SPEM) deficits in schizophrenic patients and their first degree relatives,57–61 which appear to be mediated by deficient neurotransmission through α7 nAChRs.30,62 In addition, nicotine and smoking have been shown to remediate working memory55,63,64 and attentional deficits55,65,66 in schizophrenics.
Cigarette smoking may reduce neuroleptic-induced parkinsonism67 but worsen symptoms of tardive dyskinesia,68 but these effects have not been observed in all studies,42,44,68 probably due to methodological differences. These observations are consistent with nicotine’s enhancement of subcortical DA systems, leading to reduced parkinsonism and increased tardive dyskinesia. The transdermal nicotine patch has been shown to reduce bradykinesia associated with haloperidol administration,69 lending some direct experimental support to the anti-parkinsonian effects of nicotine.
Affective Disorders
Depressive Disorders.
In clinical samples of patients with major depression,10,11,70,71 and in a population-based sample with clinically significant depressive symptoms,72 smoking prevalence is 40–60%. Glassman and colleagues73 found that 61% of smokers presenting to a smoking cessation program in New York City had a past history of major depression. In a clinical sample of 547 Latinos in San Francisco, Perez-Stable and colleagues74 reported that depressive symptoms (as determined by Center for Community Epidemiological Studies Depression Scale [CES-D] scores >16, suggesting clinically significant depression) were higher in Latino current smokers (21.9% and 39.5% for males and females, respectively) than Latino former (9.8% and 27.0%) and never (11.8% and 18.5%) smokers. Similarly, in a population-based study of depressive symptoms and smoking, Anda et al.72 found that 39% of individuals with CES-D scores >16 were current smokers. It has also been observed that smokers with depressive symptoms have a much harder time quitting,73,75 and require more smoking cessation attempts to successfully quit,76,77 and that smoking cessation is associated with the emergence of negative affective states.70 For those patients with a history of major depression, smoking cessation may lead to a reemergence of major depressive symptoms,76,78 though this phenomenon has been questioned.79,80 Furthermore, Breslau et al.81 demonstrated that the severity of tobacco withdrawal appears to be worse in individuals with major depression or anxiety disorders.
Bipolar Disorder.
There have been few studies on co-morbid smoking and bipolar disorder10,82–84 and no studies on the treatment of smoking in this disorder. Hughes and colleagues10 reported a smoking prevalence of 70% in bipolar patients from Minnesota, while in a Spanish population of chronically mentally ill patients, Gonzalez-Pinto and associates82 reported that 63% of patients had lifetime histories of smoking and 51% were current smokers, as compared to 33% in the control group. More recent studies83,84 have found similarly high rates (55–70%) of smoking in bipolar disorder. Corvin and associates84 found evidence that smoking may be more prevalent with the presence of psychotic symptoms in bipolar disorder, but this finding was not supported in another study.83 One population-based study suggested that the prevalence of current smoking in bipolar disorder was 60.6% and also found lower quit ratios in bipolar smokers as compared to those without mental illness.2 It remains to be determined whether the relationship between increased smoking prevalence and reduced success in smoking cessation attempts in patients with bipolar disorder parallel clinical findings observed in unipolar depression, though a study by Glassman and colleagues did find that bipolar patients were at a particular risk for depressive recurrence during smoking cessation.77
Anxiety Disorders
Panic Disorder.
Smoking prevalence in panic disorder varies widely across studies, ranging from 19.2%to 56%.2,85–88 Two longitudinal studies indicate that daily smoking is predictive of the onset of panic attacks, but not vice versa.89,90 Most recently, a third study prospectively examined the bidirectional relationship between smoking, nicotine dependence (ND), and anxiety disorders in adolescents and young adults.91 Prior regular smoking and ND were associated with an increased risk for new onset of panic attacks. However, due to discrepancies between the data analytic methods used, the authors could not conclusively rule out the potentially less common pathway of pre-existing panic attacks or disorders influencing the later development of ND. Additional research is needed to explore these pathways, including possible mediators to explain the progression from one disorder to the other and moderators that determine when and whether the progression occurs.
Obsessive-compulsive Disorder (OCD).
The prevalence of smoking among patients with OCD appears to be the lowest among the anxiety disorders (7.7% to 22.4%85,88,92,93) and PD in general. Reasons for the lower occurrence of smoking in OCD are unclear but could be related to the specific nature of OCD symptoms (eg, fears of disease, contamination) or a combination of factors, including the effects of nicotine, genetic factors, or the social effects of having OCD.92
Post-traumatic Stress Disorder (PTSD).
Prevalence estimates of smoking range from 53–66% in combat veterans with PTSD.94–96 Smoking prevalence has also been shown to differ based on high (56%) vs. low (39%) combat exposure.97 The majority of empirical studies on PTSD and smoking have been conducted with combat veterans. Higher rates of heavy smoking (>25 cigarettes/day) have been reported in PTSD versus non-PTSD veterans (48% vs. 28%, respectively95). Heavy smokers also report greater levels of total PTSD symptoms and Cluster C (avoidance and numbing) and Cluster D (hyperarousal) symptoms. Other studies have demonstrated that nicotine withdrawal symptoms are worse in smokers with PTSD in response to trauma-related stimuli as compared to those without PTSD.98
In women with PTSD related to physical and sexual assault, Acierno et al.99 found a smoking prevalence of 44.4% (vs. 26.1% in those without PTSD). Recently, Vlahov et al.100 examined civilian smoking rates and prevalence after the United States terrorist attacks on September 11, 2001. Similar to national smoking levels, 23.4% of participants reported current smoking. Among those who were actively smoking prior to September 11th, 41.2% increased their smoking. Those who increased their smoking were more likely to report symptoms of PTSD (24.2%) compared to smokers who did not increase their smoking (5.6%).
With respect to trauma exposure and risk of smoking, Anda et al.,101 controlling for confounding variables such as socioeconomic status and age, found that compared to individuals who never experienced an adverse childhood event, those with a history of five or more adverse events (eg, verbal, physical, or sexual abuse; divorce; a battered mother; substance abuse, mental illness, or an incarcerated household member) had greater risks of early onset smoking (OR: 5.4), ever smoking (OR: 3.1), and heavy smoking (OR: 2.8). Moreover, data from a 10-year prospective study indicated that trauma exposure predicted later development of ND (OR: 1.81) but that the risk of developing ND was significantly greater in those with PTSD (OR: 3.30).102 When examining retrospective lifetime data, only PTSD, but not trauma-exposure alone, predicted the subsequent development of ND. Collectively, these data suggest that PTSD and perhaps trauma exposure increase the risk for the later development of ND.
Attention-Deficit Disorder
Attention Deficit Hyperactivity Disorder (ADHD) is associated with higher rates and earlier onset of cigarette smoking,103 which has a pattern of familial transmission. There is evidence that nAChR mechanisms may be involved in the pathophysiology of ADHD, as the high-affinity nAChR agonist ABT-418 may be useful for the treatment of ADHD symptoms.104
SMOKING AND SUBSTANCE USE DISORDERS (SUD)
Over 75% of alcohol- and drug-dependent persons in early recovery smoke cigarettes105–107 and tend to be heavy, highly nicotine-dependent smokers;108 smoking-related mortality exceeds alcohol-related mortality in this population.5
Alcohol Use Disorders
Animal and human studies have suggested that the effects of alcohol consumption are partially mediated by nAChRs and that stimulation of nAChRs may enhance alcohol consumption.109–111 Le and colleagues112 demonstrated that exposure to nicotine enhanced alcohol consumption in rats. Blomqvist et al.110,112 have shown that the high-affinity nAChR antagonist mecamylamine decreased alcohol consumption in high- but not low-alcohol-preferring rats. More recently, Blomqvist et al.109 demonstrated that mecamylamine versus placebo pretreatment decreased alcohol consumption and the rewarding effects of alcohol in humans with no history of an alcohol, tobacco, or other drug dependence.
Consumption of an alcohol versus a placebo beverage acutely increases smoking behavior.113 Another study114 found that smoking acutely increased the reinforcing value of alcohol; however, this effect was observed only following alcohol pre-exposure in men. Moreover, in a population of smokers in alcohol treatment, urges to smoke increased during exposure to alcohol versus water cues,105,115,116 and urges to smoke and drink were positively correlated during exposure to alcohol cues.106 These data are consistent with a learning theory explanation of the association between drinking and smoking urges and use; that is, with repeated pairing of these behaviors, smoking urges (and smoking) become a conditioned response to alcohol cues which serve as unconditioned stimuli.
Cocaine
Individuals who use cocaine have high rates (~80%) of co-morbid cigarette smoking.117,118 Significant reductions in cigarette consumption have been found after cocaine discontinuation.117 The presence of cigarette smoking in individuals with cocaine dependence is associated with earlier onset of cocaine use, more severe use, more legal problems, and use by intravenous or smoked routes of administration.119
Horger and colleagues120 found that nicotine potentiates cocaine self-administration in rats, suggesting that the stimulation of nAChRs can enhance the rewarding effects of cocaine. Studies in mice121 found that pre-treatment with the nAChR antagonist mecamylamine or deletion of the β2 subunit of the high-affinity nAChR with the use of transgenic mice can reduce conditioned place preferences to cocaine but not morphine. In humans with nicotine and cocaine dependence, an acute dose of nicotine enhances cue-induced cocaine craving,122 while a single pre-treatment dose of the nicotinic receptor antagonist mecamylamine reduces cue-induced cocaine craving.123 Because nAChRs are present on mesolimbic DA neurons and nAChR stimulation augments DA release and metabolism,20 the blockade of nAChRs may modify DA responses induced by cocaine administration, thereby altering the reinforcing properties of cocaine. This may have implications for the development of treatments for cocaine addiction based on nAChR systems.
Opioids
Greater than 80%of opioid-dependent patients smoke cigarettes.118,124–128 The presence of depressive symptoms appears to increase the risk of smoking in methadone-maintained individuals.126 Increases in methadone dose may lead to increased nicotine craving and cigarette consumption.128 Conversely, heroin abstinence after detoxification has been associated with increased smoking consumption.129
Cannabis
Data from the Australian National Survey of Mental Health and Well-Being (NSMHWB) Study3 found that the prevalence of cannabis use disorders was 0.8% in never smokers, 1.0% in former smokers, and 6.4% in current smokers, with an adjusted Odds Ratio of 5.00 (95% CI; 3.35–7.45) in current smokers. The lack of additional studies of the co-morbidity between tobacco and marijuana use likely relates to the fact that cannabis is typically used in combination with cigarettes and other drugs of abuse.
SMOKING CESSATION IN PD AND SUD
Treatments for Nicotine Addiction in PD and SUD
The development of effective smoking cessation treatments in PD and SUD may depend on their ability to target pathophysiological aspects of the primary disorder. Examples of treatments that target the pathophysiology of a PD or SUD, and which may also target smoking behavior, include atypical antipsychotics for schizophrenia,43,50,51,130,131 antidepressants for depressive disorders,132,133 and naltrexone for alcoholism.134–136 For example, several studies have suggested that switching treatment-refractory schizophrenics from typical antipsychotic agents to clozapine is associated with a reduction in cigarette smoking,43,130,131 especially in heavier smokers.43 Atypical antipsychotics may also enhance smoking cessation rates in combination with a nicotine patch51 and bupropion50 in schizophrenic patients. The tricyclic antidepressant nortriptyline appears to be efficacious for smoking cessation in smokers with either a positive or negative history of major depressive disorder (MDD), and the combination of this agent with cognitive-behavioral therapy (CBT) was superior in MDD history-positive smokers,137 suggesting the potential of treatment-matching strategies in smokers with PD. While widely used for treatment of major depression, serotonin-selective reuptake inhibitors do not appear to be efficacious for smoking cessation,133 including for smokers with concurrent alcoholism and depressive symptoms138 or a past history of depression.139 Nonetheless, antide-pressant therapies for smoking cessation have not been systematically evaluated in currently depressed smokers.
Interventions that combine behavioral and pharmacological treatments for co-morbid smoking and psychiatric symptoms may have considerable promise.36,37,51,56,71,140 For example, the combination of mood-focused CBT with nicotine gum was superior to that of standard behavioral smoking treatments with nicotine gum for smoking cessation in individuals with a past history of major depression.141 Data also support the utility of mood-focused CBT programs for smoking cessation in patients with histories of combined major depression and alcohol dependence.142 Brown and colleagues143 compared standard CBT for smoking cessation alone and in combination with CBT for depression (CBT-D) in smokers with a past history of major depression. Although no overall differences were found between treatment groups, smokers with recurrent major depression or those who were heavy smokers (>25 cigarettes/day) responded better to CBT-D than standard treatment alone, suggesting that the addition of CBT for depression may be beneficial for this subset of smokers.
Recent clinical trials in smokers with SUD have suggested the benefits of nicotine replacement and intensive psychosocial supports,127,144–148 although findings indicate that persons in early recovery find it very difficult to quit smoking with long-term quit rates rarely exceeding 12%.149,150 Given that tricyclic antidepressants and bupropion may have efficacy in the treatment of cocaine and other stimulant dependence,151 controlled studies of these agents for smoking treatment in SUD populations are also warranted. Guidelines for smoking cessation in individuals with PD and SUD have been proposed.37,149,150,152,153 Based on the current state of knowledge, a summary of recommendations for smoking cessation treatment in PD and SUD is given in Table 2.
TABLE 2.
Recommendations for the Treatment of Nicotine Dependence in PD and SUD
|
Timing of Smoking Cessation and Duration of Treatment in Co-morbid Smokers
It is unclear at present whether treatment of nicotine addiction in PD and SUD should be provided at the same time as treatments for other disorders (concurrent treatment) or implemented after the initiation and stabilization of the PD/SUD (sequential treatment). The example of smoking cessation in recovering alcoholics illustrates the challenges associated with whether to implement smoking cessation interventions sequentially or concurrently. Several smaller studies have suggested that smoking cessation in clinical trials is not associated with increased alcohol and other drug use among persons in recovery.150,154–156 However, findings from a recent study of smokers in alcohol recovery suggest that it may be important for some smokers in recovery to delay smoking cessation treatment until they have established several months of sobriety. Joseph and colleagues157 randomly assigned smokers (N = 499) in alcohol treatment to concurrent or delayed (six months after enrollment) smoking cessation treatment. While smoking cessation outcomes were similar at comparable follow-up assessment points, smokers assigned to delayed treatment had significantly better alcohol outcomes. Further study of this issue is warranted.
Despite evidence that it may be prudent to address treatment of alcohol and smoking sequentially, many alcohol abusers prefer concurrent treatment for their alcohol and nicotine addiction and were confident that they could quit drinking and stop smoking within six months.158 Furthermore, the fact that a substantial proportion of alcohol- and opioid-dependent patients die of tobacco-related illness5,159 supports the assertion that smoking cessation services be rendered when these patients present to addiction treatment programs, as this offers a “teachable moment.” Thus, it would appear that consideration should be given to addressing these problems concurrently, though in practice this is often done sequentially often due to the lack of coordination of treatments for PD/SUD and smoking. This is clearly an area where more controlled study is needed. Based on the current evidence, we suggest addressing treatment of co-morbid PD/SUD and cigarette smoking in a sequential manner. Careful monitoring of relapse in PD and SUD after achievement of smoking cessation is recommended.
The optimal duration of treatment in co-morbid populations is unknown, and most active trials have not exceeded twelve weeks in both the non-comorbid and comorbid smoking treatment literature. Six-month smoking abstinence rates tend to be poor in patients with PD and SUD compared to smokers without these co-morbid disorders.36,50,51,160,161 Accordingly, the issue of maintenance treatment with nicotine replacement therapy and/or bupropion SR and behavioral therapies for the goal of smoking relapse prevention warrants consideration in PD and SUD populations,162 given their low probabilities of achieving long-term smoking abstinence. While long-term use of psychosocial and behavioral therapy support (eg, relapse-prevention therapy) might be recommended for smokers with PD and SUD, it is untested in prospective studies. Thus, there is an urgent need to obtain data on such maintenance regimens on treatment outcomes in co-morbid populations and for reducing smoking-related morbidity and mortality rates, especially in light of managed care constraints on reimbursement for providers of smoking cessation services in most states.
Difficulties with Integration of Smoking Cessation Interventions into Mental Health and Addiction Treatment Settings
Since the Joint Commission on Accreditation of Health Care Organizations mandated smoking bans in accredited facilities in 1992, there is a requirement to create smoke-free environments in treatment settings for individuals with PD and SUD. However, Bobo and colleagues145 found that only one-third of addiction counselors routinely encouraged their patients to quit smoking, and counselors who smoked themselves rarely tended to give such advice. This is problematic since conventional smoking cessation programs offered to community smokers do not appear to serve the smoking cessation treatment needs of individuals with PD or SUD.51,150 Modifications of conventional smoking cessation programs for individuals with PD36,51,56,140,145 and SUD144–147,163,164 appear to show promise for these populations.
CONCLUSIONS AND RECOMMENDATIONS FOR FUTURE RESEARCH
Cigarette smoking is highly co-morbid with PD and SUD. In some cases, specific pathophysiological relationships have emerged. The systematic study of cigarette smoking interactions with PD and SUD promises to further our understanding of the biology and treatment of each of the individual disorders. Identification of these high-risk smokers through careful screening for PD and SUD in treatment settings will allow the implementation of potentially effective interventions directed towards reducing rates of smoking and associated medical sequelae.
There is a critical need for more substantiated research on biological and psychosocial factors that suggest causal connections for co-morbid smoking in these disorders and effective treatments directed toward smoking cessation in individuals with PD and SUD. Specifically, more research is needed on the temporal onset of smoking in PD and SUD, on abnormalities in nAChR systems in PD and SUD, and on whether addressing co-morbid smoking in PD and SUD is best approached by concurrent versus sequential treatment approaches. Advances in the treatment of nicotine dependence in co-morbid smokers depends on several factors. First, as Hughes107 and Hurt and Patten165 argue, larger controlled clinical trials are needed to test standard smoking cessation treatments in these populations. Rational decisions regarding whether treatment for this population of smokers needs to be tailored can be made based on these findings. Second, data pertaining to drug use history and/or psychiatric symptoms should be collected in these trials. Third, it will be important to investigate predictor (eg, measures of disinhibition, negative affect), moderator (eg, gender), and mediator (eg, self-medication) variables that are relevant to co-morbid populations of interest. Ultimately, advances in understanding the etiology of co-morbid PD, SUD, and smoking will allow for a more rational development of treatments for nicotine addiction in these individuals.
Acknowledgments
The assistance of Taryn Allen, B.S., and Andrea H. Weinberger, Ph.D., in the preparation of this manuscript is gratefully acknowledged.
Footnotes
The preparation of this review article was supported in part by U.S. Public Health Service grants R01-DA-13672, R01-DA-14039, R01-DA-15757, and K02-DA-16611 (Dr. George), K23-DA-16376 (Dr. Morissette), and R29-DA-11713 (Dr. Kalman); a Patrick and Catherine Weldon Donaghue Medical Research Foundation Clinical and Community Issues Award (Dr. George); a NARSAD Young Investigator Award (Dr. George); and the VISN 1 MIRECC from the U.S. Department of Veterans Affairs (Drs. Kalman and George).
References
- 1.CDC Cigarette smoking among adults in the United States. MMWR (Morb Mortal Wkly) 2002;51:300–303. [PubMed] [Google Scholar]
- 2.Lasser K, Boyd JW, Woolhander S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: a population-based prevalence study. JAMA. 2000;284:2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- 3.Degenhardt L, Hall W. The relationship between tobacco use, substance-use disorders and mental health: results from the National Survey of Mental Health and Well-being. Nicotine Tob Res. 2001;3:225–234. doi: 10.1080/14622200110050457. [DOI] [PubMed] [Google Scholar]
- 4.George TP, Vessicchio JC. Nicotine addiction and schizophrenia. Psychiatric Times. 2001;18(2):39–42. [Google Scholar]
- 5.Hurt RD, Offord KP, Croghan IT, et al. Mortality following inpatient addictions treatment: role of tobacco use in a community-based cohort. JAMA. 1996;275:1097–1103. doi: 10.1001/jama.275.14.1097. [DOI] [PubMed] [Google Scholar]
- 6.Lichtermann D, Ekelund E, Pukkala E, Tanskanen A, Lonnqvist J. Incidence of cancer among persons with schizophrenia and their relatives. Arch Gen Psychiatry. 2001;58:573–578. doi: 10.1001/archpsyc.58.6.573. [DOI] [PubMed] [Google Scholar]
- 7.Tsuang MT, Perkins K, Simpson JC. Physical diseases in schizophrenia and affective disorder. J Clin Psychiatry. 1983;44:42–46. [PubMed] [Google Scholar]
- 8.Tsuang MT, Woolson RF, Fleming JA. Premature deaths in schizophrenia and affective disorders: an analysis of survival curves and variables affecting the shortened survival. Arch Gen Psychiatry. 1980;37:979–983. doi: 10.1001/archpsyc.1980.01780220017001. [DOI] [PubMed] [Google Scholar]
- 9.Dalack GW, Healy DJ, Meador-Woodruff JH. Nicotine dependence and schizophrenia: clinical phenomenon and laboratory findings. Am J Psychiatry. 1998;155:1490–1501. doi: 10.1176/ajp.155.11.1490. [DOI] [PubMed] [Google Scholar]
- 10.Hughes JR, Hatsukami DK, Mitchell JE, Dahlgreen LA. Prevalence of smoking among psychiatric outpatients. Am J Psychiatry. 1986;143:993–997. doi: 10.1176/ajp.143.8.993. [DOI] [PubMed] [Google Scholar]
- 11.Glassman AH. Cigarette smoking: implications for psychiatric illness. Am J Psychiatry. 1993;150:546–553. doi: 10.1176/ajp.150.4.546. [DOI] [PubMed] [Google Scholar]
- 12.Sacco KA, Bannon KL, George TP. Nicotinic receptor mechanisms and cognition in normal states and neuropsychiatric disorders. J Psychopharmacol. 2004;18:457–474. doi: 10.1177/0269881104047273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.George TP, Krystal JH. Comorbidity of psychiatric and substance abuse disorders. Curr Opin Psychiatry. 2000;13:327–331. [Google Scholar]
- 14.George TP, O’Malley SS. Current pharmacological treatments for nicotine dependence. Trends Pharmacol Sci. 2004;25:42–48. doi: 10.1016/j.tips.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 15.Clarke PBS, Fu DS, Jakubovic A, Fibiger HC. Evidence that mesolimbic dopamine activation underlies the locomotor stimulant action of nicotine in rats. J Pharmacol Exp Ther. 1988;246:701–708. [PubMed] [Google Scholar]
- 16.Picciotto MR. Nicotine as a modulator of behavior: beyond the inverted U. Trends Pharmacol Sci. 2003;23:494–499. doi: 10.1016/S0165-6147(03)00230-X. [DOI] [PubMed] [Google Scholar]
- 17.Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited access schedule. Psychopharmacology. 1989;99:473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- 18.Corrigall WA, Franklin KB, Coen KM, Clarke PB. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology. 1992;107:285–289. doi: 10.1007/BF02245149. [DOI] [PubMed] [Google Scholar]
- 19.Fung YK, Schmid MJ, Anderson TM, Lau Y-S. Effects of nicotine withdrawal on central dopaminergic systems. Pharmacol Biochem Behav. 1996;53:635–640. doi: 10.1016/0091-3057(95)02063-2. [DOI] [PubMed] [Google Scholar]
- 20.George TP, Verrico CD, Roth RH. Effects of repeated nicotine pre-treatment on mesoprefontal dopaminergic and behavioral responses to acute footshock stress. Brain Res. 1998;801:36–49. doi: 10.1016/s0006-8993(98)00537-x. [DOI] [PubMed] [Google Scholar]
- 21.West RJ, Russell MAH, Jarvis MJ, Pizzey T, Kadam B. Urinary adrenaline concentrations during 10 days of smoking abstinence. Psychopharmacology. 1984;84:141–142. doi: 10.1007/BF00432045. [DOI] [PubMed] [Google Scholar]
- 22.Ward KD, Garvey AJ, Bliss RE, Sparrow D, Young JB, Landsberg L. Changes in urinary catecholamine excretion after smoking cessation. Pharmacol Biochem Behav. 1991;40:937–940. doi: 10.1016/0091-3057(91)90109-f. [DOI] [PubMed] [Google Scholar]
- 23.Kendler KS, Neale MC, MacLean CJ, Heath AC, Eaves LJ, Kessler RC. Smoking and major depression: a causal analysis. Arch Gen Psychiatry. 1993;50:36–43. doi: 10.1001/archpsyc.1993.01820130038007. [DOI] [PubMed] [Google Scholar]
- 24.Swan GE, Carmelli D, Cardon LR. The consumption of tobacco, alcohol, and coffee in Caucasian male twins: a multivariate genetic analysis. J Subst Abuse. 1996;8:19–31. doi: 10.1016/s0899-3289(96)90055-3. [DOI] [PubMed] [Google Scholar]
- 25.Comings DE, Gade R, Wu S, et al. Studies of the potential role of the dopamine D1 receptor gene in addictive behaviors. Mol Psychiatry. 1997;2:44–56. doi: 10.1038/sj.mp.4000207. [DOI] [PubMed] [Google Scholar]
- 26.Comings DE, Ferry L, Bradshaw-Robinson S, Burchette R, Chui C, Muhleman D. The dopamine D2 receptor (DRD2) gene: a genetic risk factor for smoking. Pharmacogenetics. 1996;6:73–79. doi: 10.1097/00008571-199602000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Noble EP, St Jeor ST, Ritchie T, et al. D2 dopamine receptor gene and cigarette smoking: a reward gene? Med Hypotheses. 1994;42:257–260. doi: 10.1016/0306-9877(94)90127-9. [DOI] [PubMed] [Google Scholar]
- 28.Lerman C, Caporaso N, Main D, et al. Depression and self-medication with nicotine: the modifying influence of the dopamine D4 receptor gene. Health Psychol. 1998;17:56–62. doi: 10.1037//0278-6133.17.1.56. [DOI] [PubMed] [Google Scholar]
- 29.Lerman C, Caporaso NE, Audrain J, et al. Evidence suggesting the role of specific genetic factors in cigarette smoking. Health Psychol. 1999;18:14–20. doi: 10.1037//0278-6133.18.1.14. [DOI] [PubMed] [Google Scholar]
- 30.Freedman R, Coon H, Myles-Worsley M, et al. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc Nat Acad Sci USA. 1997;94:587–592. doi: 10.1073/pnas.94.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pianezza ML, Sellers EM, Tyndale RF. Nicotine metabolism defect reduces smoking. Nature. 1998;393:750. doi: 10.1038/31623. [DOI] [PubMed] [Google Scholar]
- 32.Rao Y, Hoffman E, Zia M, et al. Duplications and defects in the CYP 2A6 gene: identification, genotyping and in vivo effects on smoking. Mol Pharmacol. 2000;58:747–755. doi: 10.1124/mol.58.4.747. [DOI] [PubMed] [Google Scholar]
- 33.Lerman C, Roth D, Kaufmann V, et al. Mediating mechanisms for the impact of bupropion in smoking cessation treatment. Drug Alcohol Depend. 2002;69:219–223. doi: 10.1016/s0376-8716(02)00067-4. [DOI] [PubMed] [Google Scholar]
- 34.Lerman C, Shields PG, Wileyto EP, et al. Pharmacogenetic investigation of smoking cessation treatment. Pharmacogenetics. 2002;12:627–634. doi: 10.1097/00008571-200211000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Hughes JR, Howard TS. Nicotine and caffeine use as confounds in psychiatric studies. Biol Psychiatry. 1997;42:1184–1185. doi: 10.1016/s0006-3223(97)00344-2. [DOI] [PubMed] [Google Scholar]
- 36.Ziedonis DM, George TP. Schizophrenia and nicotine use: report of a pilot smoking cessation program and review of neurobiological and clinical issues. Schizophr Bull. 1997;23:247–254. doi: 10.1093/schbul/23.2.247. [DOI] [PubMed] [Google Scholar]
- 37.McChargue DE, Gulliver SB, Hitsman B. Would smokers with schizophrenia benefit from a more flexible approach to smoking treatment? Addiction. 2002;97:785–793. doi: 10.1046/j.1360-0443.2002.00064.x. [DOI] [PubMed] [Google Scholar]
- 38.de Leon J, Dadvand M, Canuso C, White AO, Stanilla JK, Simpson GM. Schizophrenia and smoking: an epidemiological survey in a state hospital. Am J Psychiatry. 1995;152:453–455. doi: 10.1176/ajp.152.3.453. [DOI] [PubMed] [Google Scholar]
- 39.el-Guebaly N, Hodgins D. Schizophrenia and substance abuse: prevalence issues. Can J Psychiatry. 1992;37:704–710. doi: 10.1177/070674379203701006. [DOI] [PubMed] [Google Scholar]
- 40.Masterson E, O’Shea B. Smoking and malignancy in schizophrenia. Brit J Psychiatry. 1984;145:429–432. doi: 10.1192/bjp.145.4.429. [DOI] [PubMed] [Google Scholar]
- 41.O’Farrell TJ, Connors GJ, Upper D. Addictive behaviors among hospitalized psychiatric patients. Addict Behav. 1983;8:329–333. doi: 10.1016/0306-4603(83)90032-1. [DOI] [PubMed] [Google Scholar]
- 42.Goff DC, Henderson DC, Amico E. Cigarette smoking in schizophrenia: relationship to psychopathology and medication side effects. Am J Psychiatry. 1992;149:1189–1194. doi: 10.1176/ajp.149.9.1189. [DOI] [PubMed] [Google Scholar]
- 43.George TP, Sernyak MJ, Ziedonis DM, Woods SW. Effects of clozapine on smoking in chronic schizophrenic outpatients. J Clin Psychiatry. 1995;56:344–346. [PubMed] [Google Scholar]
- 44.Menza MA, Grossman M, Van Horn M, Cody R, Forman N. Smoking and movement disorders in psychiatric patients. Biol Psychiatry. 1991;30:109–115. doi: 10.1016/0006-3223(91)90163-g. [DOI] [PubMed] [Google Scholar]
- 45.Diwan A, Castine M, Pomerleau CS, Meador-Woodruff JH, Dalack GW. Differential prevalence of cigarette smoking in patients with schizophrenia vs. mood disorders. Schizophr Res. 1998;33:113–118. doi: 10.1016/s0920-9964(98)00045-0. [DOI] [PubMed] [Google Scholar]
- 46.Chong SA, Choo HL. Smoking among Chinese patients with schizophrenia. Aust N Z J Psychiatry. 1996;30:350–353. doi: 10.3109/00048679609064998. [DOI] [PubMed] [Google Scholar]
- 47.Kelly C, McCredie RG. Smoking habits, current symptoms, and premorbid characteristics of schizophrenic patients in Nithsdale, Scotland. Am J Psychiatry. 1999;156:1751–1757. doi: 10.1176/ajp.156.11.1751. [DOI] [PubMed] [Google Scholar]
- 48.McEvoy JP, Brown S. Smoking in first-episode patients with schizophrenia. Am J Psychiatry. 1999;156:1120–1121. doi: 10.1176/ajp.156.7.1120a. [DOI] [PubMed] [Google Scholar]
- 49.Ziedonis DM, Kosten TR, Glazer WM, Frances RJ. Nicotine dependence and schizophrenia. Hosp Community Psychiatry. 1994;45:204–206. doi: 10.1176/ps.45.3.204. [DOI] [PubMed] [Google Scholar]
- 50.George TP, Vessicchio JC, Termine A, et al. A placebo-controlled study of bupropion for smoking cessation in schizophrenia. Biol Psychiatry. 2002;52:53–61. doi: 10.1016/s0006-3223(02)01339-2. [DOI] [PubMed] [Google Scholar]
- 51.George TP, Zeidonis DM, Feingold A, et al. Nicotine transdermal patch and atypical anti-psychotic medications for smoking cessation in schizophrenia. Am J Psychiatry. 2000;157:1835–1842. doi: 10.1176/appi.ajp.157.11.1835. [DOI] [PubMed] [Google Scholar]
- 52.Evins AE, Mays VK, Rigotti NA, Tisdale T, Cather C, Goff DC. A pilot trial of bupropion added to cognitive behavioral therapy for smoking cessation in schizophrenia. Nicotine Tob Res. 2001;3:397–403. doi: 10.1080/14622200110073920. [DOI] [PubMed] [Google Scholar]
- 53.Hall RG, Duhamel M, McClanahan R, et al. Level of functioning, severity of illness, and smoking status among chronic psychiatric patients. J Nerv Men Dis. 1995;183:468–471. doi: 10.1097/00005053-199507000-00008. [DOI] [PubMed] [Google Scholar]
- 54.Dalack GW, Becks L, Hill E, Pomerleau OF, Meador-Woodruff JH. Nicotine withdrawal and psychiatric symptoms in cigarette smokers with schizophrenia. Neuropsychopharmacology. 1999;21:195–202. doi: 10.1016/S0893-133X(98)00121-3. [DOI] [PubMed] [Google Scholar]
- 55.Sacco KA, Termine A, Seyal AA, et al. Effects of cigarette smoking function on spatial working memory and attentional function in schizophrenia: involvement of nicotinic receptor mechanisms. Arch Gen Psychiatry. 2005, in press. [DOI] [PubMed]
- 56.Addington J, el-Guebaly N, Campbell W, Hodgins DC, Addington D. Smoking cessation treatment for patients with schizophrenia. Am J Psychiatry. 1998;155:974–976. doi: 10.1176/ajp.155.7.974. [DOI] [PubMed] [Google Scholar]
- 57.Adler LE, Hoffer LD, Wiser A, Freedman R. Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Am J Psychiatry. 1993;150:1856–1861. doi: 10.1176/ajp.150.12.1856. [DOI] [PubMed] [Google Scholar]
- 58.Freedman R, Adler LE, Bickford P, et al. Schizophrenia and nicotinic receptors. Harv Rev Psychiatry. 1994;2:179–192. doi: 10.3109/10673229409017136. [DOI] [PubMed] [Google Scholar]
- 59.Olincy A, Ross RG, Young DA, Roath M, Freedman R. Improvement in smooth pursuit eye movements after cigarette smoking in schizophrenic patients. Neuropsychopharmacology. 1998;18:175–185. doi: 10.1016/S0893-133X(97)00095-X. [DOI] [PubMed] [Google Scholar]
- 60.Olincy A, Johnson LL, Ross RG. Differential effects of cigarette smoking on performance of a smooth pursuit and a saccadic eye movement task in schizophrenia. Psychiatry Res. 2003;117:223–236. doi: 10.1016/s0165-1781(03)00022-2. [DOI] [PubMed] [Google Scholar]
- 61.Avila MT, Sherr JD, Hong E, Myers CS, Thaker GK. Effects of nicotine on leading saccades during smooth pursuit eye movements in smokers and nonsmokers with schizophrenia. Neuropsychopharmacology. 2003;28:2184–2191. doi: 10.1038/sj.npp.1300265. [DOI] [PubMed] [Google Scholar]
- 62.Leonard S, Gault J, Hopkins J, et al. Promoter variants in the alpha-7 nicotinic acetylcholine receptor subunit gene are associated with an inhibitory deficit found in schizophrenia. Arch Gen Psychiatry. 2002;59:1085–1096. doi: 10.1001/archpsyc.59.12.1085. [DOI] [PubMed] [Google Scholar]
- 63.George TP, Vessicchio JC, Termine A, et al. Effects of smoking abstinence on visuospatial working memory function in schizophrenia. Neuropsychopharmacology. 2002;26:75–85. doi: 10.1016/S0893-133X(01)00296-2. [DOI] [PubMed] [Google Scholar]
- 64.Jacobsen LK, D’Souza DC, Mencl WE, Pugh KR, Skudlarski P, Krystal JH. Nicotine effects on brain function and functional connectivity in schizophrenia. Biol Psychiatry. 2004;55:850–858. doi: 10.1016/j.biopsych.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 65.Levin ED, Wilson W, Rose J, McEvoy J. Nicotine-haloperidol interactions and cognitive performance in schizophrenics. Neuropsychopharmacology. 1996;15:429–436. doi: 10.1016/S0893-133X(96)00018-8. [DOI] [PubMed] [Google Scholar]
- 66.Depatie L, Driscoll GA, Holahan A-LV, et al. Nicotine and behavioral markers of risk for schizophrenia: a double-blind, placebo-controlled, cross-over study. Neuropsychopharmacology. 2002;27:1056–1070. doi: 10.1016/S0893-133X(02)00372-X. [DOI] [PubMed] [Google Scholar]
- 67.Decina P, Caracci G, Sandik R, Berman W, Mukerjee S, Scapicchio P. Cigarette smoking and neuroleptic-induced parkinsonism. Biol Psychiatry. 1990;28:502–508. doi: 10.1016/0006-3223(90)90483-i. [DOI] [PubMed] [Google Scholar]
- 68.Yassa R, Lal S, Korpassy A, Ally J. Nicotine exposure and tardive dyskinesia. Biol Psychiatry. 1987;30:109–115. doi: 10.1016/0006-3223(87)90131-4. [DOI] [PubMed] [Google Scholar]
- 69.Yang YK, Nelson L, Kamaraju L, Wilson W, McEvoy JP. Nicotine decreases bradykinesia-rigidity in haloperidol-treated patients with schizophenia. Neuropsychopharmacology. 2002;27:684–686. doi: 10.1016/S0893-133X(02)00325-1. [DOI] [PubMed] [Google Scholar]
- 70.Hall SM, Munoz RF, Reus VI, et al. Nicotine, negative affect and depression. J Consul Clin Psychol. 1993;61:761–767. doi: 10.1037//0022-006x.61.5.761. [DOI] [PubMed] [Google Scholar]
- 71.Matthew RJ, Weinman ML, Mirabi M. Physical symptoms of depression. Br J Psychiatry. 1981;139:293–296. [PubMed] [Google Scholar]
- 72.Anda RF, Williamson DF, Escobedo LG, et al. Depression and the dynamics of smoking. JAMA. 1990;264:1541–1545. [PubMed] [Google Scholar]
- 73.Glassman AH, Stetner F, Walsh BT, et al. Heavy smokers, smoking cessation, and clonidine. JAMA. 1988;259:2863–2866. [PubMed] [Google Scholar]
- 74.Perez-Stable EJ, Marin G, Marin BV, Katz MH. Depressive symptoms and cigarette smoking among Latinos in San Francisco. Am J Public Health. 1990;80:1500–1502. doi: 10.2105/ajph.80.12.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Niaura R, Britt DM, Shadel WM, Goldstein M, Abrams D, Brown R. Symptoms of depression and survival experience among three samples of smokers trying to quit. Psychol Addict Behav. 2001;15:13–17. doi: 10.1037/0893-164x.15.1.13. [DOI] [PubMed] [Google Scholar]
- 76.Glassman AH, Helzer JE, Covey LS, et al. Smoking, smoking cessation and major depression. JAMA. 1990;264:1546–1549. [PubMed] [Google Scholar]
- 77.Glassman AH, Covey LS, Dalack GW, et al. Smoking cessation, clonidine, and vulnerability to nicotine among dependent smokers. Clin Pharmacol Ther. 1993;54:670–679. doi: 10.1038/clpt.1993.205. [DOI] [PubMed] [Google Scholar]
- 78.Covey LS, Glassman AH, Stetner F. Major depression following smoking cessation. Am J Psychiatry. 1997;154:263–265. doi: 10.1176/ajp.154.2.263. [DOI] [PubMed] [Google Scholar]
- 79.Thorsteinsson HS, Gillin JC, Patten CA, et al. The effects of transdermal nicotine therapy for smoking cessation on depressive symptoms in patients with major depression. Neuropsychopharmacology. 2001;24:350–358. doi: 10.1016/S0893-133X(00)00217-7. [DOI] [PubMed] [Google Scholar]
- 80.Tsoh JY, Humfleet GL, Munoz RF, Reus VI, Hartz DT, Hall SM. Development of major depression after treatment for smoking cessation. Am J Psychiatry. 2000;157:368–374. doi: 10.1176/appi.ajp.157.3.368. [DOI] [PubMed] [Google Scholar]
- 81.Breslau N, Kilbey MM, Andreski P. Nicotine withdrawal symptoms and psychiatric disorders: findings from an epidemiologic study of young adults. Am J Psychiatry. 1992;149:464–469. doi: 10.1176/ajp.149.4.464. [DOI] [PubMed] [Google Scholar]
- 82.Gonzalez-Pinto A, Gutierrez M, Ezcurra J, et al. Tobacco smoking and bipolar disorder. J Clin Psychiatry. 1998;59:225–228. doi: 10.4088/jcp.v59n0503. [DOI] [PubMed] [Google Scholar]
- 83.Cassidy F, McEvoy JP, Yang Y-K, Wilson WH. Smoking and psychosis in patients with bipolar I disorder. Compr Psychiatry. 2002;43:63–64. doi: 10.1053/comp.2002.29847. [DOI] [PubMed] [Google Scholar]
- 84.Corvin A, O’Mahoney E, O’Regan M, et al. Cigarette smoking and psychotic symptoms in bipolar affective disorder. Brit J Psychiatry. 2001;179:35–38. doi: 10.1192/bjp.179.1.35. [DOI] [PubMed] [Google Scholar]
- 85.Baker-Morissette SL, Gulliver SB, Wiegel M, Barlow DH. Prevalence of smoking in anxiety disorders uncomplicated by comorbid alcohol or substance abuse. J Psychopathol Behav Assess. 2004;26:107–112. [Google Scholar]
- 86.Amering M, Bankier B, Berger P, Griengl H, Windhaber J, Katsching H. Panic disorder and cigarette smoking behavior. Compr Psychiatry. 1999;40:35–38. doi: 10.1016/s0010-440x(99)90074-3. [DOI] [PubMed] [Google Scholar]
- 87.Pohl R, Yergani VK, Balon R, Lycaki H, McBride R. Smoking in patients with panic disorder. Psychiatry Res. 1992;43:253–262. doi: 10.1016/0165-1781(92)90058-b. [DOI] [PubMed] [Google Scholar]
- 88.Himle J, Thyer BA, Fischer DJ. Prevalence of smoking among anxious outpatients. Phobia Pract Res J. 1988;1:25–31. [Google Scholar]
- 89.Breslau N, Klein DF. Smoking and panic attacks: an epidemiologic investigation. Arch Gen Psychiatry. 1999;56:1141–1147. doi: 10.1001/archpsyc.56.12.1141. [DOI] [PubMed] [Google Scholar]
- 90.Johnson JG, Cohen P, Pine DS, Klein DF, Kasen S, Brook JS. Association between cigarette smoking and anxiety disorders during adolescence and early adulthood. JAMA. 2000;284:2348–2351. doi: 10.1001/jama.284.18.2348. [DOI] [PubMed] [Google Scholar]
- 91.Isensee B, Wittchen H, Stein MB, Hofler M, Lieb R. Smoking increases the risk of panic: findings from a prospective community sample. Arch Gen Psychiatry. 2003;60:692–700. doi: 10.1001/archpsyc.60.7.692. [DOI] [PubMed] [Google Scholar]
- 92.Bejerot S, Humble M. Low prevalence of smoking among patients with obsessive-compulsive disorder. Compl Psychiatry. 1999;40:268–272. doi: 10.1016/s0010-440x(99)90126-8. [DOI] [PubMed] [Google Scholar]
- 93.McCabe RE, Chudzik SM, Antony MM. Smoking behaviors across anxiety disorders. J Anxiety Disord. 2004;18:7–18. doi: 10.1016/j.janxdis.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 94.Beckham JC, Roodman AA, Shipley RH, et al. Smoking in Vietnam combat veterans with posttraumatic stress disorder. J Trauma Stress. 1995;8:461–472. doi: 10.1007/BF02102970. [DOI] [PubMed] [Google Scholar]
- 95.Beckham JC, Kirby AC, Feldman ME, et al. Prevalence and correlates of heavy smoking in Vietnam veterans with chronic posttraumatic stress disorder. Addict Behav. 1997;22:637–647. doi: 10.1016/s0306-4603(96)00071-8. [DOI] [PubMed] [Google Scholar]
- 96.Op den Velde W, Aarts PGH, Falger PRJ, et al. Alcohol use, cigarette consumption and chronic post-traumatic stress disorder. Alcohol Alcohol. 2002;37:355–361. doi: 10.1093/alcalc/37.4.355. [DOI] [PubMed] [Google Scholar]
- 97.Stellman JM, Stellman SD, Sommer JF. Social and behavioral consequences of the Vietnam experience among American Legionnaires. Environ Res. 1988;47:129–149. doi: 10.1016/s0013-9351(88)80038-0. [DOI] [PubMed] [Google Scholar]
- 98.Beckham JC, Lytle BL, Vrana SR, Hertzberg MA, Feldman ME, Shipley RH. Smoking withdrawal symptoms in response to trauma-related stimuli among Vietnam combat veterans with posttraumatic stress. Addic Behav. 1995;20:1–9. doi: 10.1016/0306-4603(95)00038-0. [DOI] [PubMed] [Google Scholar]
- 99.Acierno RA, Kilpatrick DG, Resnick HS, Saunders BE, Best CL. Violent assault, posttraumatic stress disorder, and depression: risk factors for cigarette use among adult women. Behavior Modification. 1996;20:363–384. doi: 10.1177/01454455960204001. [DOI] [PubMed] [Google Scholar]
- 100.Vlahov D, Galea S, Resnick H, et al. Increased use of cigarettes, alcohol and marijuana among Manhattan, New York residents after the September 11th terrorist attacks. Am J Pub Health. 2002;155:988–996. doi: 10.1093/aje/155.11.988. [DOI] [PubMed] [Google Scholar]
- 101.Anda RF, Croft JB, Felitti VJ, et al. Adverse childhood experiences and smoking during adolescence and adulthood. JAMA. 1999;282:1652–1658. doi: 10.1001/jama.282.17.1652. [DOI] [PubMed] [Google Scholar]
- 102.Breslau N, Davis GC, Schultz LR. Posttraumatic stress disorder and the incidence of nicotine, alcohol, and other drug disorders in persons who have experienced trauma. Arch Gen Psychiatry. 2003;60:289–294. doi: 10.1001/archpsyc.60.3.289. [DOI] [PubMed] [Google Scholar]
- 103.Milberger S, Biederman J, Faraone SV, Chen L, Jones J. ADHD is associated with early initiation of cigarette smoking in children and adolescents. J Am Acad Child Adolesc Psychiatry. 1997;36:37–44. doi: 10.1097/00004583-199701000-00015. [DOI] [PubMed] [Google Scholar]
- 104.Wilens TE, Biederman J, Spencer TJ, et al. A pilot controlled clinical trial of ABT-418, a cholinergic agonist, in the treatment of adults with attention deficit hyperactivity disorder. Am J Psychiatry. 1999;156:1931–1937. doi: 10.1176/ajp.156.12.1931. [DOI] [PubMed] [Google Scholar]
- 105.Gulliver SB, Rohsenow DJ, Colby SM, et al. Interrelationship of smoking and alcohol dependence, use and urges to use. J Stud Alcohol. 1995;56:202–206. doi: 10.15288/jsa.1995.56.202. [DOI] [PubMed] [Google Scholar]
- 106.Gulliver SB, Kalman D, Rohsenow DJ, Colby SM, Monti PM, Eaton CA. Smoking and drinking among alcoholics in treatment: cross-sectional and longitudinal relationships. J Stud Alcohol. 2000;61:157–163. doi: 10.15288/jsa.2000.61.157. [DOI] [PubMed] [Google Scholar]
- 107.Hughes JR. Clinical implications of the association between smoking and alcoholism. In: Fertig J, Fuller R, eds. Alcohol and Tobacco: From Basic Science to Policy. Washington, DC: NIAAA Research Monograph; 1995:171–181.
- 108.Kalman D, Tirch D, Kahler C, Penk W, Monti P, Kaschub C. Preliminary finding from an investigation of high dose nicotine patch therapy for heavy smokers with a past history of alcohol dependence. Psychology of Addictive Behaviors. 2004;18:78–82. doi: 10.1037/0893-164X.18.1.78. [DOI] [PubMed] [Google Scholar]
- 109.Blomqvist O, Hernandez-Avila CA, Van Kirk J, Rose JE, Kranzler HR. Mecamylamine modifies the pharmacokinetics and reinforcing effects of alcohol. Alcohol Clin Exp Res. 2002;26:326–331. [PubMed] [Google Scholar]
- 110.Soderpalm B, Ericson M, Olausson P, Blomqvist O, Engel JA. Nicotinic mechanisms involved in the dopamine activating and reinforcing properties of ethanol. Behav Brain Res. 2000;113:85–96. doi: 10.1016/s0166-4328(00)00203-5. [DOI] [PubMed] [Google Scholar]
- 111.Le AD, Corrigall WA, Watchus J, Harding J, Juzytsch S, Li T-K. Involvement of nicotinic receptors in alcohol self-administration. Alcoholism: Clin Exp Res. 2000;24:155–163. doi: 10.1111/j.1530-0277.2000.tb04585.x. [DOI] [PubMed] [Google Scholar]
- 112.Blomqvist O, Ericson M, Johnson DH, Engel JA, Solderpalm B. Voluntary ethanol intake in the rat: effects of nicotinic acetylcholine receptor blockade or subchronic nicotine treatment. J Pharmacol. 1996;314:257–267. doi: 10.1016/s0014-2999(96)00583-3. [DOI] [PubMed] [Google Scholar]
- 113.Mitchell SH, deWit H, Zacny JP. Effects of varying ethanol dose on cigarette consumption in healthy normal volunteers. Behav Pharmacol. 1995;6:356–359. [PubMed] [Google Scholar]
- 114.Perkins KA, Fonte C, Ashcom J, Broge M, Wilson A. Subjective responses to nicotine in smokers may be associated with responses to caffeine and alcohol. Exp Clin Psychopharmacol. 2001;9:91–100. doi: 10.1037/1064-1297.9.1.91. [DOI] [PubMed] [Google Scholar]
- 115.Cooney JL, Cooney NL, Pilkey DT, Kranzler HK, Onken CA. Effects of nicotine deprivation on urges to drink and smoke in alcoholic smokers. Addiction. 2003;98:913–921. doi: 10.1046/j.1360-0443.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- 116.Rohsenow DJ, Monti PM, Colby SM, et al. Effects of alcohol cues on smoking urges and topography among alcoholic men. Alcohol Clin Exp Res. 1997;21:101–107. [PubMed] [Google Scholar]
- 117.Budney AJ, Higgins ST, Hughes JR, Bickel WK. Nictotine and caffeine use in cocaine-dependent individuals. J Subst Abuse. 1993;5:117–130. doi: 10.1016/0899-3289(93)90056-h. [DOI] [PubMed] [Google Scholar]
- 118.Patkar AA, Lundy A, DeMaria P, Purcell C, Leone FT, Weinstein SP. A comparison of nicotine dependence among cocaine and opiate abusers. 6th Annual Meeting of the Society for Research on Nicotine and Tobacco, February 18–21, 2000, Arlington, VA.
- 119.Roll JM, Higgins ST, Budney AJ, Bickel WK, Badger GJ. A comparison of cocaine-dependent cigarette smokers and non-smokers on demographic, drug use and other characteristics. Drug Alcohol Depend. 1996;40:195–201. doi: 10.1016/0376-8716(96)01219-7. [DOI] [PubMed] [Google Scholar]
- 120.Horger BA, Giles MK, Schenk S. Preexposure to amphetamine and nicotine pre-disposes rats to self-administer a low dose of cocaine. Psychopharmacology. 1992;107:271–276. doi: 10.1007/BF02245147. [DOI] [PubMed] [Google Scholar]
- 121.Zachariou V, Weathers-Lowin A, Caldarone BJ, George TP, Changeaux J-P, Picciotto MR. Nicotine receptor inactivation can decrease sensitivity to cocaine. Neuropsychopharmacology. 2001;24:576–589. doi: 10.1016/S0893-133X(00)00224-4. [DOI] [PubMed] [Google Scholar]
- 122.Reid MS, Mickalian JD, Delucchi KL, Hall SM, Berger SP. An acute dose of nicotine enhances cue-induced cocaine craving. Drug Alcohol Depend. 1998;49:95–104. doi: 10.1016/s0376-8716(97)00144-0. [DOI] [PubMed] [Google Scholar]
- 123.Reid MS, Mickalian JD, Delucchi KL, Berger SP. A nicotinic antagonist, mecamylamine, reduces cue-induced cocaine craving in cocaine-dependent subjects. Neuropsychopharmacology. 1999;20:297–307. doi: 10.1016/S0893-133X(98)00076-1. [DOI] [PubMed] [Google Scholar]
- 124.Clemmey P, Brooner R, Chutuape MA, Kidorf M, Stitzer M. Smoking habits and attitudes in a methadone maintenance treatment population. Drug Alcohol Depend. 1997;44:123–132. doi: 10.1016/s0376-8716(96)01331-2. [DOI] [PubMed] [Google Scholar]
- 125.Berger H, Schweigler M. Smoking characteristics of methadone patients. JAMA. 1972;222:705. [PubMed] [Google Scholar]
- 126.Meyer TJ, Lin MM, Brown LS., Jr Nicotine dependence and depression among methadone maintenance patients. J Nat Med Assoc. 1996;88:800–804. [PMC free article] [PubMed] [Google Scholar]
- 127.Shoptaw S, Jarvik ME, Ling W, Rawson RA. Contingency management for tobacco smoking in methadone-maintained opiate addicts. Addict Behav. 1996;21:409–412. doi: 10.1016/0306-4603(95)00066-6. [DOI] [PubMed] [Google Scholar]
- 128.Story J, Stark MJ. Treating cigarette smoking in methadone maintenance clients. J Psychoactive Drugs. 1991;23:203–215. doi: 10.1080/02791072.1991.10472237. [DOI] [PubMed] [Google Scholar]
- 129.Conner BT, Stein JA, Longshore D, Stacy AW. Associations between drug abuse treatment and cigarette use: evidence of substance replacement. Exp Clin Psychopharmacol. 1999;7:64–71. doi: 10.1037//1064-1297.7.1.64. [DOI] [PubMed] [Google Scholar]
- 130.McEvoy J, Freudenreich O, McGee M, VanderZwaag C, Levin E, Rose J. Clozapine decreases smoking in patients with chronic schizophrenia. Biol Psychiatry. 1995;37:550–552. doi: 10.1016/0006-3223(94)00365-A. [DOI] [PubMed] [Google Scholar]
- 131.McEvoy JP, Freudenreich O, Wilson WH. Smoking and therapeutic response to clozapine in patients with schizophrenia. Biol Psychiatry. 1999;46:125–129. doi: 10.1016/s0006-3223(98)00377-1. [DOI] [PubMed] [Google Scholar]
- 132.Ascher JA, Cole JO, Colin JN, et al. Bupropion: a review of its mechanism of antidepressant activity. J Clin Psychiatry. 1995;56:395–401. [PubMed] [Google Scholar]
- 133.Niaura R, Borreli B, Hedeker D, et al. Multi-center trial of fluoxetine as an adjunct to behavioral smoking cessation treatment. J Consult Clin Psychology. 2002;70:887–896. doi: 10.1037//0022-006X.70.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Volpicelli JR, Alterman AI, Hayashida M, O’Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876–880. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- 135.O’Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B. Naltrexone and coping skills therapy for alcohol dependence: a controlled study. Arch Gen Psychiatry. 1992;49:881–887. doi: 10.1001/archpsyc.1992.01820110045007. [DOI] [PubMed] [Google Scholar]
- 136.Krishnan-Sarin S, Meandjiza B, O’Malley SS. Nicotine patch and naltrexone for smoking cessation: a preliminary study. Nicotine Tob Res. 2003;5:851–857. doi: 10.1080/14622200310001614601. [DOI] [PubMed] [Google Scholar]
- 137.Hall SM, Reus VI, Munoz RF, et al. Nortriptyline and cognitive-behavioral therapy in the treatment of cigarette smoking. Arch Gen Psychiatry. 1998;55:683–690. doi: 10.1001/archpsyc.55.8.683. [DOI] [PubMed] [Google Scholar]
- 138.Cornelius JR, Perkins KA, Salloum IM, Thase ME, Moss HB. Fluoxetine vs. placebo to decrease the smoking of depressed alcoholic patients. J Clin Psychopharmacol. 1999;19:183–184. doi: 10.1097/00004714-199904000-00015. [DOI] [PubMed] [Google Scholar]
- 139.Covey LS, Glassman AH, Stetner F, Rivelli S. A trial of sertraline for smokers with past major depression. Am J Psychiatry. 2002;159:1731–1737. doi: 10.1176/appi.ajp.159.10.1731. [DOI] [PubMed] [Google Scholar]
- 140.Addington J. Group treatment for smoking cessation among persons with schizophrenia. Psychiatri Serv. 1998;49:925–928. doi: 10.1176/ps.49.7.925. [DOI] [PubMed] [Google Scholar]
- 141.Hall SM, Munoz RF, Reus VI. Cognitive-behavioral intervention increases abstinence rates for depressive-history smokers. J Consult Clin Psychol. 1994;62:141–146. doi: 10.1037//0022-006x.62.1.141. [DOI] [PubMed] [Google Scholar]
- 142.Patten CA, Martin JE, Myers MG, Calfas KJ, Williams CD. Effectiveness of cognitive-behavioral therapy for smokers with histories of alcohol dependence and depression. J Stud Alcohol. 1998;59:327–335. doi: 10.15288/jsa.1998.59.327. [DOI] [PubMed] [Google Scholar]
- 143.Brown RA, Kahler CW, Niaura R, et al. Cognitive-behavioral treatment for depression in smoking cessation. J Consult Clin Psychol. 2001;3:471–480. doi: 10.1037//0022-006x.69.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Burling TA, Salvio MA, Seidner AL, Ramsey TG. Cigarette smoking in alcohol and cocaine abusers. J Subst Abuse. 1996;8:445–452. doi: 10.1016/s0899-3289(96)90005-x. [DOI] [PubMed] [Google Scholar]
- 145.Bobo JK, Slade J, Hoffman AL. Nicotine addiction counseling for chemical dependent patients. Psychiatri Serv. 1995;46:945–947. doi: 10.1176/ps.46.9.945. [DOI] [PubMed] [Google Scholar]
- 146.Campbell BK, Wander N, Stark MJ, Holbert T. Treating cigarette smoking in drug-abusing clients. J Subst Abuse Treatment. 1995;12:89–94. doi: 10.1016/0740-5472(95)00002-m. [DOI] [PubMed] [Google Scholar]
- 147.Campbell BK, Krumenacker J, Stark MJ. Smoking cessation for clients in chemical dependence treatment: a demonstration project. J Subst Abuse Treatment. 1998;15:313–318. doi: 10.1016/s0740-5472(97)00197-9. [DOI] [PubMed] [Google Scholar]
- 148.Schmitz JM, Rhoades H, Grabowski J. Contingent reinforcement for reducing carbon monoxide level in methadone maintained patients. Addict Behav. 1995;20:171–179. doi: 10.1016/0306-4603(94)00059-x. [DOI] [PubMed] [Google Scholar]
- 149.Sussman S. Smoking cessation among persons in recovery. Subst Use Misuse. 2002;37:1275–1298. doi: 10.1081/ja-120004185. [DOI] [PubMed] [Google Scholar]
- 150.Kalman D. Smoking cessation treatment for substance misusers in early recovery: a review of the literature and recommendations for practice. Subst Use Misuse. 1998;33:2021–2047. doi: 10.3109/10826089809069815. [DOI] [PubMed] [Google Scholar]
- 151.O’Brien CP. Principles of the pharmacotherapy of substance abuse disorders. In: Charney DS, Nestler EJ, Bunney BS, eds. Neurobiology of Mental Illness. New York, NY: Oxford University Press; 1999:627–638.
- 152.Burling TA, Ramsey TG, Seidner AL, Kondo CS. Issues related to smoking cessation among substance abusers. J Subst Abuse Treat. 1997;9:27–40. doi: 10.1016/s0899-3289(97)90004-3. [DOI] [PubMed] [Google Scholar]
- 153.George TP, Seyal AA, Dolan SL, Dudas MM, Termine A, Vessicchio JC. Nicotine addiction and schizophrenia: a clinical approach. Primary Psychiatry. 2002;9:48–53. [Google Scholar]
- 154.Kalman D, Hayes K, Colby SM, Eaton CA, Rohsenow DJ, Monti PM. Concurrent versus delayed smoking cessation treatment for persons in early alcohol recovery: a pilot study. J Subst Abuse Treatment. 2001;20:233–238. doi: 10.1016/s0740-5472(00)00174-4. [DOI] [PubMed] [Google Scholar]
- 155.Burling TA, Burling AS, Latini D. A controlled smoking cessation trial for substance-dependent inpatients. J Consult Clin Psychol. 2001;69:295–304. doi: 10.1037//0022-006x.69.2.295. [DOI] [PubMed] [Google Scholar]
- 156.Kalman D, Kahler C, Tirch D, Penk W, Kaschib C, Monti PM. Twelve-week outcomes from an investigation of high dose nicotine patch therapy for heavy smokers with a past history of alcohol dependence. Psychol Addictive Behav. 2004;18:78–82. doi: 10.1037/0893-164X.18.1.78. [DOI] [PubMed] [Google Scholar]
- 157.Joseph A, Willenbring M, Nugent S, Nelson D. Timing of alcohol and smoking cessation study. 9th Annual Meeting of the Society for Research on Nicotine & Tobacco, February 19–22, 2003, New Orleans, LA.
- 158.Ellingstad TP, Sobell LC, Sobell MB, Cleland PA, Agrawal S. Alcohol abusers who want to quit smoking: implications for clinical treatment. Drug Alcohol Depend. 1999;54:259–265. doi: 10.1016/s0376-8716(98)00180-x. [DOI] [PubMed] [Google Scholar]
- 159.Hser YI, McCarthy WJ, Anglin MD. Tobacco use as a distal predictor of mortality among long-term narcotic addicts. Prev Medi. 1994;23:61–69. doi: 10.1006/pmed.1994.1009. [DOI] [PubMed] [Google Scholar]
- 160.Burling TA, Marshall GD, Seidner AL. Smoking cessation for substance abuse inpatients. J Subst Abuse. 1991;3:269–276. doi: 10.1016/s0899-3289(10)80011-2. [DOI] [PubMed] [Google Scholar]
- 161.Evins AE, Cather C, Rigotti NA, et al. Two-year follow-up of a smoking cessation trial in patients with schizophrenia: increased rates of smoking cessation and reduction. J Clin Psychiatry. 2004;65:307–311. doi: 10.4088/jcp.v65n0304. [DOI] [PubMed] [Google Scholar]
- 162.Warner KE, Slade J, Sweanor DT. The emerging market for long-term nicotine maintenance. JAMA. 1997;278:1087–1092. [PubMed] [Google Scholar]
- 163.Allebeck P. Schizophrenia: a life-shortening disease. Schizophr Bull. 1989;15:81–89. doi: 10.1093/schbul/15.1.81. [DOI] [PubMed] [Google Scholar]
- 164.Bobo JK, Lando HA, Walker RD, McIlvain HE. Predictors of tobacco quit attempts among recovering alcoholics. J Subst Abuse. 1996;8:431–443. doi: 10.1016/s0899-3289(96)90004-8. [DOI] [PubMed] [Google Scholar]
- 165.Hurt RD, Patten CA. Treatment of tobacco dependence in alcoholics. Recent Dev Alcohol. 2003;16:335–359. doi: 10.1007/0-306-47939-7_23. [DOI] [PubMed] [Google Scholar]


